 Found 77 hits Enz. Inhib. hit(s) with all data for entry = 50000713
Found 77 hits Enz. Inhib. hit(s) with all data for entry = 50000713 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50259267
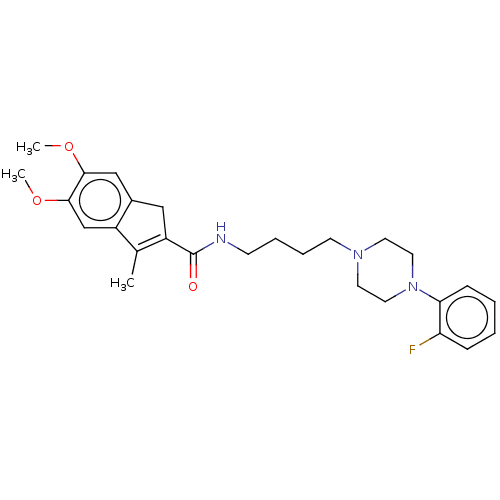
(CHEMBL4099779)Show SMILES COc1cc2CC(C(=O)NCCCCN3CCN(CC3)c3ccccc3F)=C(C)c2cc1OC |t:28| Show InChI InChI=1S/C27H34FN3O3/c1-19-21-18-26(34-3)25(33-2)17-20(21)16-22(19)27(32)29-10-6-7-11-30-12-14-31(15-13-30)24-9-5-4-8-23(24)28/h4-5,8-9,17-18H,6-7,10-16H2,1-3H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50325744

(CHEMBL1223683 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3ccc4ccccc4n3)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-19-7-5-9-21(23(19)25)29-16-14-28(15-17-29)13-4-3-12-26-22-11-10-18-6-1-2-8-20(18)27-22/h1-2,5-11H,3-4,12-17H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50325748

(CHEMBL1223750 | N-(4-(4-(3-(trifluoromethyl)phenyl...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCCNc2nccc3ccccc23)CC1 Show InChI InChI=1S/C24H27F3N4/c25-24(26,27)20-7-5-8-21(18-20)31-16-14-30(15-17-31)13-4-3-11-28-23-22-9-2-1-6-19(22)10-12-29-23/h1-2,5-10,12,18H,3-4,11,13-17H2,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50325746
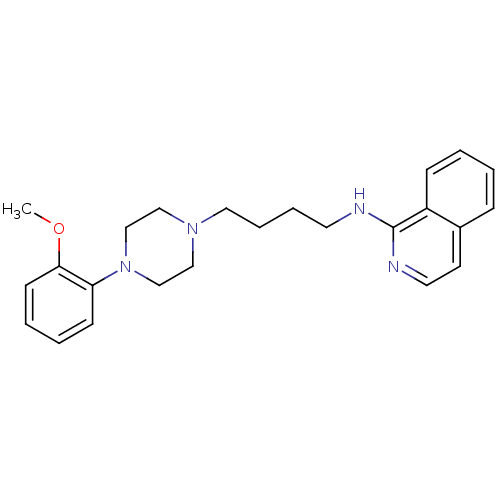
(CHEMBL1223748 | N-(4-(4-(2-methoxyphenyl)piperazin...)Show InChI InChI=1S/C24H30N4O/c1-29-23-11-5-4-10-22(23)28-18-16-27(17-19-28)15-7-6-13-25-24-21-9-3-2-8-20(21)12-14-26-24/h2-5,8-12,14H,6-7,13,15-19H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259291

(CHEMBL4102970)Show SMILES [O-][N+](=O)c1cccc(c1)C(OCCCc1c[nH]cn1)c1ccccc1 Show InChI InChI=1S/C19H19N3O3/c23-22(24)18-10-4-8-16(12-18)19(15-6-2-1-3-7-15)25-11-5-9-17-13-20-14-21-17/h1-4,6-8,10,12-14,19H,5,9,11H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259291

