

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50172414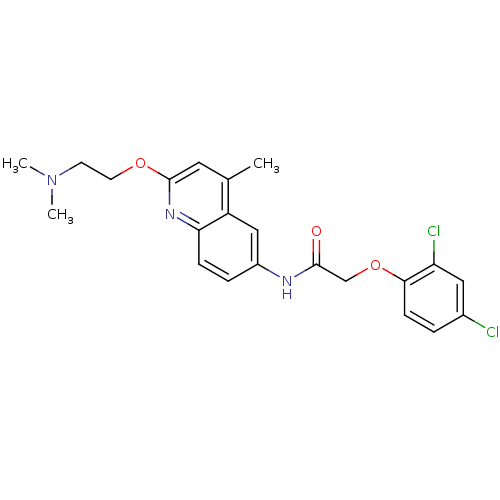 (2-(2,4-Dichloro-phenoxy)-N-[2-(2-dimethylamino-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S Curated by ChEMBL | Assay Description Inhibitory concentration against histamine H2 receptor | J Med Chem 48: 5684-97 (2005) Article DOI: 10.1021/jm050103y BindingDB Entry DOI: 10.7270/Q2H41QZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM22568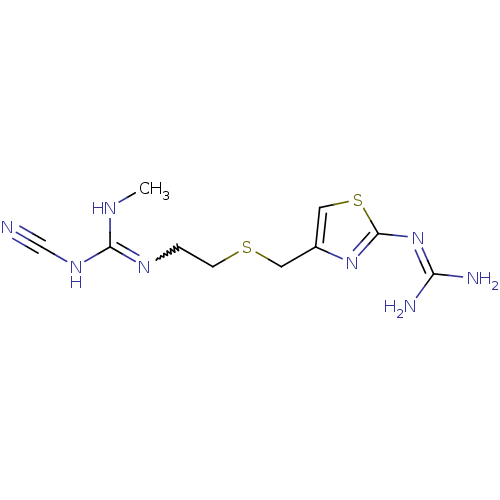 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Aminopotentidine from human recombinant histamine H2 receptor expressed in CHOK1 cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156855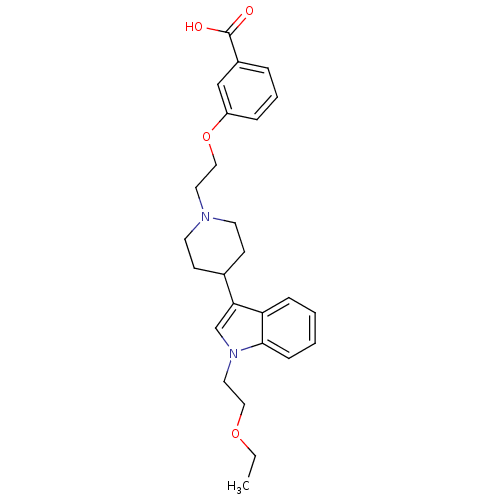 (3-(2-(4-(1-(2-ethoxyethyl)-1H-indol-3-yl)piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50403559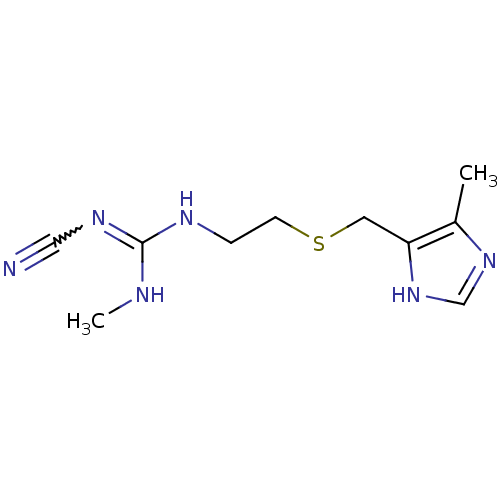 (Brumetadina | CIMETIDINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human histamine H2 receptor by radioligand displacement assay | Eur J Med Chem 63: 85-94 (2013) Article DOI: 10.1016/j.ejmech.2013.01.044 BindingDB Entry DOI: 