

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21842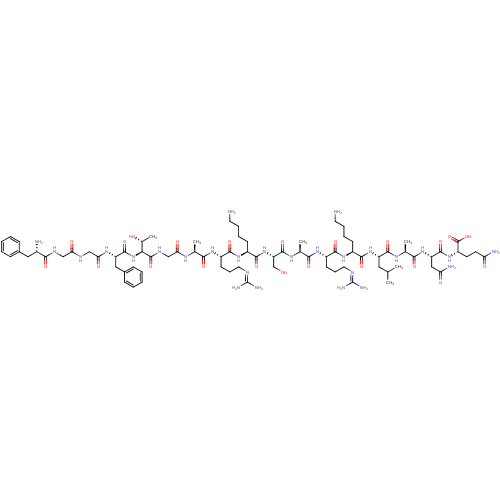 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.130 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239934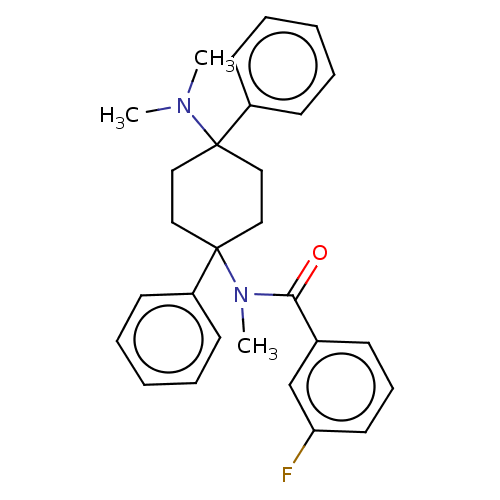 (US9403767, 117) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21844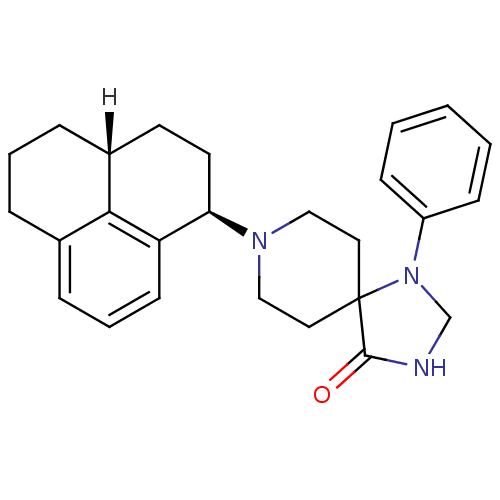 (8-[(1R,3aR)-2,3,3a,4,5,6-hexahydro-1H-phenalen-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239921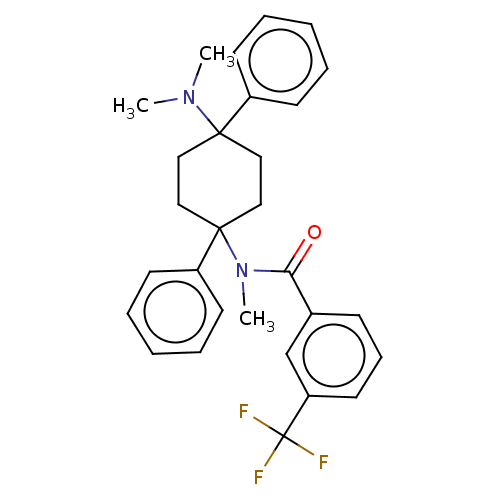 (US9403767, 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239925 (US9403767, 104 | US9403767, 105) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239911 (US9403767, 76 | US9403767, 77) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239914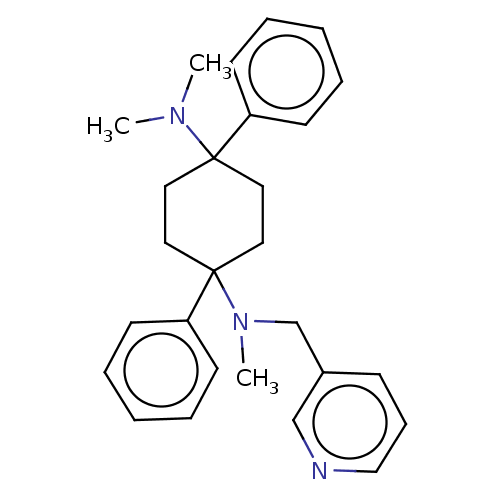 (US9403767, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM97714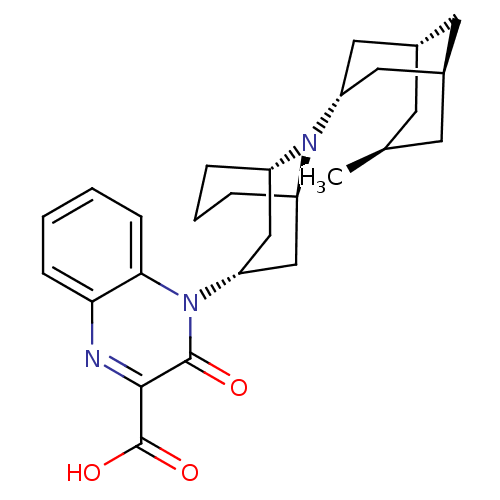 (US8476271, 405) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P.; Shionogi & Co., Ltd. US Patent | Assay Description In vitro binding assay using ORL-1m Mu-opioid, kappa-opioid, and delta-opioid receptor. | US Patent US8476271 (2013) BindingDB Entry DOI: 10.7270/Q2416VNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239892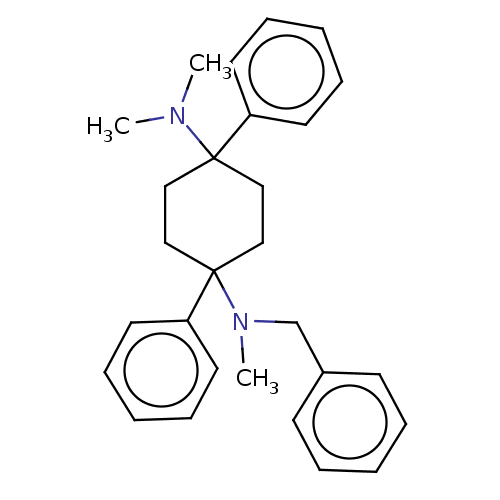 (US9403767, 50 | US9403767, 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.28 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239935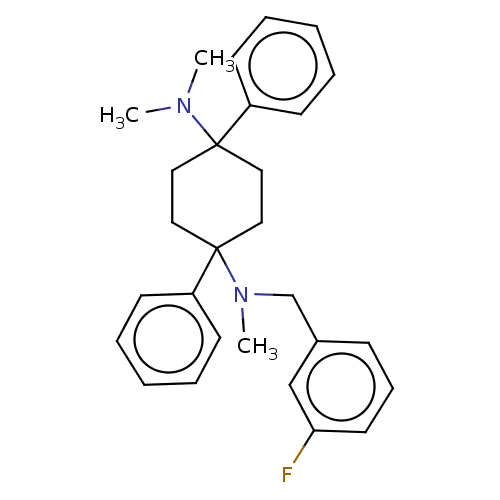 (US9403767, 119) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239890 (US9403767, 48 | US9403767, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239873 (US9403767, 29 | US9403767, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26906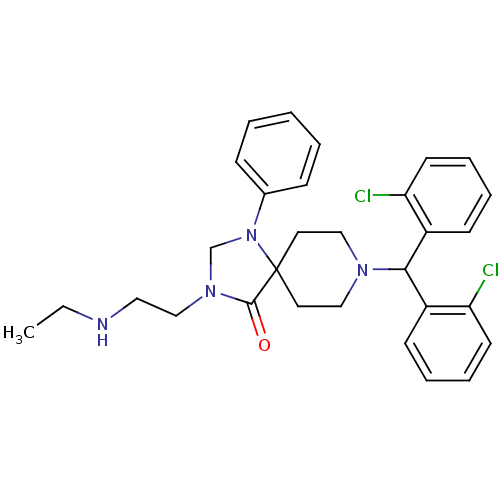 (8-[bis(2-chlorophenyl)methyl]-3-[2-(ethylamino)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26913 (8-[bis(2-chlorophenyl)methyl]-3-[2-(butylamino)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.15 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26916 (8-[bis(2-chlorophenyl)methyl]-1-phenyl-3-[2-(pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.25 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM97710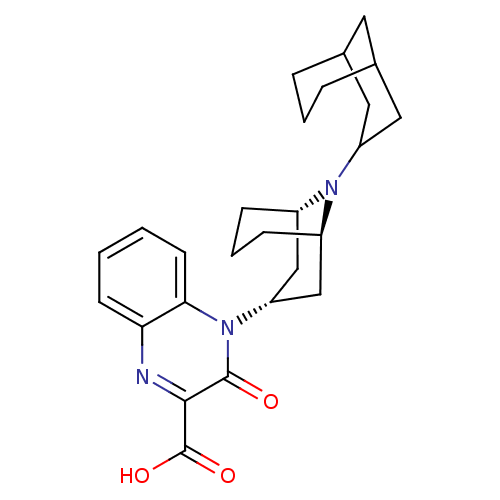 (US8476271, 16 | US9145408, 362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.40 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P.; Shionogi & Co., Ltd. US Patent | Assay Description In vitro binding assay using ORL-1m Mu-opioid, kappa-opioid, and delta-opioid receptor. | US Patent US8476271 (2013) BindingDB Entry DOI: 10.7270/Q2416VNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239923 (US9403767, 101 | US9403767, 102) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26907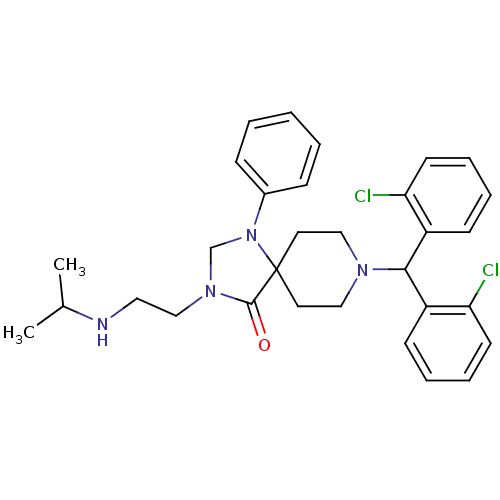 (8-[bis(2-chlorophenyl)methyl]-1-phenyl-3-[2-(propa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.55 