(CHEMBL4102970)Show SMILES [O-][N+](=O)c1cccc(c1)C(OCCCc1c[nH]cn1)c1ccccc1 Show InChI InChI=1S/C19H19N3O3/c23-22(24)18-10-4-8-16(12-18)19(15-6-2-1-3-7-15)25-11-5-9-17-13-20-14-21-17/h1-4,6-8,10,12-14,19H,5,9,11H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50259290
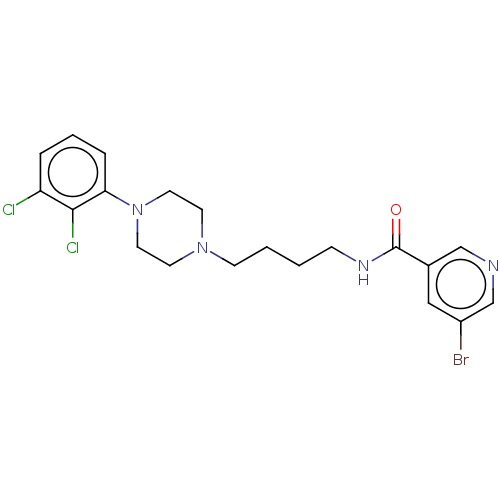
(CHEMBL4078910)Show SMILES Clc1cccc(N2CCN(CCCCNC(=O)c3cncc(Br)c3)CC2)c1Cl Show InChI InChI=1S/C20H23BrCl2N4O/c21-16-12-15(13-24-14-16)20(28)25-6-1-2-7-26-8-10-27(11-9-26)18-5-3-4-17(22)19(18)23/h3-5,12-14H,1-2,6-11H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259257

(CHEMBL4065099)Show InChI InChI=1S/C19H19IN2O/c20-17-10-8-16(9-11-17)19(15-5-2-1-3-6-15)23-12-4-7-18-13-21-14-22-18/h1-3,5-6,8-11,13-14,19H,4,7,12H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259257

(CHEMBL4065099)Show InChI InChI=1S/C19H19IN2O/c20-17-10-8-16(9-11-17)19(15-5-2-1-3-6-15)23-12-4-7-18-13-21-14-22-18/h1-3,5-6,8-11,13-14,19H,4,7,12H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50325747

(CHEMBL1223749 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3nccc4ccccc34)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-20-8-5-9-21(22(20)25)29-16-14-28(15-17-29)13-4-3-11-26-23-19-7-2-1-6-18(19)10-12-27-23/h1-2,5-10,12H,3-4,11,13-17H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at D3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259258
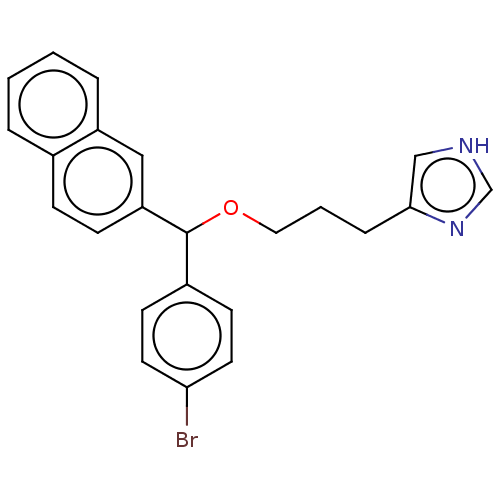
(CHEMBL4098585)Show SMILES Brc1ccc(cc1)C(OCCCc1c[nH]cn1)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H21BrN2O/c24-21-11-9-18(10-12-21)23(27-13-3-6-22-15-25-16-26-22)20-8-7-17-4-1-2-5-19(17)14-20/h1-2,4-5,7-12,14-16,23H,3,6,13H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259258

(CHEMBL4098585)Show SMILES Brc1ccc(cc1)C(OCCCc1c[nH]cn1)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H21BrN2O/c24-21-11-9-18(10-12-21)23(27-13-3-6-22-15-25-16-26-22)20-8-7-17-4-1-2-5-19(17)14-20/h1-2,4-5,7-12,14-16,23H,3,6,13H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259268

(CHEMBL4084820)Show InChI InChI=1S/C21H30N4O/c22-19-7-10-21(24-17-19)23-12-11-18-5-8-20(9-6-18)26-16-4-15-25-13-2-1-3-14-25/h5-10,17H,1-4,11-16,22H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325747

(CHEMBL1223749 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3nccc4ccccc34)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-20-8-5-9-21(22(20)25)29-16-14-28(15-17-29)13-4-3-11-26-23-19-7-2-1-6-18(19)10-12-27-23/h1-2,5-10,12H,3-4,11,13-17H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325747

(CHEMBL1223749 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3nccc4ccccc34)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-20-8-5-9-21(22(20)25)29-16-14-28(15-17-29)13-4-3-11-26-23-19-7-2-1-6-18(19)10-12-27-23/h1-2,5-10,12H,3-4,11,13-17H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325748