10.7270/Q2JH3NKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [125I]APT from human recombinant histamine H2 receptor after 120 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156856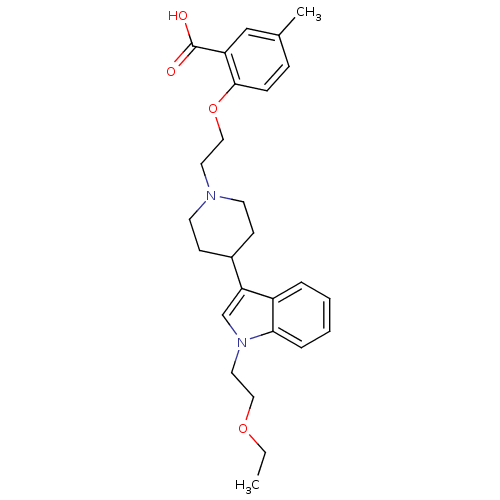 (2-(2-(4-(1-(2-ethoxyethyl)-1H-indol-3-yl)piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156857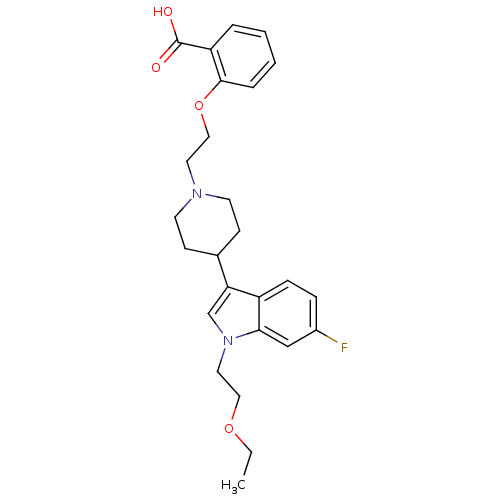 (2-(2-(4-(1-(2-ethoxyethyl)-6-fluoro-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50403559 (Brumetadina | CIMETIDINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis Curated by ChEMBL | Assay Description Binding affinity to human histamine H2 receptor by radioligand displacement assay | Bioorg Med Chem 21: 2764-71 (2013) Article DOI: 10.1016/j.bmc.2013.03.016 BindingDB Entry DOI: 10.7270/Q2N87C5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50105105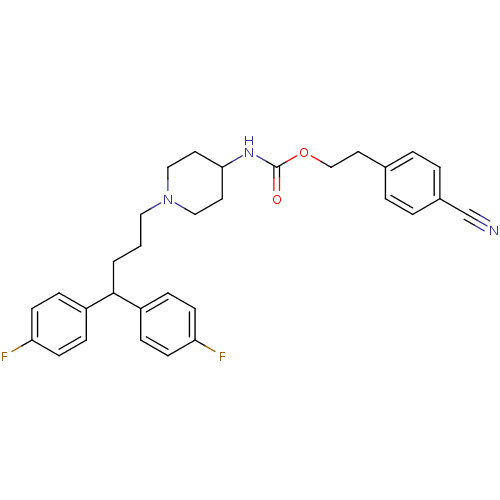 (CHEMBL116463 | {1-[4,4-Bis-(4-fluoro-phenyl)-butyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156900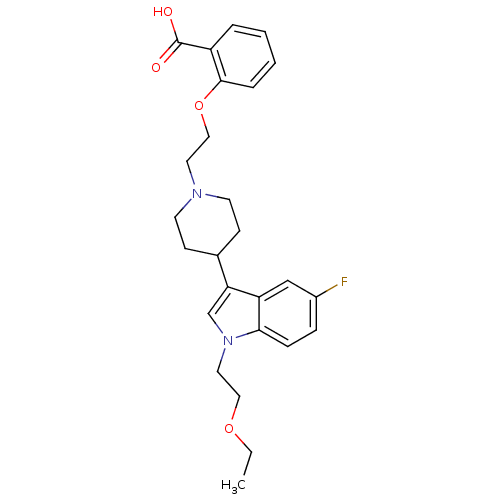 (2-(2-(4-(1-(2-ethoxyethyl)-5-fluoro-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM181119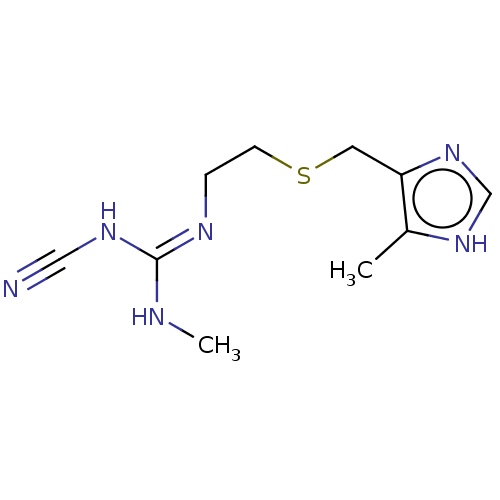 (US9138393, Cimetidine | US9144538, Cimetidine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 60: 349-361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01422 BindingDB Entry DOI: 10.7270/Q27M0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156892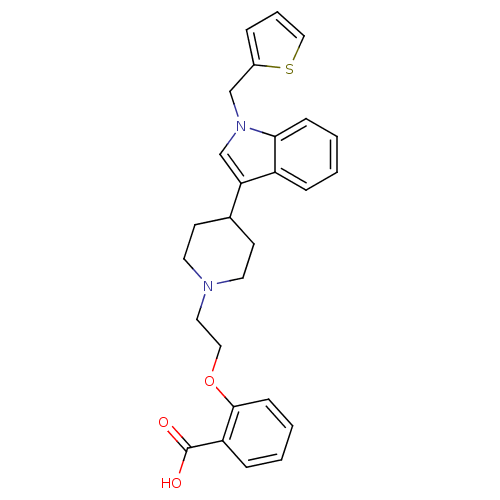 (2-(2-(4-(1-(thiophen-2-ylmethyl)-1H-indol-3-yl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156878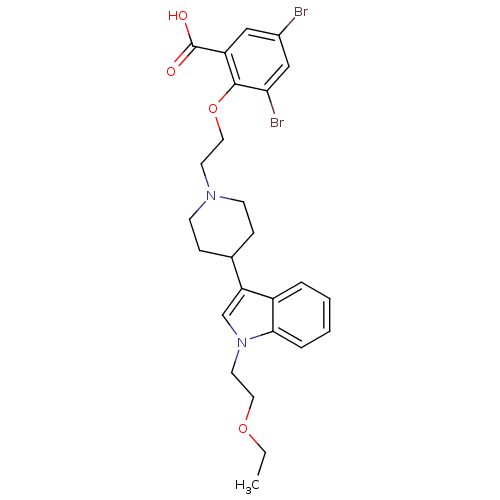 (2-(2-(4-(1-(2-ethoxyethyl)-1H-indol-3-yl)piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156887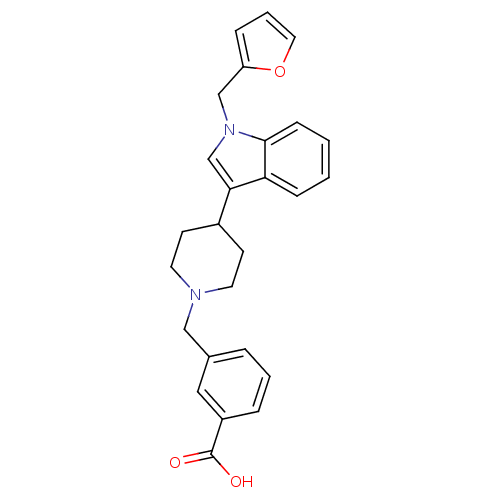 (3-[4-(1-furan-2-ylmethyl-1H-indol-3-yl)piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156894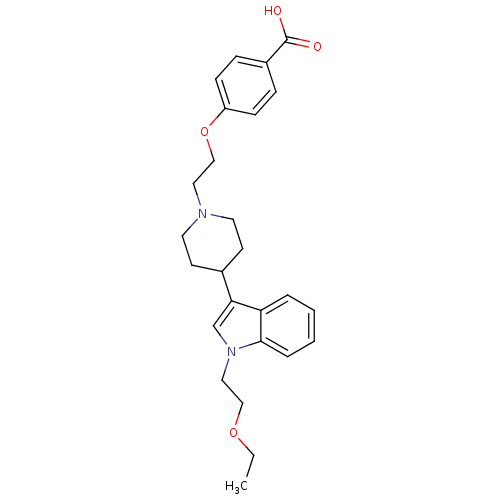 (4-(2-(4-(1-(2-ethoxyethyl)-1H-indol-3-yl)piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 691 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50105102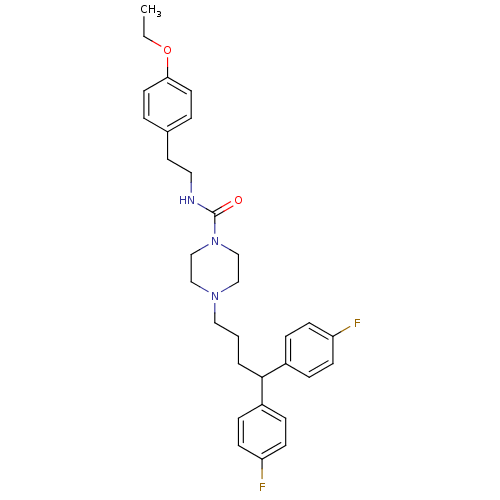 (4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 757 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156893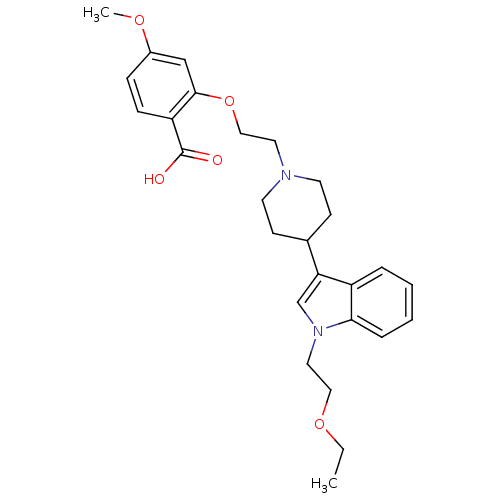 (2-(2-{4-[1-(2-Ethoxy-ethyl)-1H-indol-3-yl]-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 