

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402202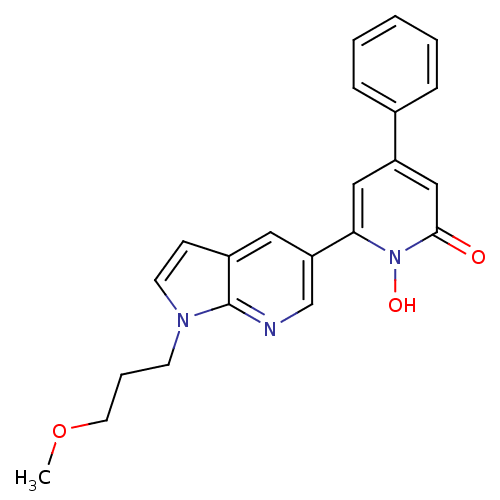 (CHEMBL2203964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402204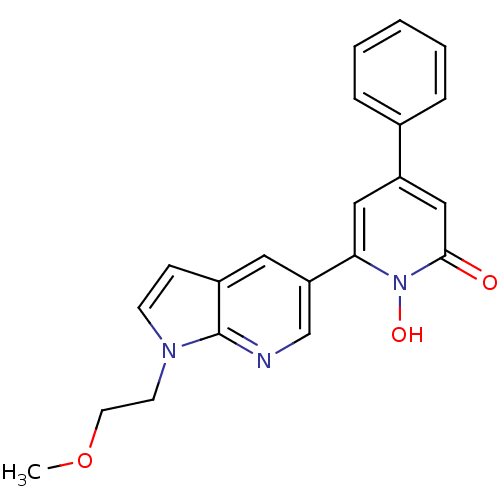 (CHEMBL2203978) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402206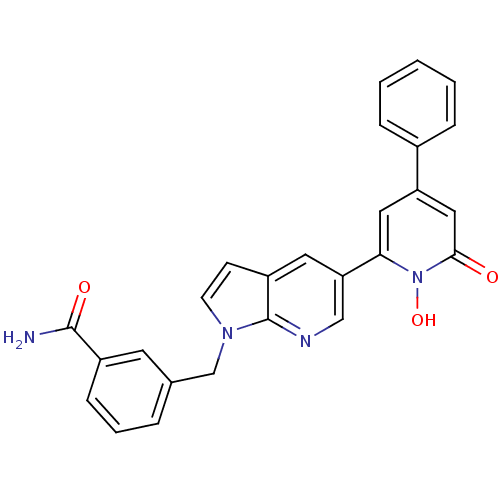 (CHEMBL2203976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402207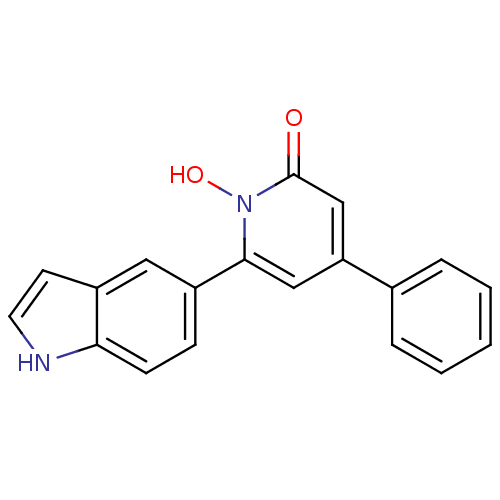 (CHEMBL2203975) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402203 (CHEMBL2203963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402208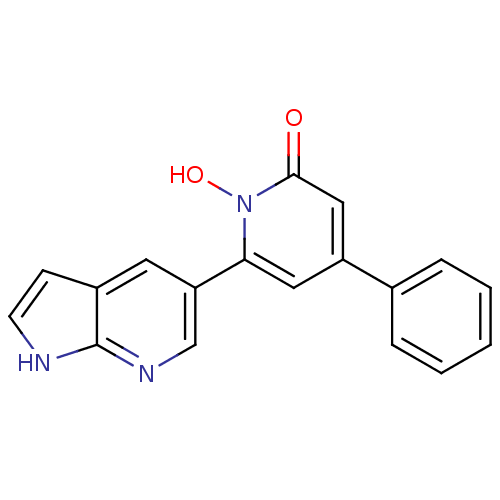 (CHEMBL2203974) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402201 (CHEMBL2203965) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402200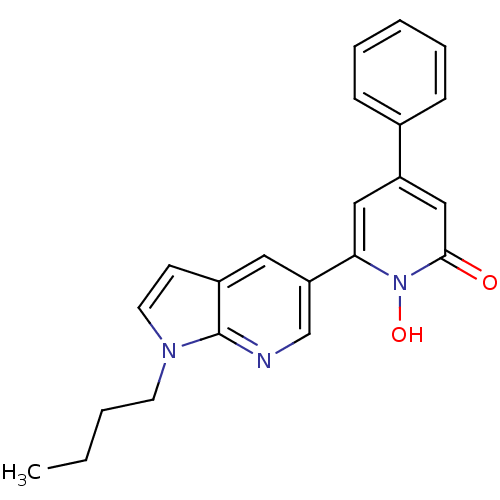 (CHEMBL2203966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517468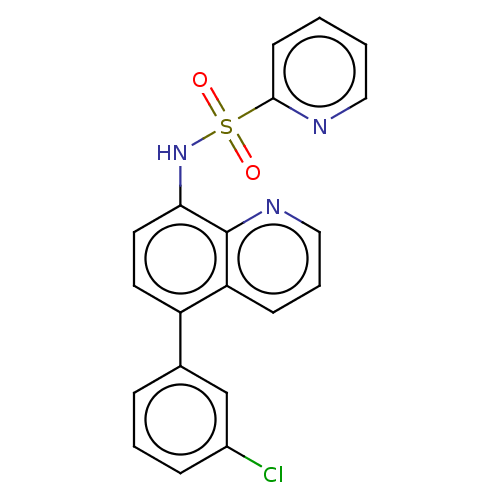 (CHEMBL4559294) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517488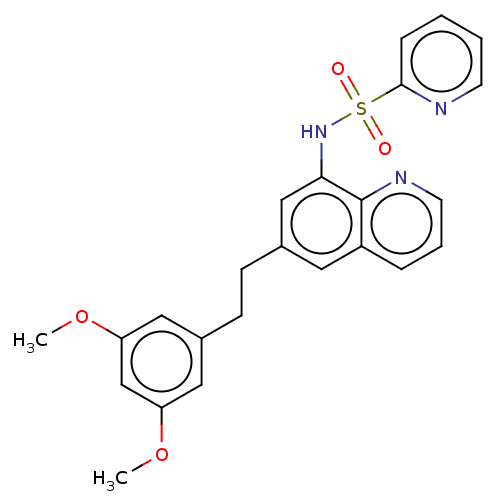 (CHEMBL4516514) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402205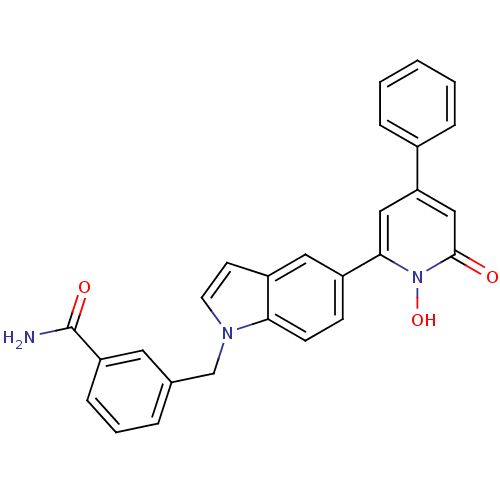 (CHEMBL2203977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517469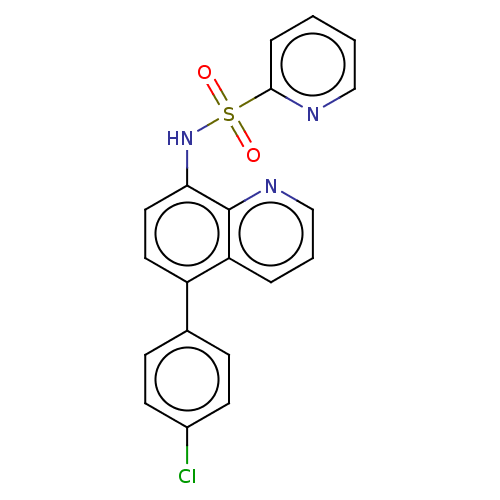 (CHEMBL4543065) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517474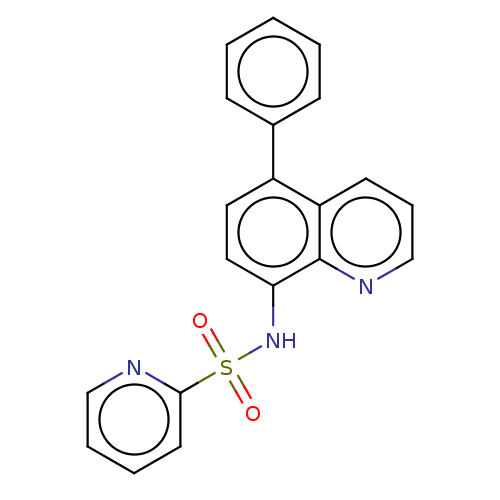 (CHEMBL4539074) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402199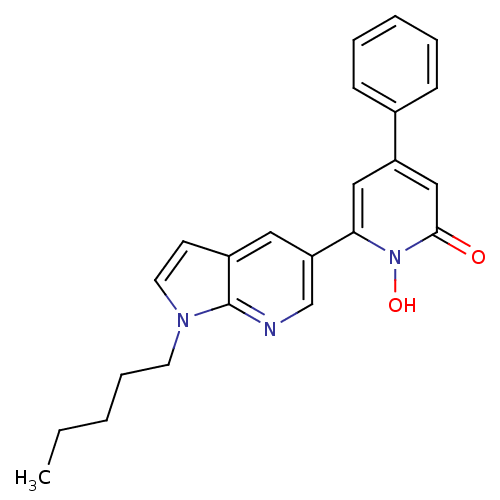 (CHEMBL2203967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517465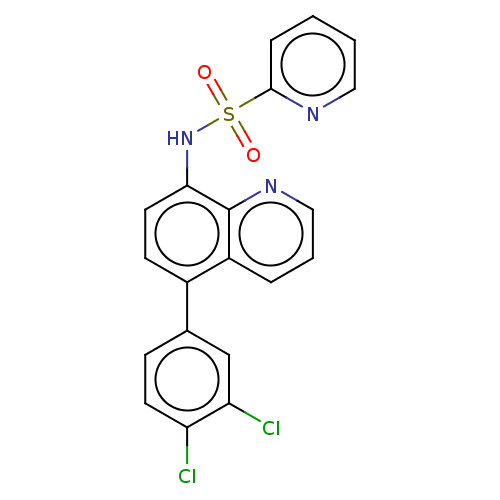 (CHEMBL4457949) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi... | Bioorg Med Chem Lett 21: 4337-42 (2011) Article DOI: 10.1016/j.bmcl.2011.05.046 BindingDB Entry DOI: 10.7270/Q2WW7J38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus system | Bioorg Med Chem 16: 3969-75 (2008) Article DOI: 10.1016/j.bmc.2008.01.031 BindingDB Entry DOI: 10.7270/Q2Z037XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517466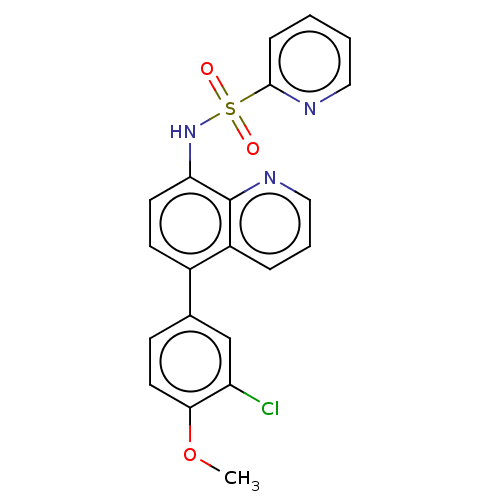 (CHEMBL4571524) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517473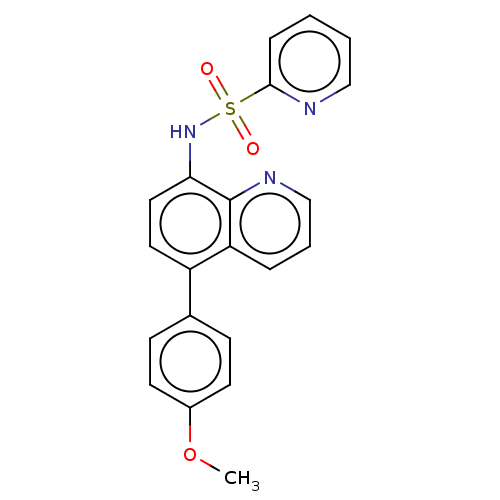 (CHEMBL4573731) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517471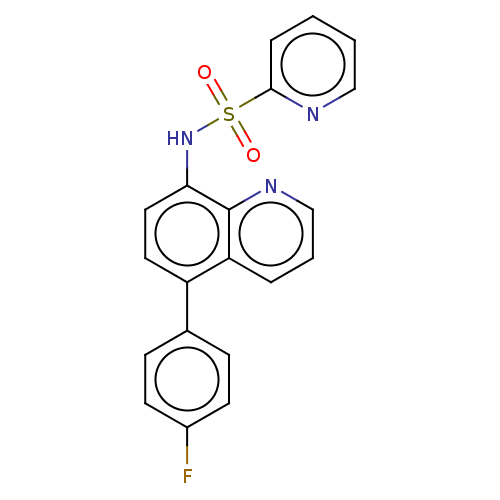 (CHEMBL4466017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50045936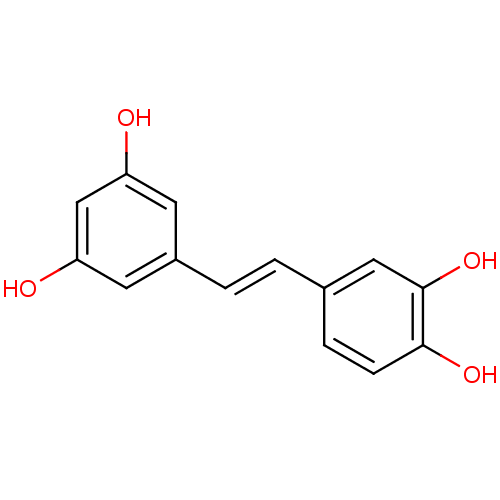 ((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged GLO1 expressed in baculovirus infected sf21 cells assessed as reduction in S-D-lactoylglutathione formatio... | Bioorg Med Chem Lett 27: 1169-1174 (2017) Article DOI: 10.1016/j.bmcl.2017.01.070 BindingDB Entry DOI: 10.7270/Q29W0HQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517437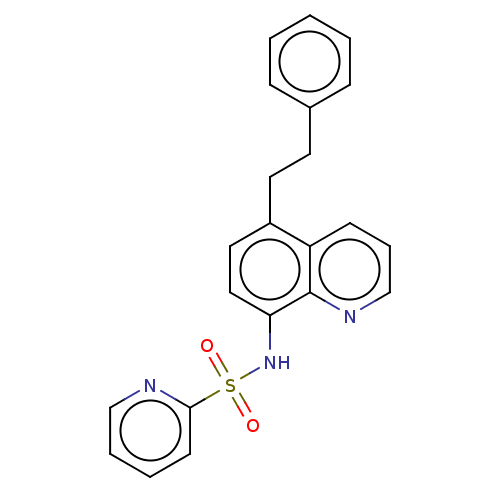 (CHEMBL4514413) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517463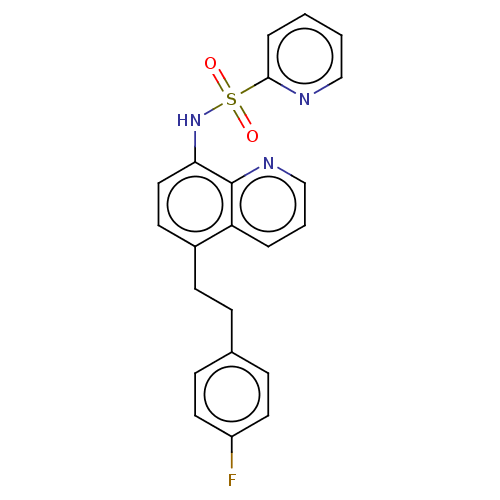 (CHEMBL4532036) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517467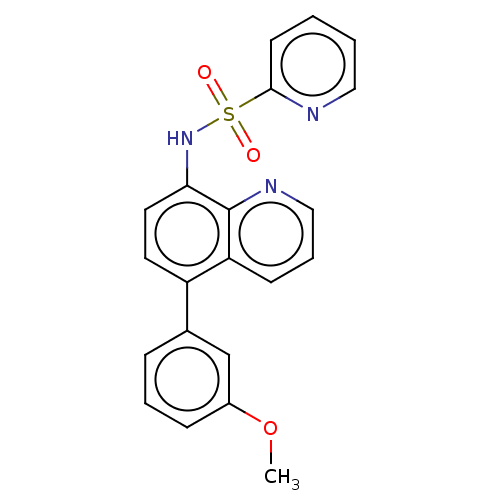 (CHEMBL4580349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50560159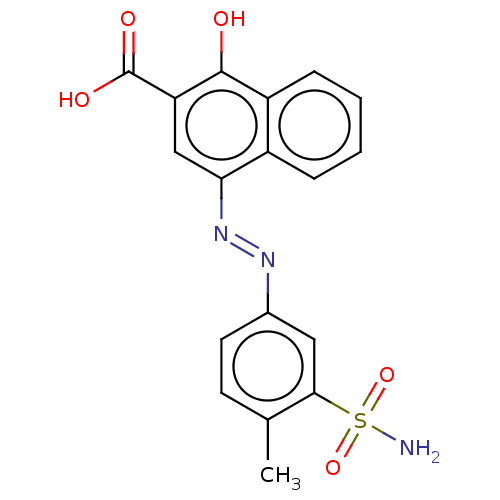 (CHEMBL4752509) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115608 BindingDB Entry DOI: 10.7270/Q2C53QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402215 (CHEMBL1624076) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517461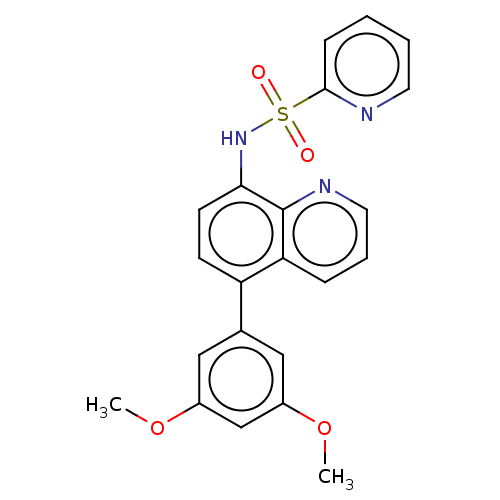 (CHEMBL4575972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50241121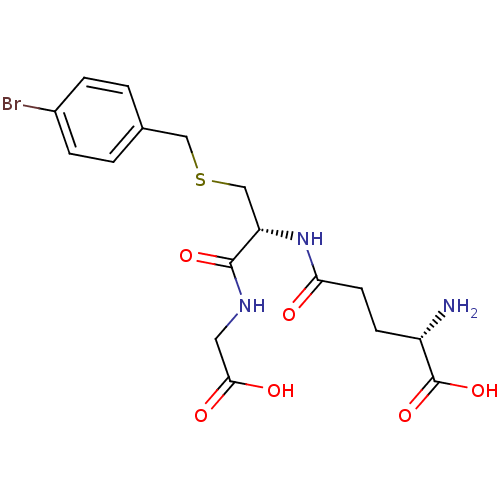 ((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517462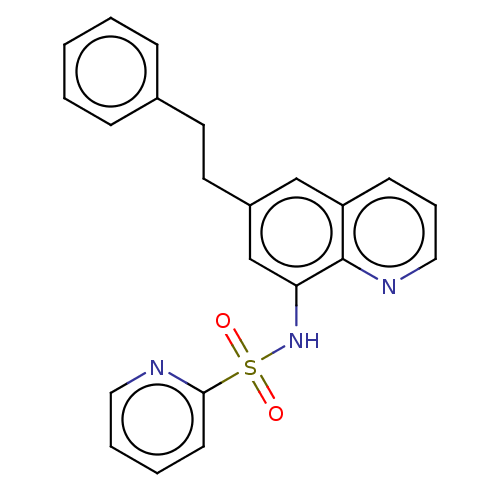 (CHEMBL4467660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50560166 (CHEMBL4799336) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115608 BindingDB Entry DOI: 10.7270/Q2C53QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50560150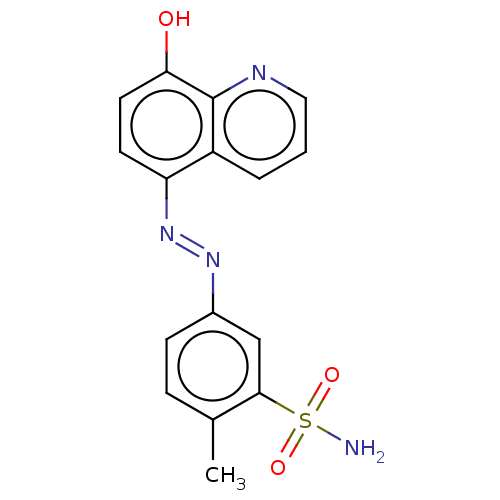 (CHEMBL4779039) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115608 BindingDB Entry DOI: 10.7270/Q2C53QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517483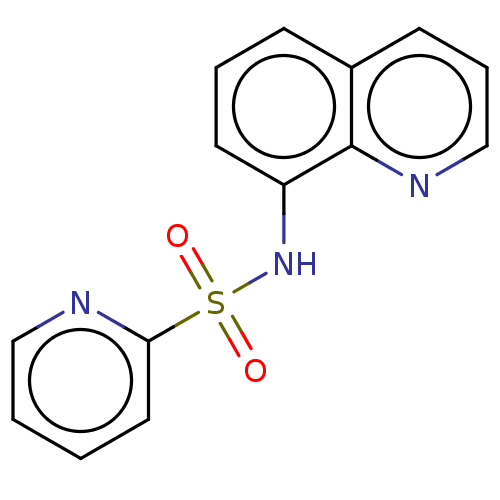 (CHEMBL3981698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517489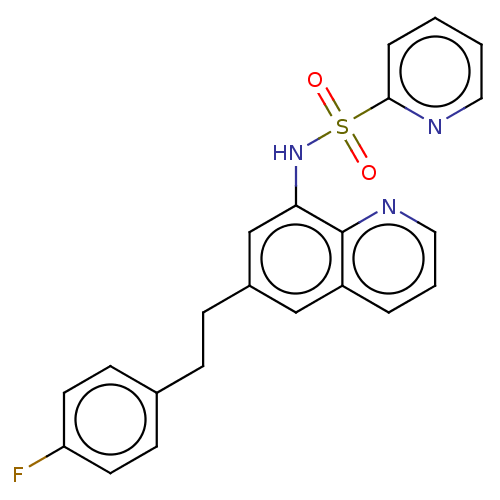 (CHEMBL4454726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50560167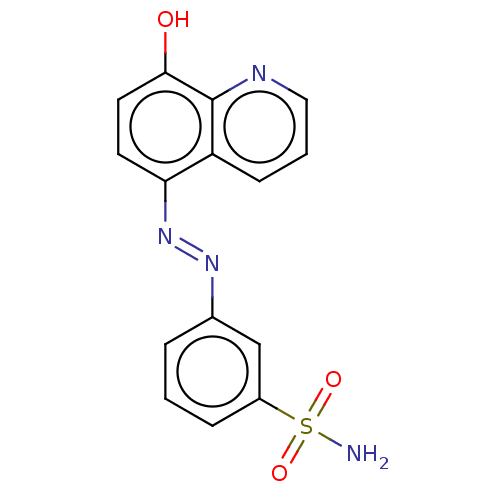 (CHEMBL4749730) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115608 BindingDB Entry DOI: 10.7270/Q2C53QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402214 (CHEMBL2203968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... | Bioorg Med Chem Lett 22: 7486-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.045 