(CHEMBL1223750 | N-(4-(4-(3-(trifluoromethyl)phenyl...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCCNc2nccc3ccccc23)CC1 Show InChI InChI=1S/C24H27F3N4/c25-24(26,27)20-7-5-8-21(18-20)31-16-14-30(15-17-31)13-4-3-11-28-23-22-9-2-1-6-19(22)10-12-29-23/h1-2,5-10,12,18H,3-4,11,13-17H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325748

(CHEMBL1223750 | N-(4-(4-(3-(trifluoromethyl)phenyl...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCCNc2nccc3ccccc23)CC1 Show InChI InChI=1S/C24H27F3N4/c25-24(26,27)20-7-5-8-21(18-20)31-16-14-30(15-17-31)13-4-3-11-28-23-22-9-2-1-6-19(22)10-12-29-23/h1-2,5-10,12,18H,3-4,11,13-17H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259293
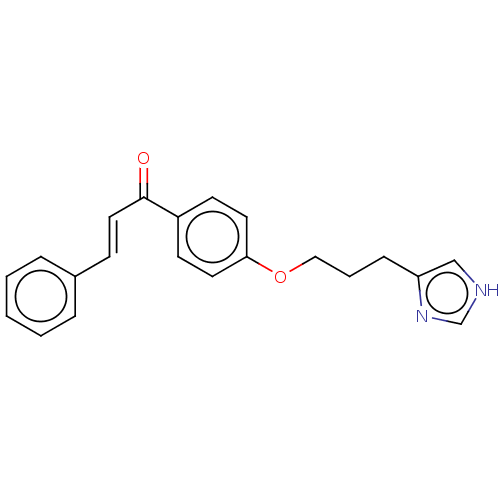
(CHEMBL4063170)Show InChI InChI=1S/C21H20N2O2/c24-21(13-8-17-5-2-1-3-6-17)18-9-11-20(12-10-18)25-14-4-7-19-15-22-16-23-19/h1-3,5-6,8-13,15-16H,4,7,14H2,(H,22,23)/b13-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259291

(CHEMBL4102970)Show SMILES [O-][N+](=O)c1cccc(c1)C(OCCCc1c[nH]cn1)c1ccccc1 Show InChI InChI=1S/C19H19N3O3/c23-22(24)18-10-4-8-16(12-18)19(15-6-2-1-3-7-15)25-11-5-9-17-13-20-14-21-17/h1-4,6-8,10,12-14,19H,5,9,11H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325744

(CHEMBL1223683 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3ccc4ccccc4n3)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-19-7-5-9-21(23(19)25)29-16-14-28(15-17-29)13-4-3-12-26-22-11-10-18-6-1-2-8-20(18)27-22/h1-2,5-11H,3-4,12-17H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259293

(CHEMBL4063170)Show InChI InChI=1S/C21H20N2O2/c24-21(13-8-17-5-2-1-3-6-17)18-9-11-20(12-10-18)25-14-4-7-19-15-22-16-23-19/h1-3,5-6,8-13,15-16H,4,7,14H2,(H,22,23)/b13-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259293

(CHEMBL4063170)Show InChI InChI=1S/C21H20N2O2/c24-21(13-8-17-5-2-1-3-6-17)18-9-11-20(12-10-18)25-14-4-7-19-15-22-16-23-19/h1-3,5-6,8-13,15-16H,4,7,14H2,(H,22,23)/b13-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259257

(CHEMBL4065099)Show InChI InChI=1S/C19H19IN2O/c20-17-10-8-16(9-11-17)19(15-5-2-1-3-6-15)23-12-4-7-18-13-21-14-22-18/h1-3,5-6,8-11,13-14,19H,4,7,12H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259249
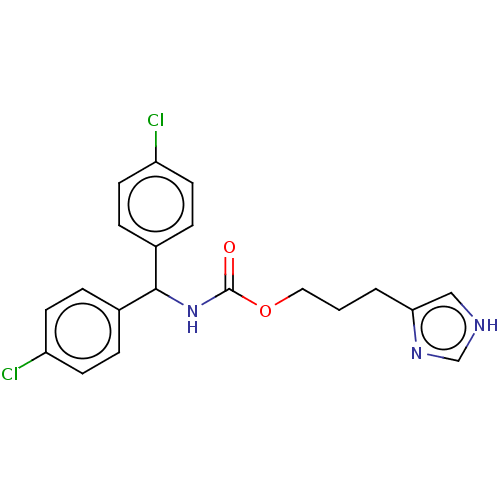
(CHEMBL4095224)Show SMILES Clc1ccc(cc1)C(NC(=O)OCCCc1c[nH]cn1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H19Cl2N3O2/c21-16-7-3-14(4-8-16)19(15-5-9-17(22)10-6-15)25-20(26)27-11-1-2-18-12-23-13-24-18/h3-10,12-13,19H,1-2,11H2,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259249