779 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156875 (2-(2-(4-(1-(2-ethoxyethyl)-1H-indol-3-yl)piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50172421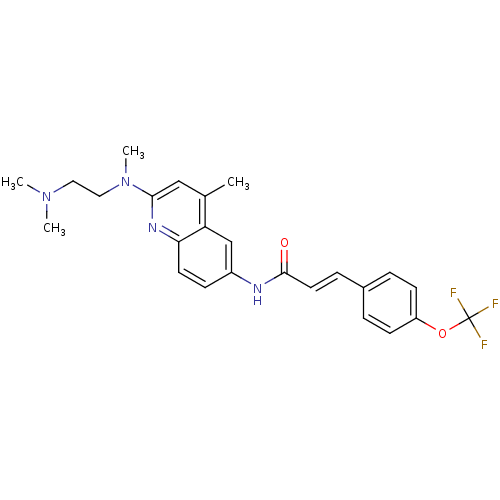 ((E)-N-{2-[(2-Dimethylamino-ethyl)-methyl-amino]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S Curated by ChEMBL | Assay Description Inhibitory concentration against Histamine H2 receptor | J Med Chem 48: 5684-97 (2005) Article DOI: 10.1021/jm050103y BindingDB Entry DOI: 10.7270/Q2H41QZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156886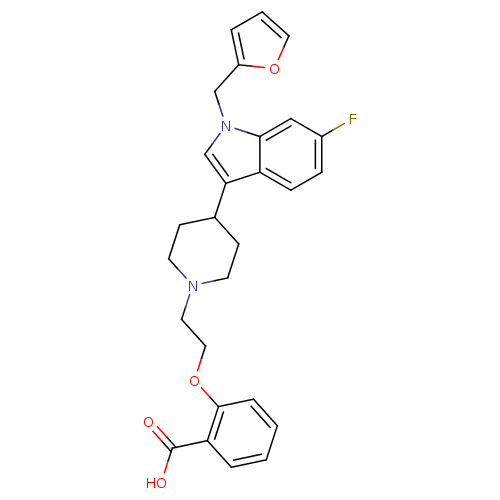 (2-{2-[4-(6-fluoro-1-furan-2-ylmethyl-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156876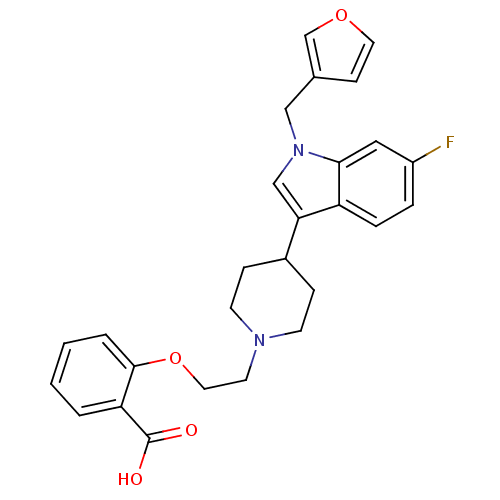 (2-{2-[4-(6-fluoro-1-furan-3-ylmethyl-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156890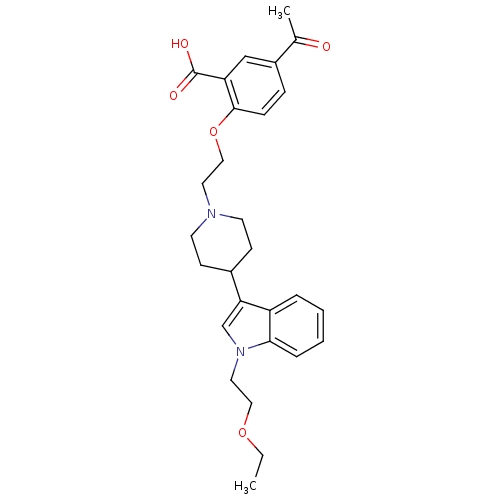 (5-acetyl-2-(2-(4-(1-(2-ethoxyethyl)-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50103595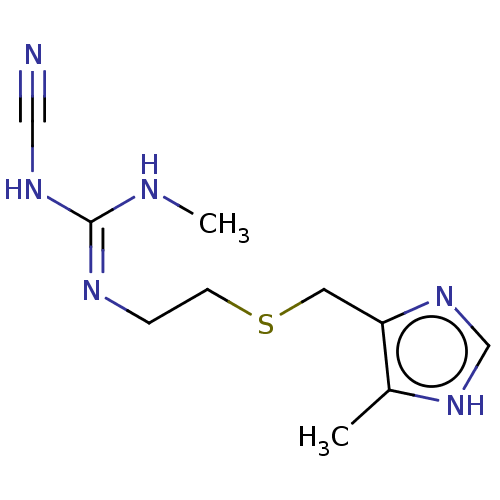 (Brumetadina | CHEBI:3699 | Cimetidine | Tagamet | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]APT