

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063910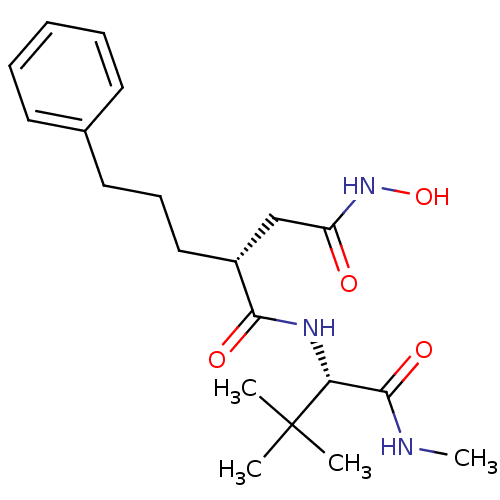 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097273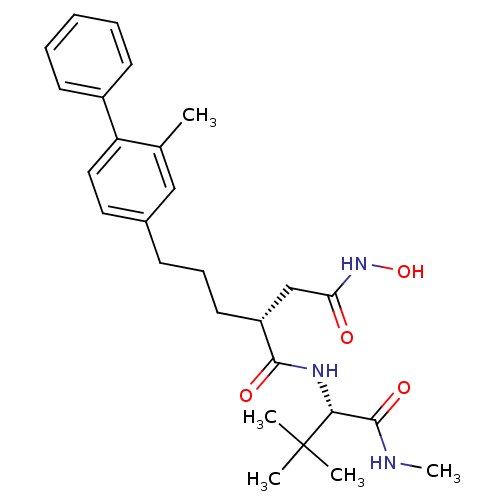 ((R)-N*1*-((S)-2,2-dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097273 ((R)-N*1*-((S)-2,2-dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097270 ((R)-2-(3-Biphenyl-4-yl-propyl)-N*1*-[(S)-2,2-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097261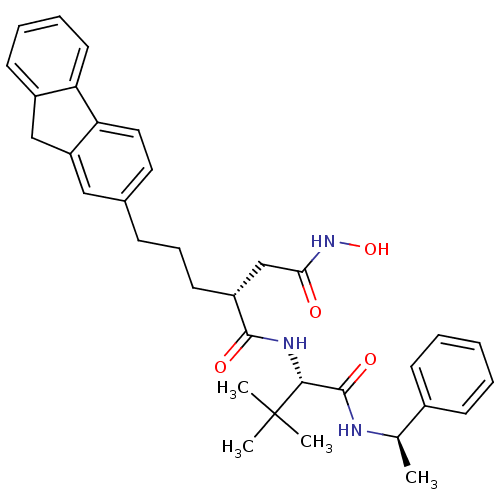 ((R)-2-(3-(9H-fluoren-2-yl)propyl)-N1-((S)-3,3-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097263 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097272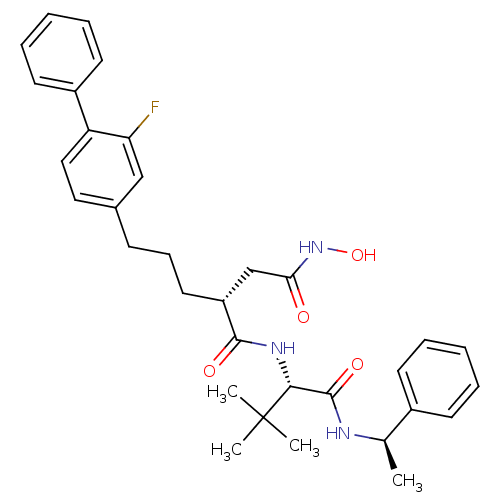 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097268 ((R)-N*1*-[(S)-1-(Benzhydryl-carbamoyl)-2,2-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097267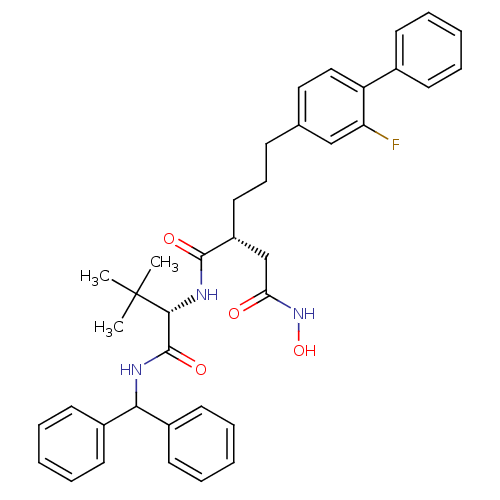 ((R)-N*1*-[(S)-1-(benzhydryl-carbamoyl)-2,2-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097261 ((R)-2-(3-(9H-fluoren-2-yl)propyl)-N1-((S)-3,3-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097271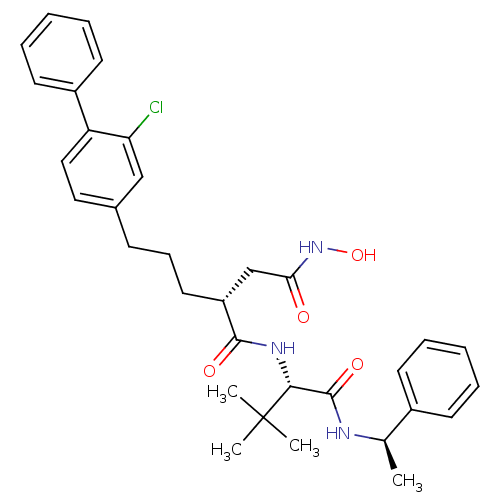 ((R)-2-[3-(2-chloro-biphenyl-4-yl)-propyl]-N*1*-[(S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097262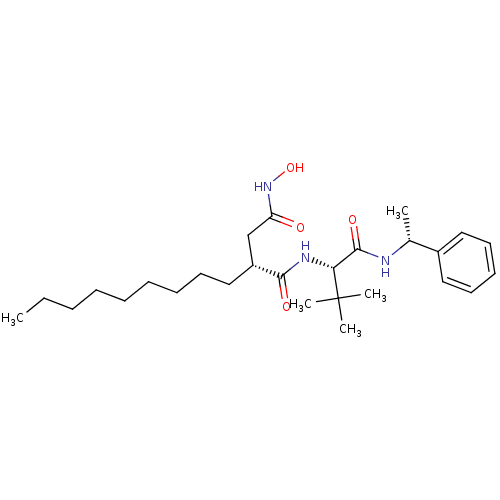 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097265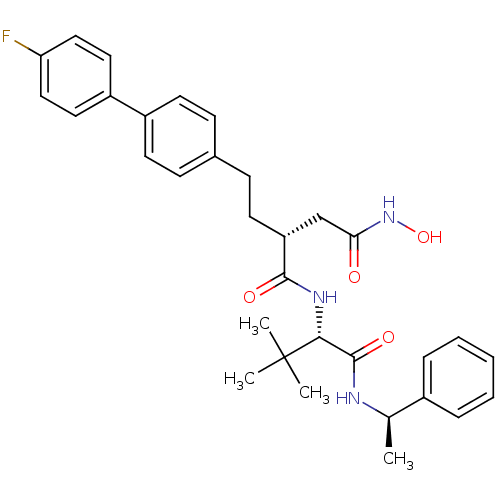 ((R)-N*1*-[(S)-2,2-dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097270 ((R)-2-(3-Biphenyl-4-yl-propyl)-N*1*-[(S)-2,2-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097252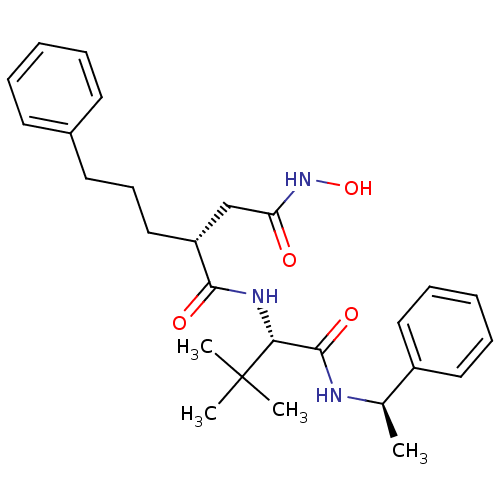 ((R)-N'-((S)-3,3-dimethyl-1-oxo-1-((R)-1-phenylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097260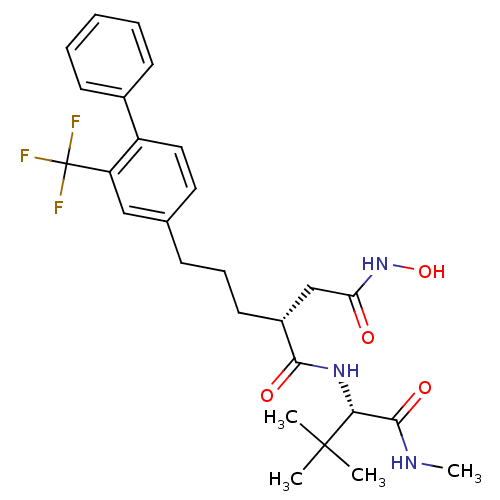 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097267 ((R)-N*1*-[(S)-1-(benzhydryl-carbamoyl)-2,2-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097239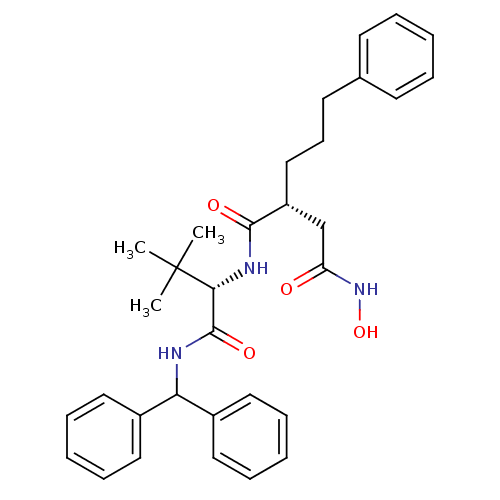 ((R)-N*1*-[(S)-1-(Benzhydryl-carbamoyl)-2,2-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097252 ((R)-N'-((S)-3,3-dimethyl-1-oxo-1-((R)-1-phenylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50097263 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-13 | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097260 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097262 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097266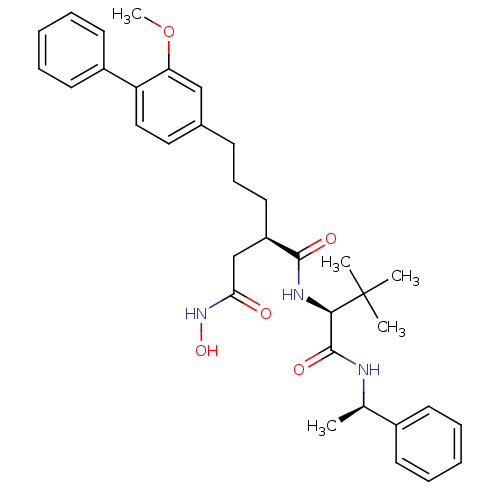 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097259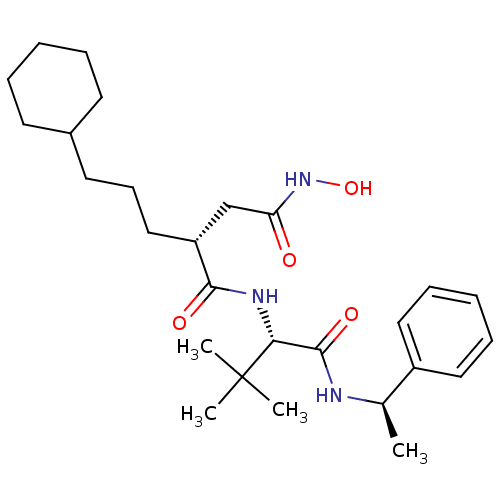 ((R)-2-(3-Cyclohexyl-propyl)-N*1*-[(S)-2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097265 ((R)-N*1*-[(S)-2,2-dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097268 ((R)-N*1*-[(S)-1-(Benzhydryl-carbamoyl)-2,2-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097272 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097239 ((R)-N*1*-[(S)-1-(Benzhydryl-carbamoyl)-2,2-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 414 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097264 ((R)-N*1*-[(S)-2,2-dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50097269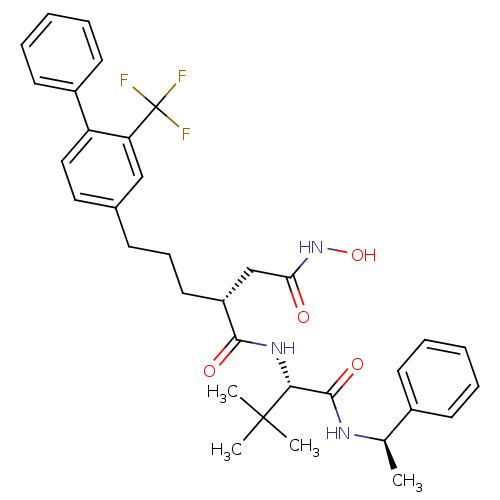 ((R)-N*1*-[(S)-2,2-dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50097263 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-9 | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097259 ((R)-2-(3-Cyclohexyl-propyl)-N*1*-[(S)-2,2-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097271 ((R)-2-[3-(2-chloro-biphenyl-4-yl)-propyl]-N*1*-[(S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097266 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097263 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50097263 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-14 | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097264 ((R)-N*1*-[(S)-2,2-dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50097263 ((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-1 | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50097269 ((R)-N*1*-[(S)-2,2-dimethyl-1-((R)-1-phenyl-ethylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||