(CHEMBL4095224)Show SMILES Clc1ccc(cc1)C(NC(=O)OCCCc1c[nH]cn1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H19Cl2N3O2/c21-16-7-3-14(4-8-16)19(15-5-9-17(22)10-6-15)25-20(26)27-11-1-2-18-12-23-13-24-18/h3-10,12-13,19H,1-2,11H2,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259265

(CHEMBL4082297)Show SMILES CC(C)(CN=C(c1ccccc1O)c1ccccc1O)c1c[nH]cn1 |(6.49,-2.55,;7.27,-3.89,;8.04,-2.55,;8.61,-4.66,;8.62,-6.2,;9.95,-6.97,;9.96,-8.51,;11.29,-9.27,;11.3,-10.82,;9.96,-11.59,;8.63,-10.82,;8.63,-9.28,;7.3,-8.51,;11.29,-6.19,;11.27,-4.66,;12.6,-3.88,;13.94,-4.65,;13.95,-6.19,;12.62,-6.96,;12.62,-8.5,;5.95,-4.67,;4.54,-4.04,;3.51,-5.19,;4.28,-6.52,;5.79,-6.2,)| Show InChI InChI=1S/C20H21N3O2/c1-20(2,18-11-21-13-23-18)12-22-19(14-7-3-5-9-16(14)24)15-8-4-6-10-17(15)25/h3-11,13,24-25H,12H2,1-2H3,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259265

(CHEMBL4082297)Show SMILES CC(C)(CN=C(c1ccccc1O)c1ccccc1O)c1c[nH]cn1 |(6.49,-2.55,;7.27,-3.89,;8.04,-2.55,;8.61,-4.66,;8.62,-6.2,;9.95,-6.97,;9.96,-8.51,;11.29,-9.27,;11.3,-10.82,;9.96,-11.59,;8.63,-10.82,;8.63,-9.28,;7.3,-8.51,;11.29,-6.19,;11.27,-4.66,;12.6,-3.88,;13.94,-4.65,;13.95,-6.19,;12.62,-6.96,;12.62,-8.5,;5.95,-4.67,;4.54,-4.04,;3.51,-5.19,;4.28,-6.52,;5.79,-6.2,)| Show InChI InChI=1S/C20H21N3O2/c1-20(2,18-11-21-13-23-18)12-22-19(14-7-3-5-9-16(14)24)15-8-4-6-10-17(15)25/h3-11,13,24-25H,12H2,1-2H3,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259250

(CHEMBL4062122)Show InChI InChI=1S/C22H27NO2/c1-17-4-3-13-23(14-17)15-19-5-7-20(8-6-19)16-25-22-11-9-21(10-12-22)18(2)24/h5-12,17H,3-4,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50198913

(CHEMBL1179089)Show InChI InChI=1S/C19H20N2O/c1-3-8-16(9-4-1)19(17-10-5-2-6-11-17)22-13-7-12-18-14-20-15-21-18/h1-6,8-11,14-15,19H,7,12-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50198913

(CHEMBL1179089)Show InChI InChI=1S/C19H20N2O/c1-3-8-16(9-4-1)19(17-10-5-2-6-11-17)22-13-7-12-18-14-20-15-21-18/h1-6,8-11,14-15,19H,7,12-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50198913

(CHEMBL1179089)Show InChI InChI=1S/C19H20N2O/c1-3-8-16(9-4-1)19(17-10-5-2-6-11-17)22-13-7-12-18-14-20-15-21-18/h1-6,8-11,14-15,19H,7,12-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259264

(CHEMBL4100315)Show InChI InChI=1S/C21H24N2O/c1-2-7-19(8-3-1)20-12-10-18(11-13-20)6-4-14-24-15-5-9-21-16-22-17-23-21/h1-3,7-8,10-13,16-17H,4-6,9,14-15H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259264

(CHEMBL4100315)Show InChI InChI=1S/C21H24N2O/c1-2-7-19(8-3-1)20-12-10-18(11-13-20)6-4-14-24-15-5-9-21-16-22-17-23-21/h1-3,7-8,10-13,16-17H,4-6,9,14-15H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325747