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239865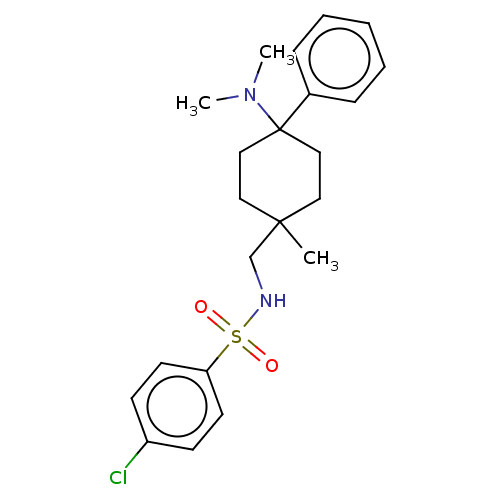 (US9403767, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26914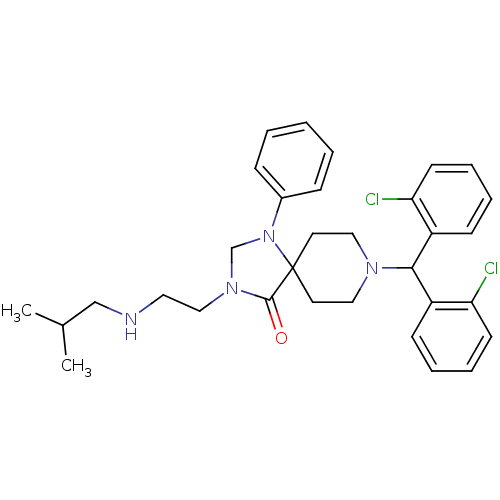 (8-[bis(2-chlorophenyl)methyl]-3-{2-[(2-methylpropy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.75 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21845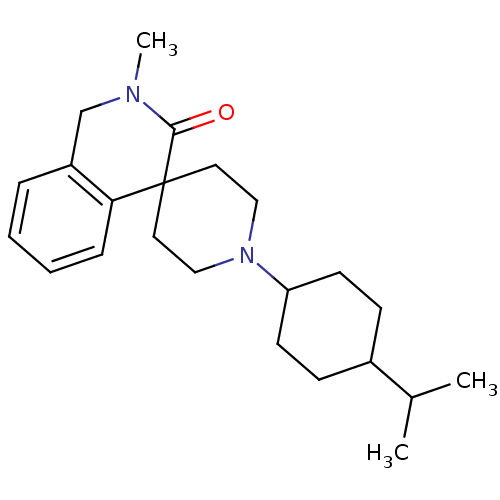 (2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26917 (8-[bis(2-chlorophenyl)methyl]-1-phenyl-3-[3-(pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26910 (8-[bis(2-chlorophenyl)methyl]-3-[2-(dimethylamino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26911 (8-[bis(2-chlorophenyl)methyl]-3-[2-(cyclopropylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26915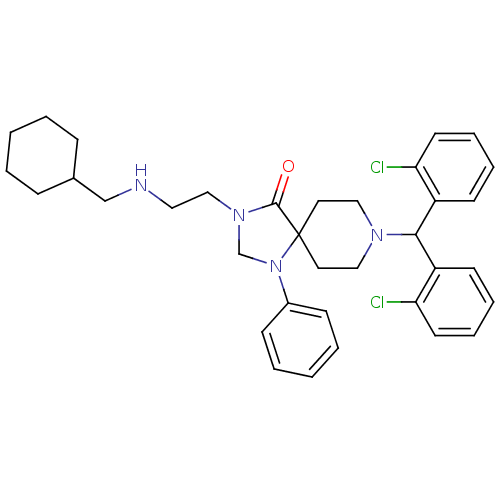 (8-[bis(2-chlorophenyl)methyl]-3-{2-[(cyclohexylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM116216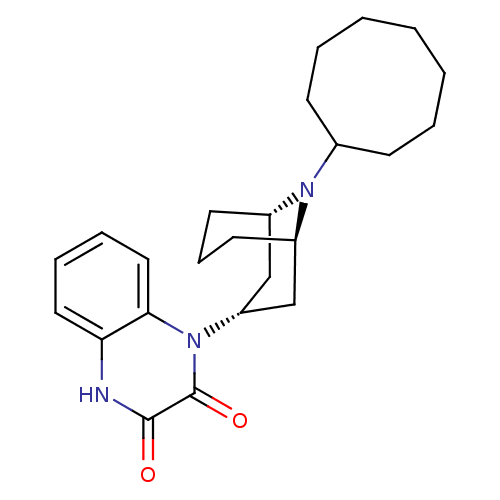 (US8637502, 56) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purde Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description ORL-1 Receptor Binding Assay Procedures: Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Recepto... | US Patent US8637502 (2014) BindingDB Entry DOI: 10.7270/Q2P55M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26905 (8-[bis(2-chlorophenyl)methyl]-3-[2-(methylamino)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.05 