from human recombinant H2 receptor expressed in CHO cells measured after 120 mins by scintillation counting method | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50103595 (Brumetadina | CHEBI:3699 | Cimetidine | Tagamet | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50330441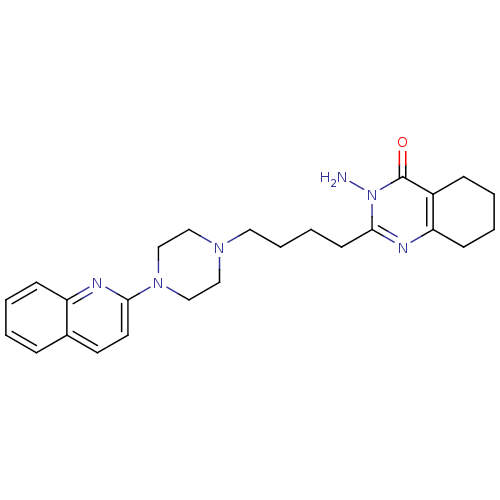 (3-amino-2-(4-(4-(quinolin-2-yl)piperazin-1-yl)buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Displacement of [125I]APT from human recombinant histamine H2 receptor | J Med Chem 53: 7549-63 (2010) Article DOI: 10.1021/jm1002292 BindingDB Entry DOI: 10.7270/Q27082D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50260582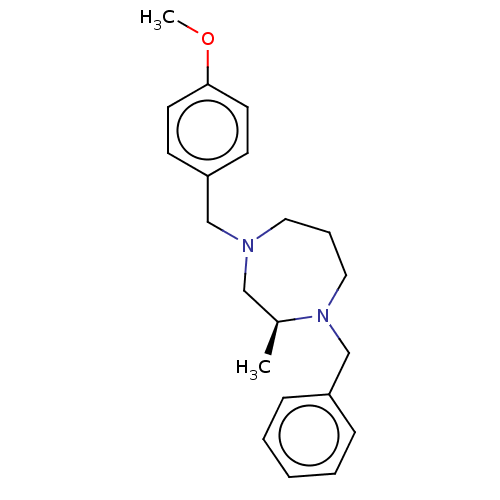 (CHEMBL4086541) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [125I]-aminopotentidine receptor from histamine H2 receptor (unknown origin) | Bioorg Med Chem 25: 4778-4799 (2017) Article DOI: 10.1016/j.bmc.2017.07.027 BindingDB Entry DOI: 10.7270/Q26M3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156861 (2-(2-(4-(1-((5-chlorothiophen-2-yl)methyl)-6-fluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50105109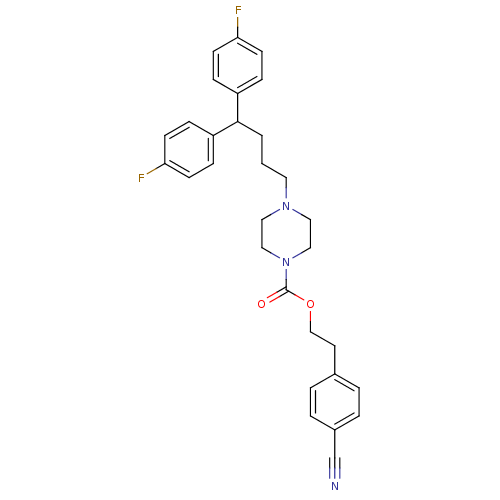 (4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156863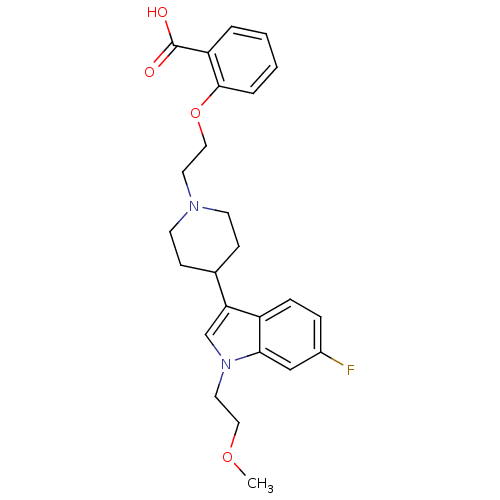 (2-(2-{4-[6-fluoro-1-(2-methoxyethyl)-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50105101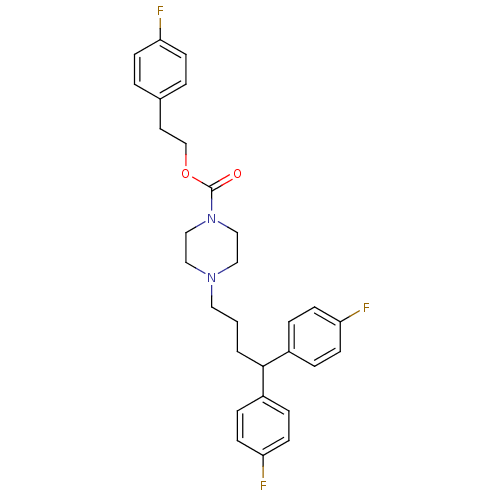 (4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazine-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156884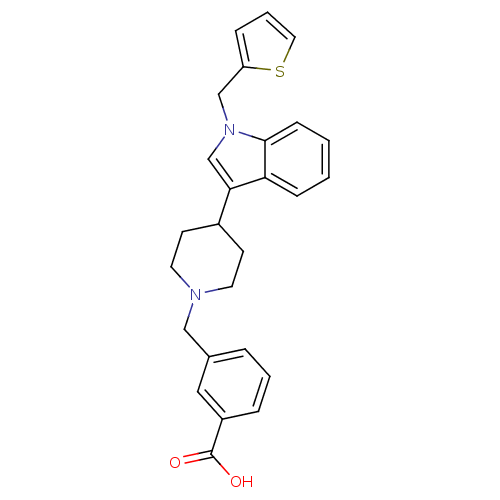 (3-[4-(1-thiophen-2-ylmethyl-1H-indol-3-yl)piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50105103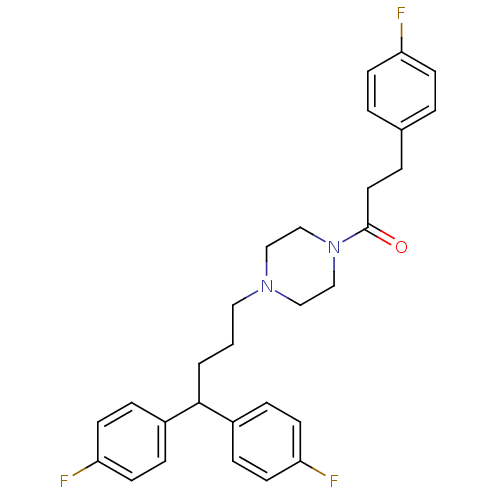 (1-{4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156860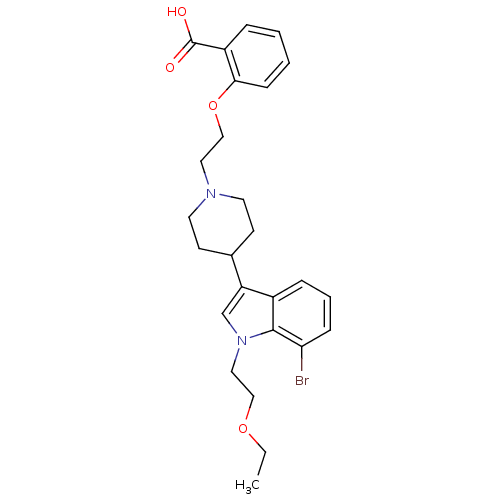 (2-(2-(4-(7-bromo-1-(2-ethoxyethyl)-1H-indol-3-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156877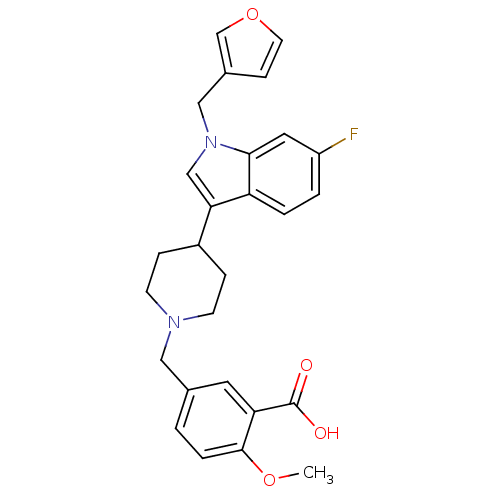 (5-[4-(6-fluoro-1-furan-3-ylmethyl-1H-indol-3-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156859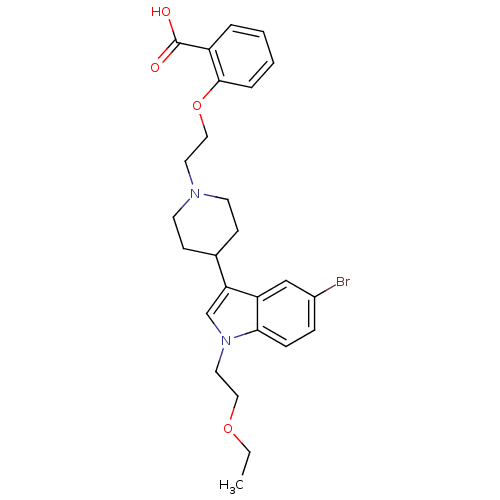 (2-(2-(4-(5-bromo-1-(2-ethoxyethyl)-1H-indol-3-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156871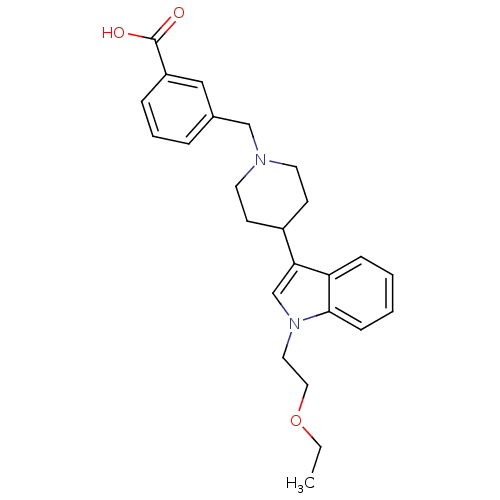 (3-{4-[1-(2-Ethoxy-ethyl)-1H-indol-3-yl]-piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156882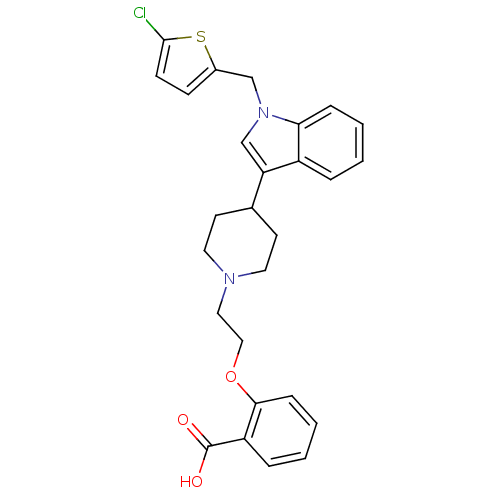 (2-(2-(4-(1-((5-chlorothiophen-2-yl)methyl)-1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156891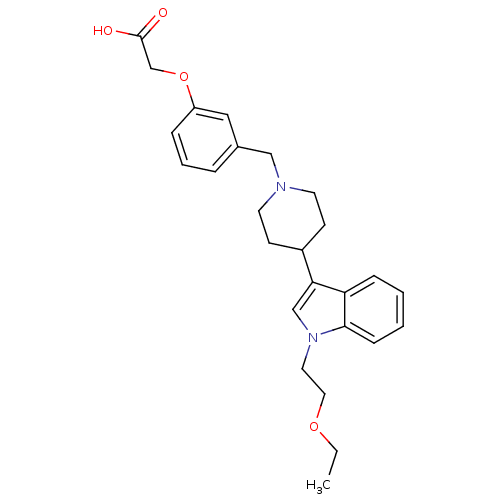 (2-(3-((4-(1-(2-ethoxyethyl)-1H-indol-3-yl)piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50105107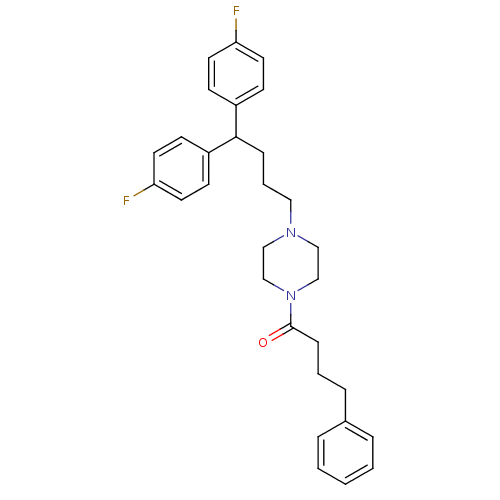 (1-{4-[4,4-Bis-(4-fluoro-phenyl)-butyl]-piperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor | J Med Chem 44: 3391-401 (2001) BindingDB Entry DOI: 10.7270/Q2057GN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156881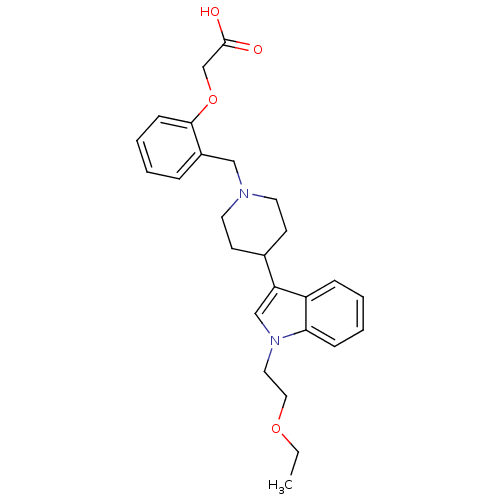 (2-(2-((4-(1-(2-ethoxyethyl)-1H-indol-3-yl)piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156864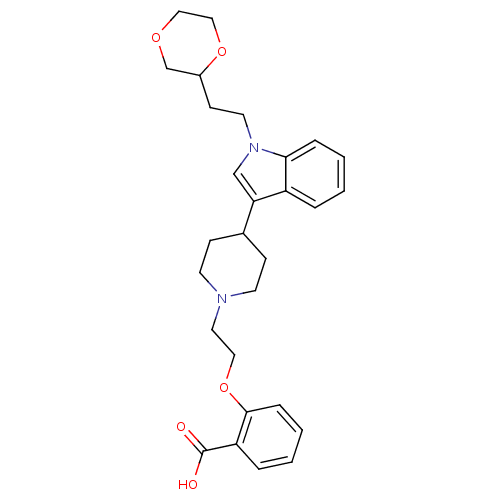 (2-(2-(4-(1-(2-(1,4-dioxan-2-yl)ethyl)-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156879 (2-{2-[4-(1-butyl-6-fluoro-1H-indol-3-yl)piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156852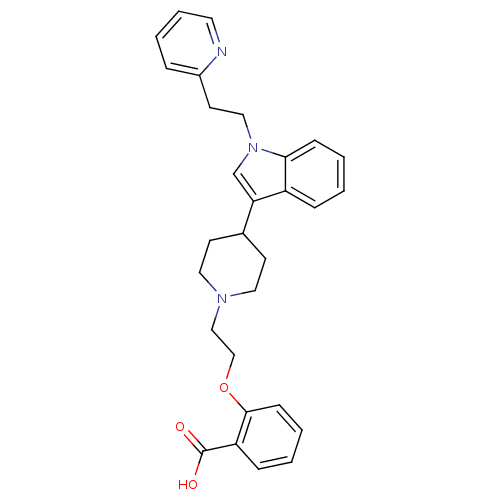 (2-(2-(4-(1-(2-(pyridin-2-yl)ethyl)-1H-indol-3-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156895 (2-(2-(4-(1-(2-ethoxyethyl)-6-(trifluoromethyl)-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156866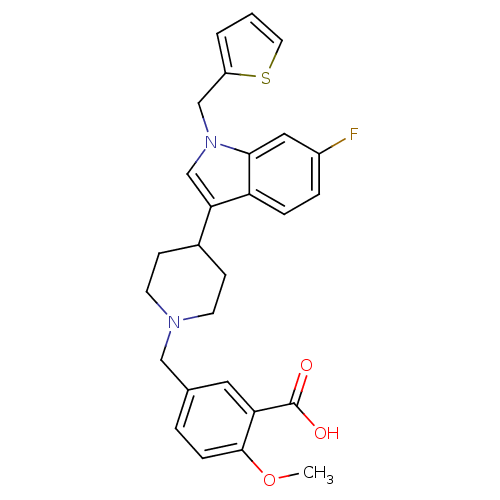 (5-[4-(6-fluoro-1-thiophen-2-ylmethyl-1H-indol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156854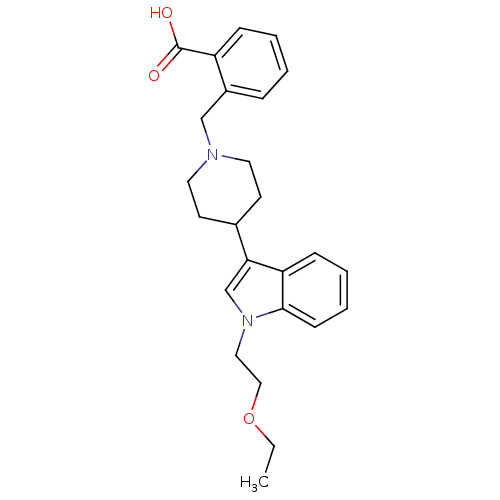 (2-((4-(1-(2-ethoxyethyl)-1H-indol-3-yl)piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156874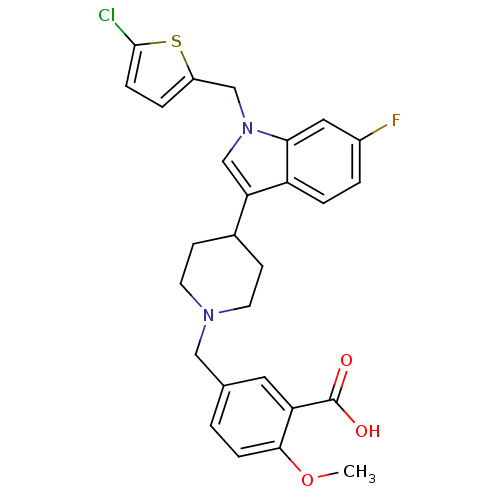 (5-{4-[1-(5-chlorothiophen-2-ylmethyl)-6-fluoro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156888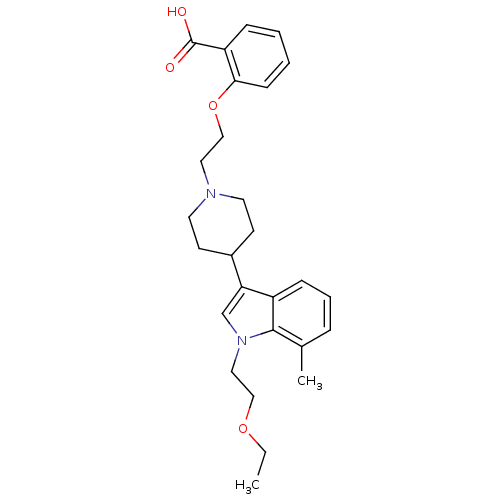 (2-(2-(4-(1-(2-ethoxyethyl)-7-methyl-1H-indol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156872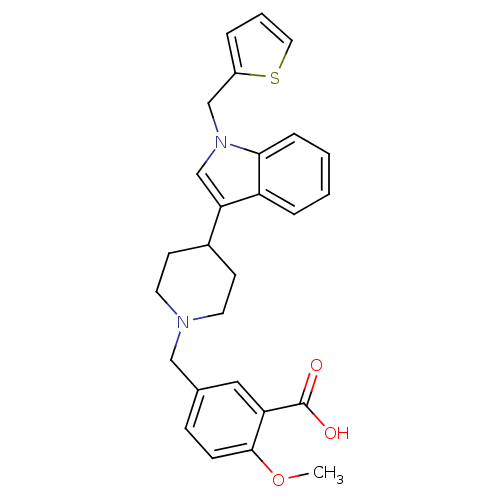 (2-methoxy-5-[4-(1-thiophen-2-ylmethyl-1H-indol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50156867 (2-(2-{4-[1-(2-Ethoxy-ethyl)-1H-indol-3-yl]-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from histamine H2 receptor in human cortex membranes | J Med Chem 47: 6326-37 (2004) Article DOI: 10.1021/jm0498203 BindingDB Entry DOI: 10.7270/Q2HM57XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |