

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 4.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human TPA by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 7a by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human u-PA by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C by Michaelis Menten equation analysis | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358889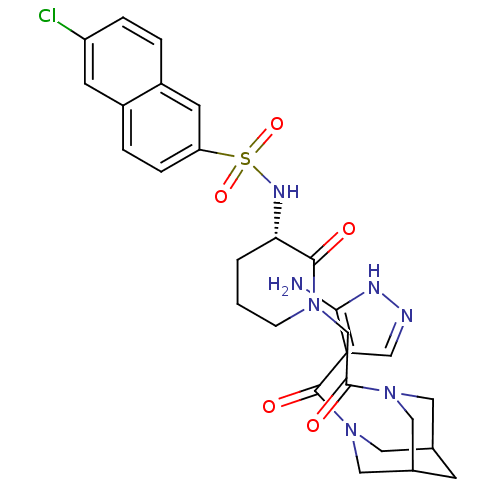 (CHEMBL1923563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328724 ((S)-N2,N2-dimethyl-N5-((2-methylbenzofuran-5-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358887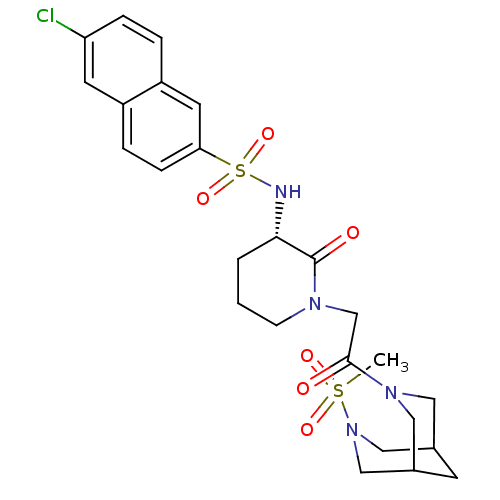 (CHEMBL1923561) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358884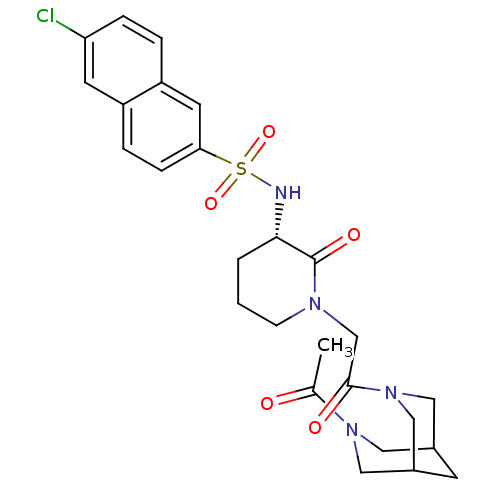 (CHEMBL1923469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358888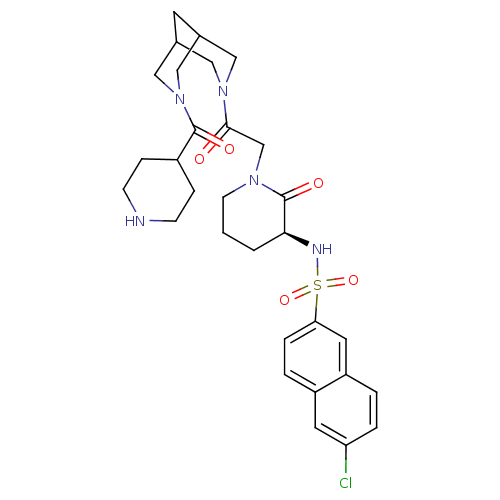 (CHEMBL1923562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358883 (CHEMBL1923468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358871 (CHEMBL1923457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358885 (CHEMBL1923470) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358869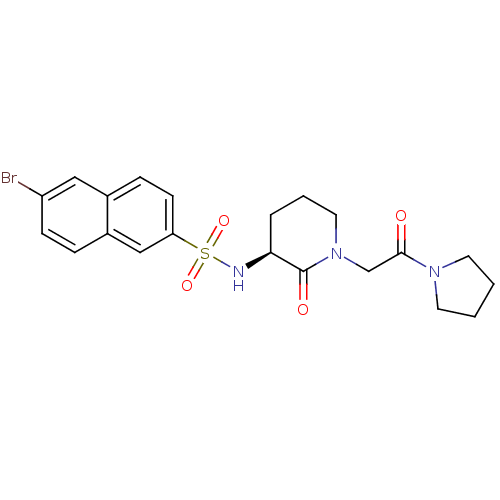 (CHEMBL1923454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358882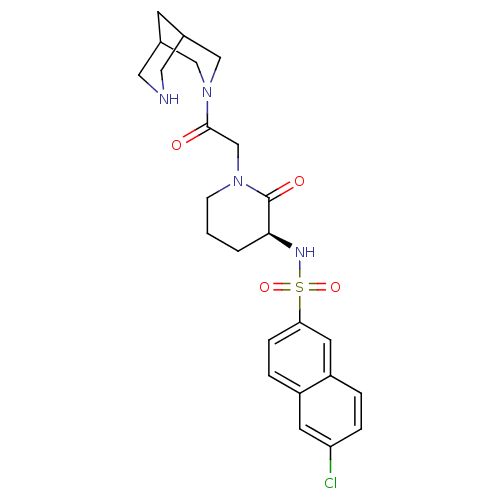 (CHEMBL1923467) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM26351 (2-methylbenzofuran compound, 2 | 2-{[(2-methyl-1-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358886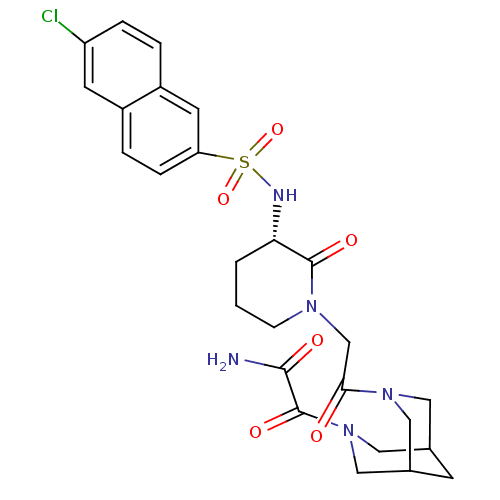 (CHEMBL1923560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358867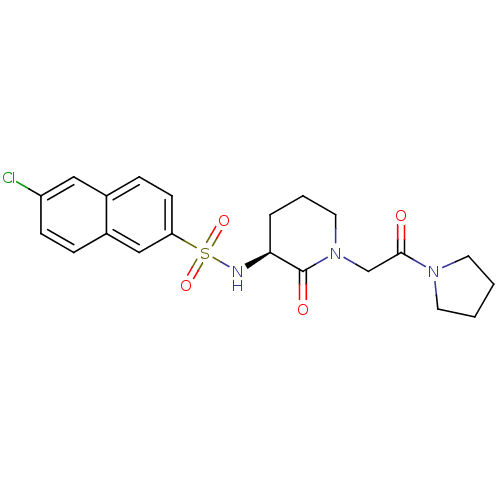 (CHEMBL1923453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358870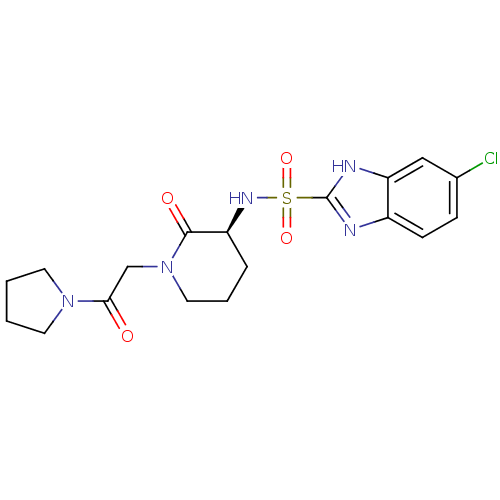 (CHEMBL1923455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358872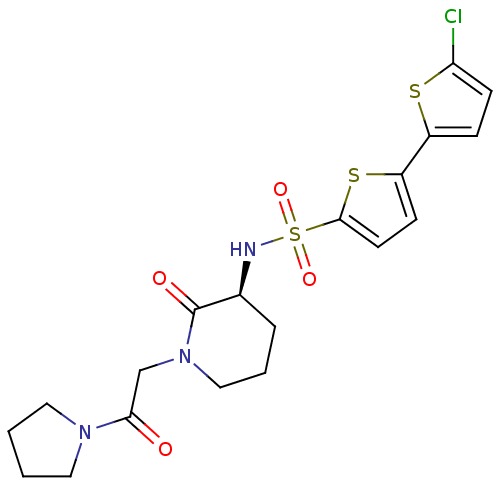 (CHEMBL1923456) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358875 (CHEMBL1923461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358863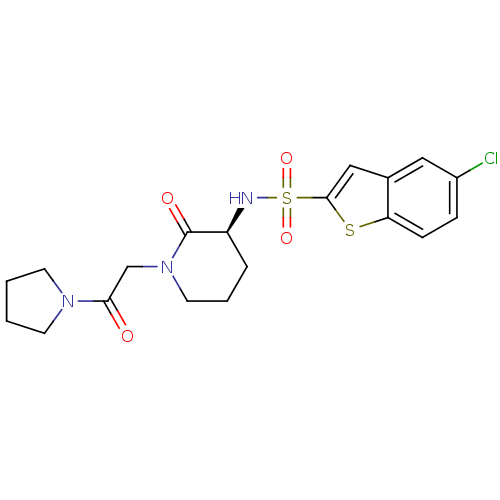 (CHEMBL1923448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358859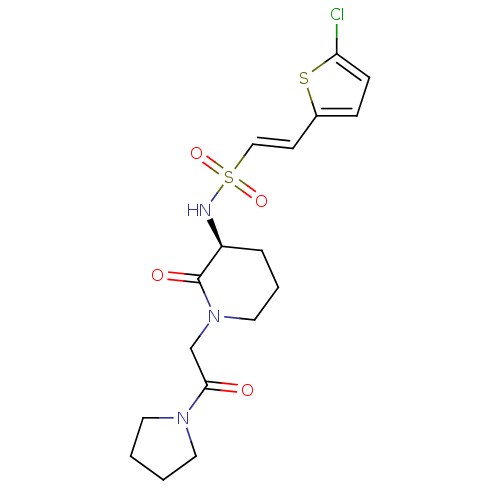 (CHEMBL1923444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358877 (CHEMBL1923462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358862 (CHEMBL1923447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358874 (CHEMBL1923459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358873 (CHEMBL1923458) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358880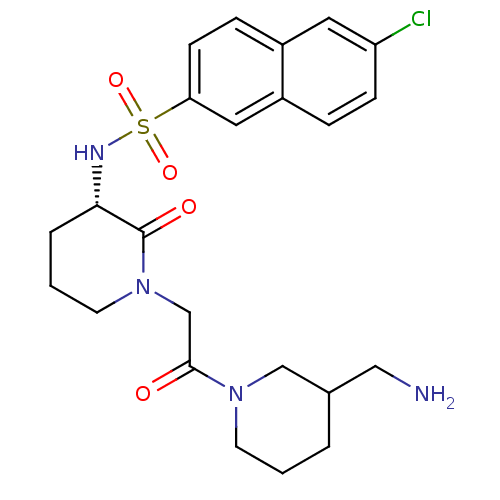 (CHEMBL1923465) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358876 (CHEMBL1923460) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 337 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358878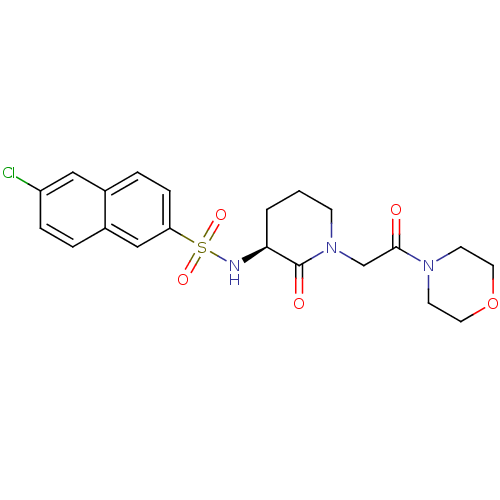 (CHEMBL1923463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 674 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358868 (CHEMBL1923452) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358860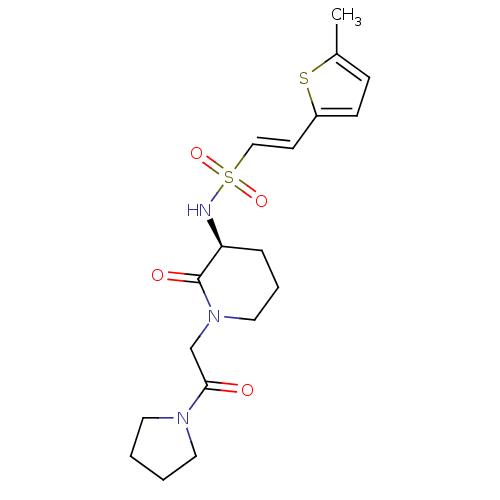 (CHEMBL1923445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358856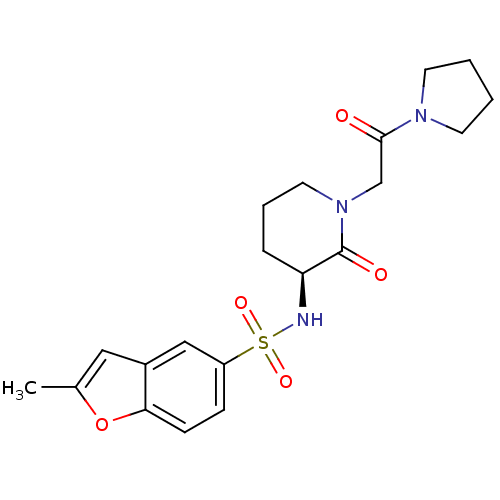 (CHEMBL1923441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358881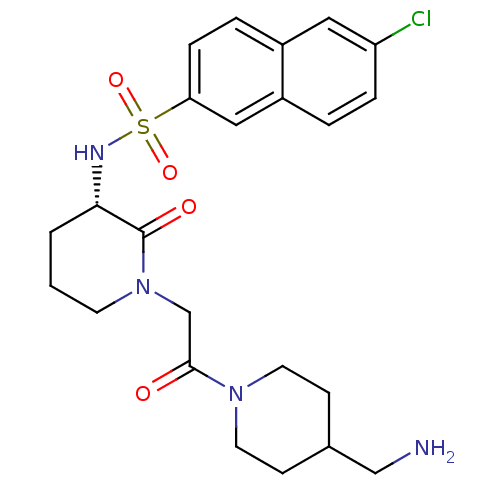 (CHEMBL1923466) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358865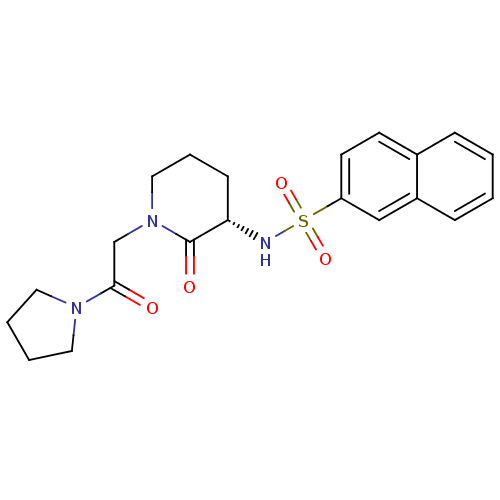 (CHEMBL1923450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358864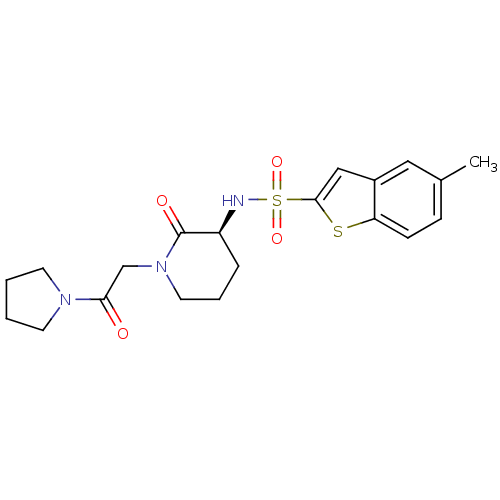 (CHEMBL1923449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358879 (CHEMBL1923464) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358866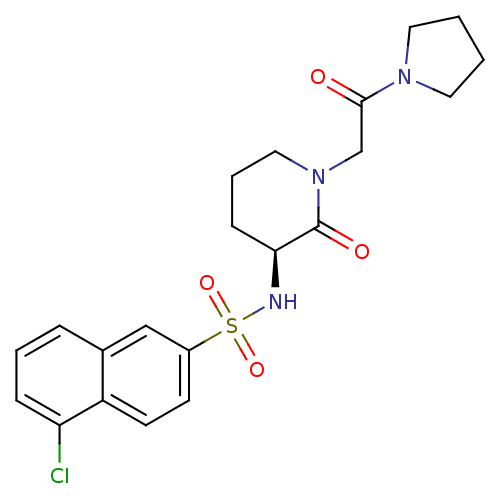 (CHEMBL1923451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358861 (CHEMBL1923446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358857 (CHEMBL1923442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50358858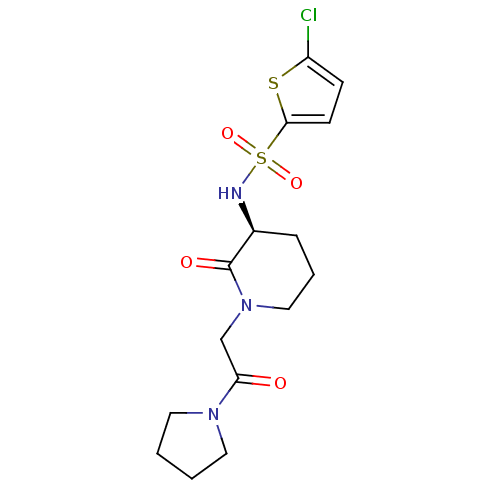 (CHEMBL1923443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a using phenyl-Ile-Glu-Gly-Arg-pNA as substrate preincubated for 3 mins prior to substrate addition by spectrophotometry | Bioorg Med Chem Lett 21: 7516-21 (2011) Article DOI: 10.1016/j.bmcl.2011.06.098 BindingDB Entry DOI: 10.7270/Q2MK6DBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||