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21857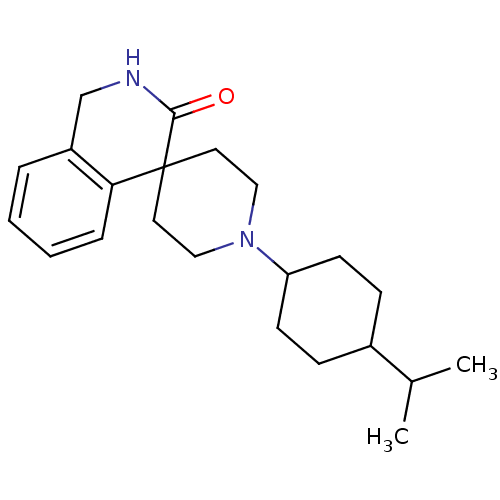 (1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydro-1H-spir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239869 (US9403767, 24 | US9403767, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26909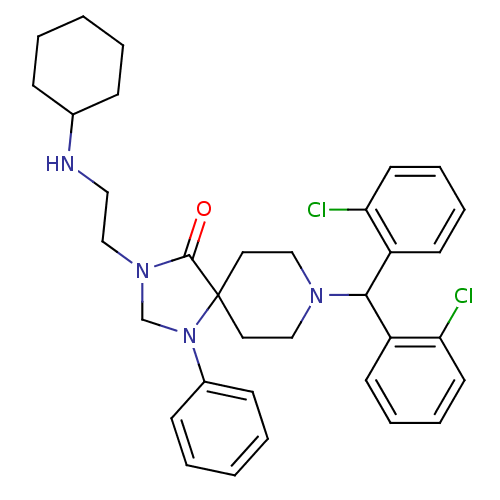 (8-[bis(2-chlorophenyl)methyl]-3-[2-(cyclohexylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239917 (US9403767, 91) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.35 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM116220 (US8637502, 60) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.60 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purde Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description ORL-1 Receptor Binding Assay Procedures: Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Recepto... | US Patent US8637502 (2014) BindingDB Entry DOI: 10.7270/Q2P55M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM97711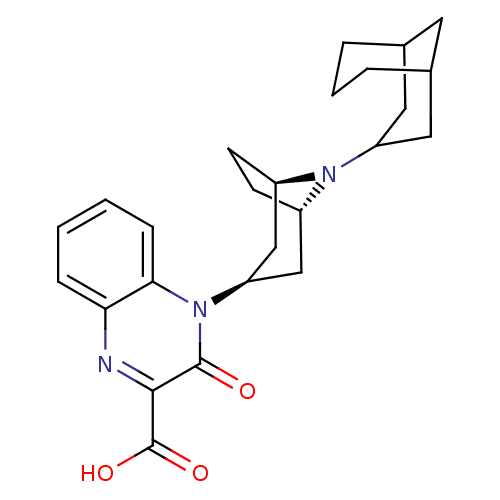 (US8476271, 17 | US8846929, 360 | US8846929, 361 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.70 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P.; Shionogi & Co., Ltd. US Patent | Assay Description In vitro binding assay using ORL-1m Mu-opioid, kappa-opioid, and delta-opioid receptor. | US Patent US8476271 (2013) BindingDB Entry DOI: 