(CHEMBL1223749 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3nccc4ccccc34)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-20-8-5-9-21(22(20)25)29-16-14-28(15-17-29)13-4-3-11-26-23-19-7-2-1-6-18(19)10-12-27-23/h1-2,5-10,12H,3-4,11,13-17H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50050165

(4-(4-Iodo-phenyl)-butyric acid 3-(1H-imidazol-4-yl...)Show InChI InChI=1S/C16H19IN2O2/c17-14-8-6-13(7-9-14)3-1-5-16(20)21-10-2-4-15-11-18-12-19-15/h6-9,11-12H,1-5,10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259258

(CHEMBL4098585)Show SMILES Brc1ccc(cc1)C(OCCCc1c[nH]cn1)c1ccc2ccccc2c1 Show InChI InChI=1S/C23H21BrN2O/c24-21-11-9-18(10-12-21)23(27-13-3-6-22-15-25-16-26-22)20-8-7-17-4-1-2-5-19(17)14-20/h1-2,4-5,7-12,14-16,23H,3,6,13H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325746

(CHEMBL1223748 | N-(4-(4-(2-methoxyphenyl)piperazin...)Show InChI InChI=1S/C24H30N4O/c1-29-23-11-5-4-10-22(23)28-18-16-27(17-19-28)15-7-6-13-25-24-21-9-3-2-8-20(21)12-14-26-24/h2-5,8-12,14H,6-7,13,15-19H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325746

(CHEMBL1223748 | N-(4-(4-(2-methoxyphenyl)piperazin...)Show InChI InChI=1S/C24H30N4O/c1-29-23-11-5-4-10-22(23)28-18-16-27(17-19-28)15-7-6-13-25-24-21-9-3-2-8-20(21)12-14-26-24/h2-5,8-12,14H,6-7,13,15-19H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259292

(CHEMBL4084275)Show SMILES OC(=O)C(Cc1cccc2ccccc12)NC(=O)[C@@H](CS)Cc1ccccc1 |r| Show InChI InChI=1S/C23H23NO3S/c25-22(19(15-28)13-16-7-2-1-3-8-16)24-21(23(26)27)14-18-11-6-10-17-9-4-5-12-20(17)18/h1-12,19,21,28H,13-15H2,(H,24,25)(H,26,27)/t19-,21?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259292

(CHEMBL4084275)Show SMILES OC(=O)C(Cc1cccc2ccccc12)NC(=O)[C@@H](CS)Cc1ccccc1 |r| Show InChI InChI=1S/C23H23NO3S/c25-22(19(15-28)13-16-7-2-1-3-8-16)24-21(23(26)27)14-18-11-6-10-17-9-4-5-12-20(17)18/h1-12,19,21,28H,13-15H2,(H,24,25)(H,26,27)/t19-,21?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50259264

(CHEMBL4100315)Show InChI InChI=1S/C21H24N2O/c1-2-7-19(8-3-1)20-12-10-18(11-13-20)6-4-14-24-15-5-9-21-16-22-17-23-21/h1-3,7-8,10-13,16-17H,4-6,9,14-15H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H3 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50050165

(4-(4-Iodo-phenyl)-butyric acid 3-(1H-imidazol-4-yl...)Show InChI InChI=1S/C16H19IN2O2/c17-14-8-6-13(7-9-14)3-1-5-16(20)21-10-2-4-15-11-18-12-19-15/h6-9,11-12H,1-5,10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at human H4 receptor expressed in CHO cells co-expressing Galphai2 assessed as inhibition of imetit-induced GTPgamma[35S] binding... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50050165

(4-(4-Iodo-phenyl)-butyric acid 3-(1H-imidazol-4-yl...)Show InChI InChI=1S/C16H19IN2O2/c17-14-8-6-13(7-9-14)3-1-5-16(20)21-10-2-4-15-11-18-12-19-15/h6-9,11-12H,1-5,10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Effective concentration for activation of RNase L |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325744

(CHEMBL1223683 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3ccc4ccccc4n3)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-19-7-5-9-21(23(19)25)29-16-14-28(15-17-29)13-4-3-12-26-22-11-10-18-6-1-2-8-20(18)27-22/h1-2,5-11H,3-4,12-17H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50325744