BindingDB Entry DOI: 10.7270/Q2RX9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50326997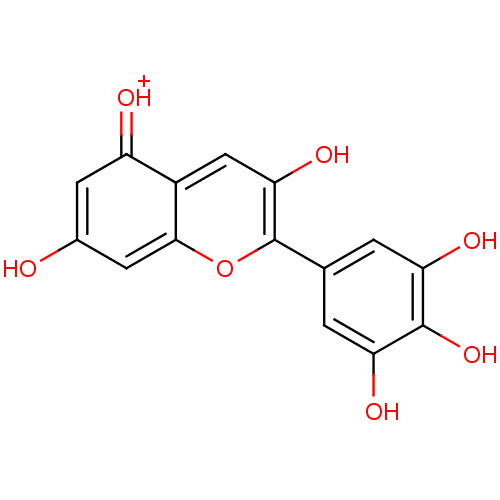 (CHEMBL590878 | Delphinidin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant glyoxalase 1 assessed as S-D-lactoylglutathione after 15 mins by spectrophotometric analysis | Bioorg Med Chem 18: 7029-33 (2010) Article DOI: 10.1016/j.bmc.2010.08.012 BindingDB Entry DOI: 10.7270/Q2PC32K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50355547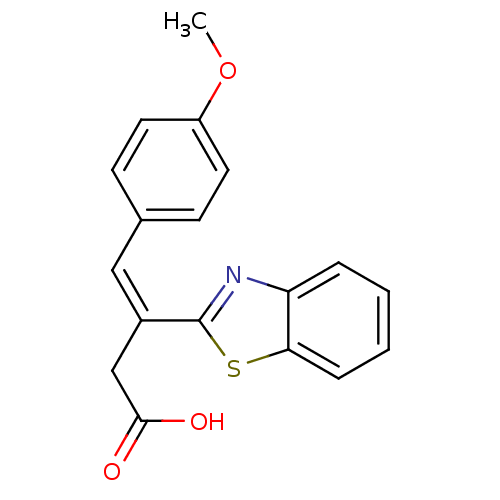 (CHEMBL1910548) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi... | Bioorg Med Chem Lett 21: 4337-42 (2011) Article DOI: 10.1016/j.bmcl.2011.05.046 BindingDB Entry DOI: 10.7270/Q2WW7J38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569120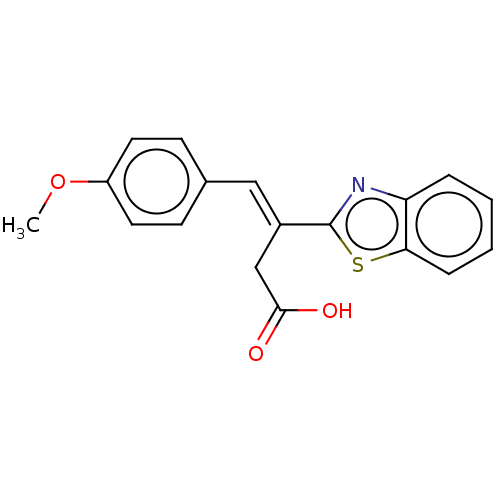 (CHEMBL4861582) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569128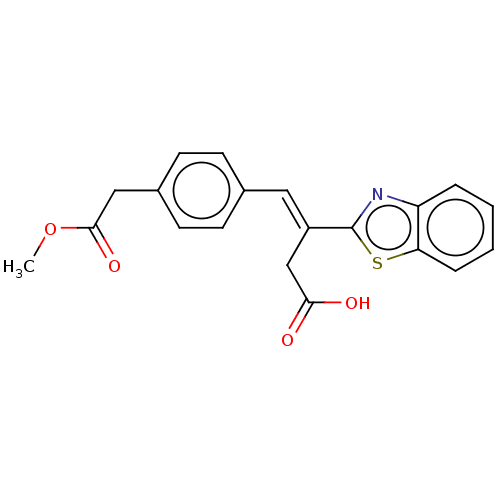 (CHEMBL4853249) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569133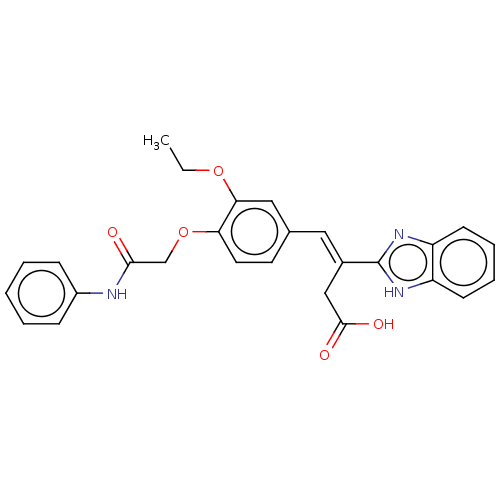 (CHEMBL4875229) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569134 (CHEMBL4863408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569131 (CHEMBL4852940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus system | Bioorg Med Chem 16: 3969-75 (2008) Article DOI: 10.1016/j.bmc.2008.01.031 BindingDB Entry DOI: 10.7270/Q2Z037XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569129 (CHEMBL4851336) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569125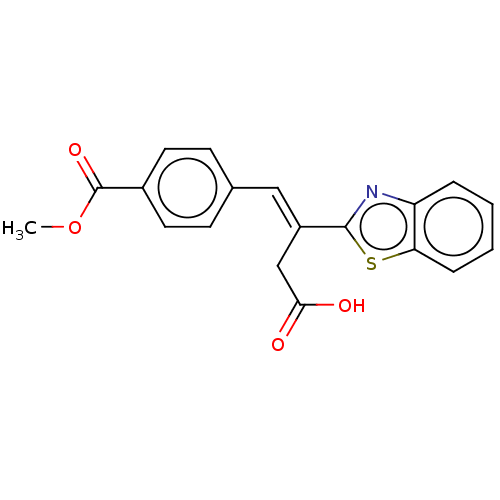 (CHEMBL4871220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using glutathione and methylglyoxal as substra... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115608 BindingDB Entry DOI: 10.7270/Q2C53QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50569130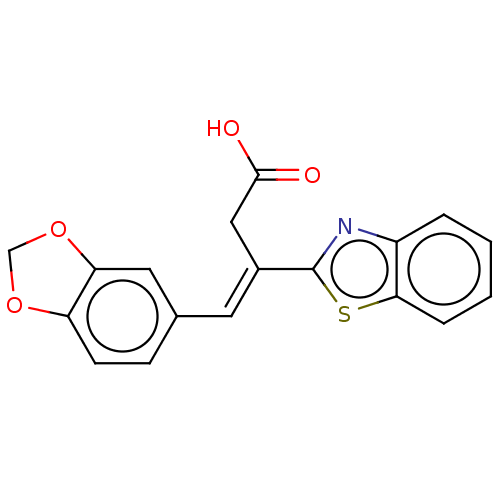 (CHEMBL1303934) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127918 BindingDB Entry DOI: 10.7270/Q23R0XNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517447 (CHEMBL3392484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50560173 (CHEMBL4747412) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal Met and 6-His-tagged Glyoxalase-1 (Ala2 to Met184 residues) using methylglyoxal and reduced glutathione as... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115608 BindingDB Entry DOI: 10.7270/Q2C53QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517446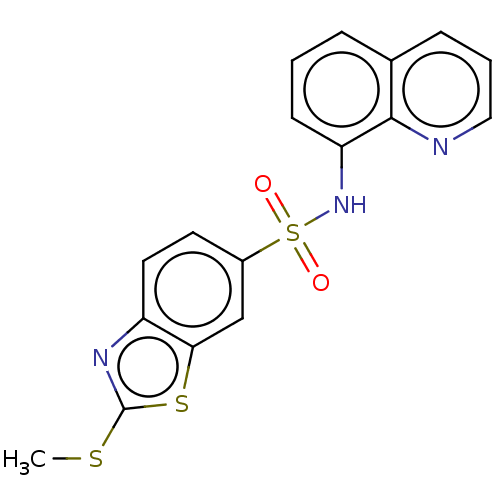 (CHEMBL4582242) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition recombinant human N-terminal 6His-tagged GLO1 (2 to 184 residues) expressed in Escherichia coli using MG as substrate preincubated for 15 ... | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 153 total ) | Next | Last >> |