10.7270/Q2416VNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239861 (US9403767, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21851 (2-benzyl-1'-[4-(propan-2-yl)cyclohexyl]-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.60 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239938 (US9403767, 122) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26908 (8-[bis(2-chlorophenyl)methyl]-3-[2-(cyclopentylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.65 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM116200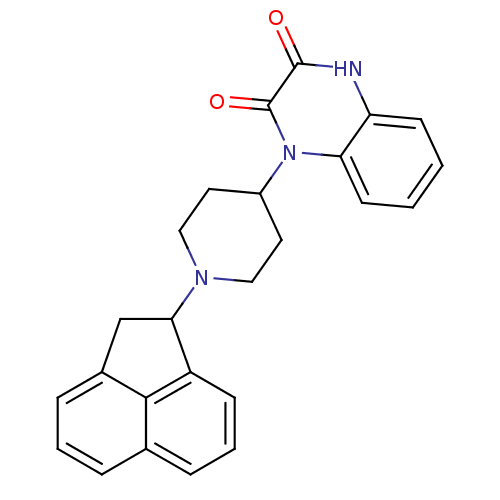 (US8637502, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8.90 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purde Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description ORL-1 Receptor Binding Assay Procedures: Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Recepto... | US Patent US8637502 (2014) BindingDB Entry DOI: 10.7270/Q2P55M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21847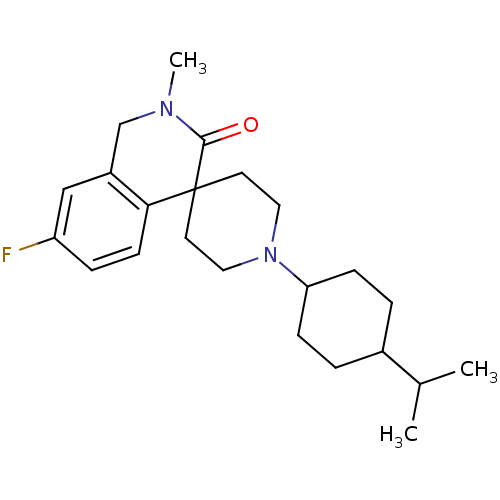 (7-fluoro-2-methyl-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.90 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM97712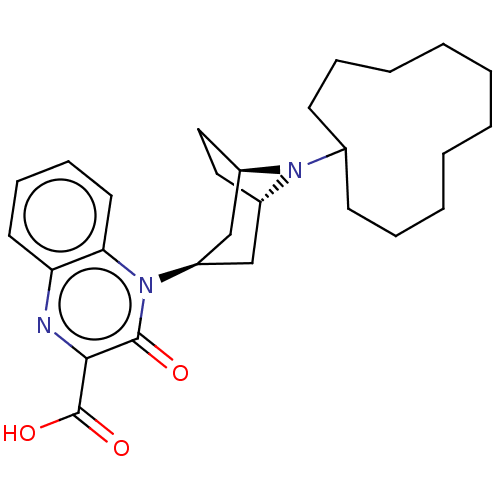 (US8476271, 18 | US8846929, 358 | US9145408, 358) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P.; Shionogi & Co., Ltd. US Patent | Assay Description In vitro binding assay using ORL-1m Mu-opioid, kappa-opioid, and delta-opioid receptor. | US Patent US8476271 (2013) BindingDB Entry DOI: 10.7270/Q2416VNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239894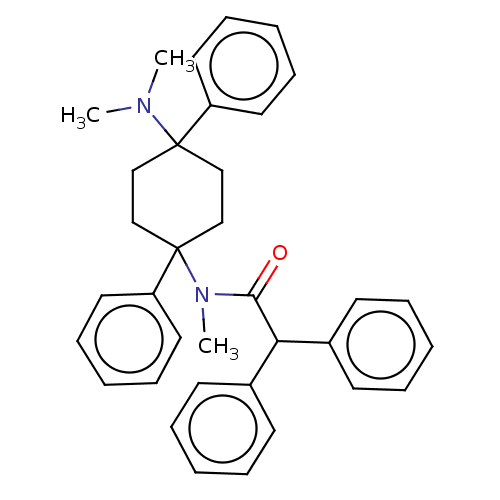 (US9403767, 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239867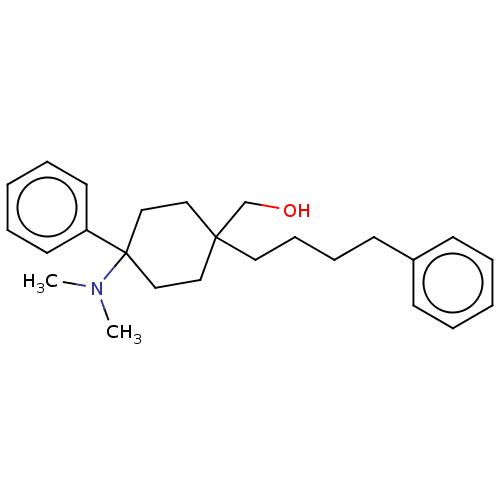 (US9403767, 20 | US9403767, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM116198 (US8637502, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11.5 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purde Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description ORL-1 Receptor Binding Assay Procedures: Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Recepto... | US Patent US8637502 (2014) BindingDB Entry DOI: 10.7270/Q2P55M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21852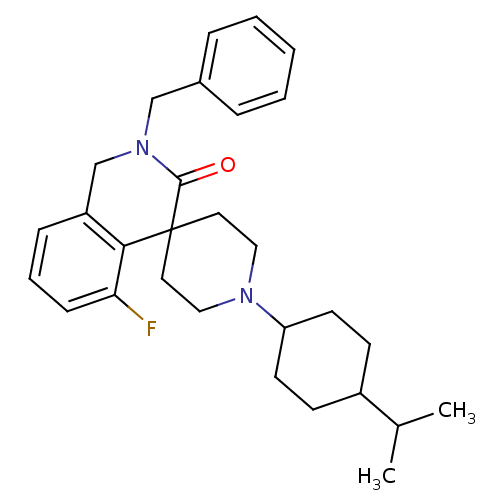 (2-benzyl-5-fluoro-1'-[4-(propan-2-yl)cyclohexyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Istituto Superiore di Sanita | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | J Med Chem 51: 1058-62 (2008) Article DOI: 10.1021/jm7009606 BindingDB Entry DOI: 10.7270/Q2HT2MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26912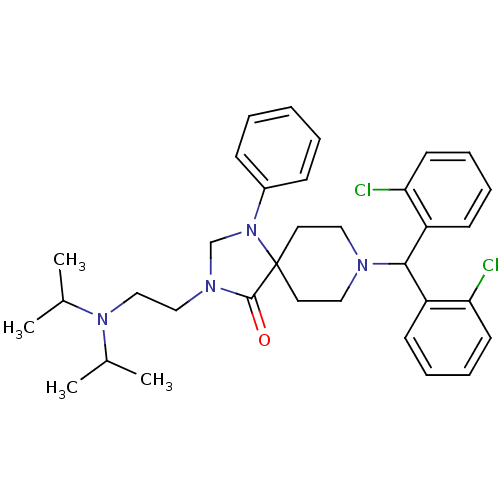 (8-[bis(2-chlorophenyl)methyl]-3-{2-[bis(propan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.1 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Schering-Plough Research Institute | Assay Description IC50 values were obtained by fitting the competition binding curves according to a 4-parameter logistic model. Inhibition constants Ki were derived f... | Bioorg Med Chem Lett 19: 1164-7 (2009) Article DOI: 10.1016/j.bmcl.2008.12.092 BindingDB Entry DOI: 10.7270/Q2TT4P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM116217 (US8637502, 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 13 | -41.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purde Pharma L.P.; Shionogi & Co., Ltd. US Patent | Assay Description ORL-1 Receptor Binding Assay Procedures: Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Recepto... | US Patent US8637502 (2014) BindingDB Entry DOI: 10.7270/Q2P55M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239931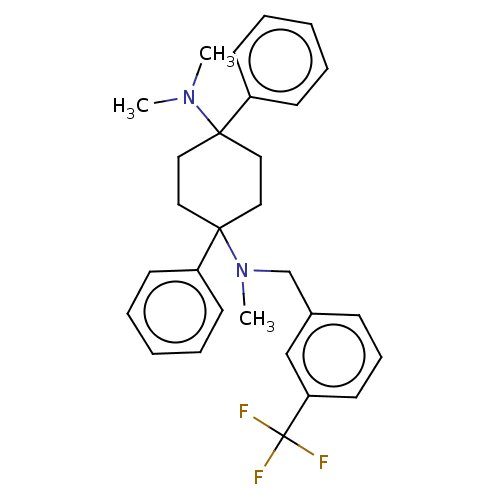 (US9403767, 114) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239918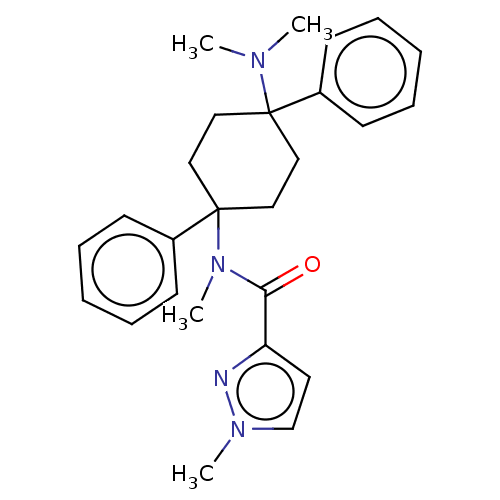 (US9403767, 92 | US9403767, 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239866 (US9403767, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM239860 (US9403767, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GRUENENTHAL GMBH US Patent | Assay Description The compounds were examined with membranes of recombinant CHO-ORL 1 cells in a receptor binding assay with 3H-nociceptin/orphanin FQ. This test syste... | US Patent US9403767 (2016) BindingDB Entry DOI: 10.7270/Q23R0RSJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 265 total ) | Next | Last >> |