(CHEMBL1223683 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Clc1cccc(N2CCN(CCCCNc3ccc4ccccc4n3)CC2)c1Cl Show InChI InChI=1S/C23H26Cl2N4/c24-19-7-5-9-21(23(19)25)29-16-14-28(15-17-29)13-4-3-12-26-22-11-10-18-6-1-2-8-20(18)27-22/h1-2,5-11H,3-4,12-17H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at human H4 receptor expressed in CHO cells co-expressing Galphai2 assessed as inhibition of imetit-induced GTPgamma[35S] binding... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259251
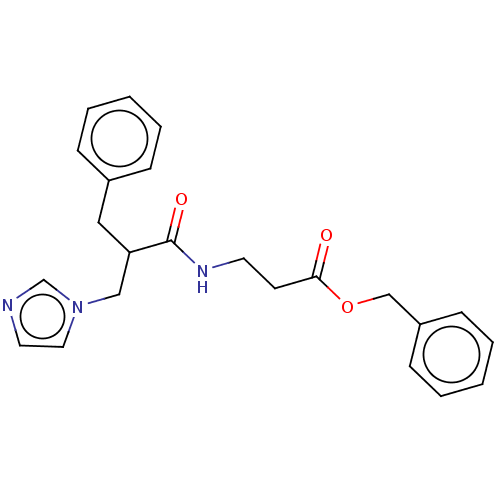
(CHEMBL4083336)Show SMILES O=C(CCNC(=O)C(Cc1ccccc1)Cn1ccnc1)OCc1ccccc1 Show InChI InChI=1S/C23H25N3O3/c27-22(29-17-20-9-5-2-6-10-20)11-12-25-23(28)21(16-26-14-13-24-18-26)15-19-7-3-1-4-8-19/h1-10,13-14,18,21H,11-12,15-17H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259251

(CHEMBL4083336)Show SMILES O=C(CCNC(=O)C(Cc1ccccc1)Cn1ccnc1)OCc1ccccc1 Show InChI InChI=1S/C23H25N3O3/c27-22(29-17-20-9-5-2-6-10-20)11-12-25-23(28)21(16-26-14-13-24-18-26)15-19-7-3-1-4-8-19/h1-10,13-14,18,21H,11-12,15-17H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor in human SH-SY5Y cells assessed as inhibition of imetit-induced GTPgamma[35S] binding after 30 mins by microbeta s... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259266
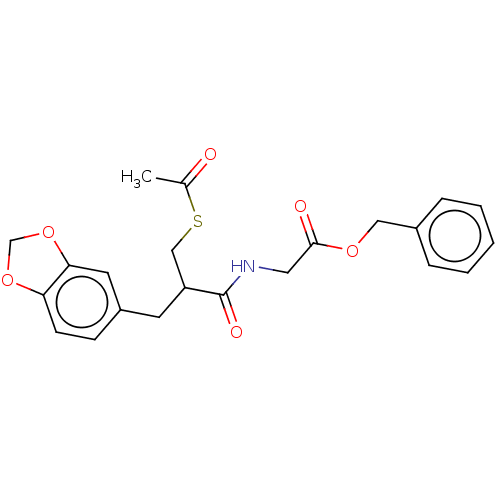
(CHEMBL4102243)Show SMILES CC(=O)SCC(Cc1ccc2OCOc2c1)C(=O)NCC(=O)OCc1ccccc1 Show InChI InChI=1S/C22H23NO6S/c1-15(24)30-13-18(9-17-7-8-19-20(10-17)29-14-28-19)22(26)23-11-21(25)27-12-16-5-3-2-4-6-16/h2-8,10,18H,9,11-14H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50259266

(CHEMBL4102243)Show SMILES CC(=O)SCC(Cc1ccc2OCOc2c1)C(=O)NCC(=O)OCc1ccccc1 Show InChI InChI=1S/C22H23NO6S/c1-15(24)30-13-18(9-17-7-8-19-20(10-17)29-14-28-19)22(26)23-11-21(25)27-12-16-5-3-2-4-6-16/h2-8,10,18H,9,11-14H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at human H4 receptor expressed in CHO cells co-expressing Galphai2 assessed as inhibition of imetit-induced GTPgamma[35S] binding... |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50146144

(4-(3-Trityloxy-propyl)-1H-imidazole | CHEMBL91576)Show SMILES C(COC(c1ccccc1)(c1ccccc1)c1ccccc1)Cc1cnc[nH]1 Show InChI InChI=1S/C25H24N2O/c1-4-11-21(12-5-1)25(22-13-6-2-7-14-22,23-15-8-3-9-16-23)28-18-10-17-24-19-26-20-27-24/h1-9,11-16,19-20H,10,17-18H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at H4 receptor (unknown origin) |
Eur J Med Chem 125: 565-572 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.074
BindingDB Entry DOI: 10.7270/Q20C4Z66 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data