

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501684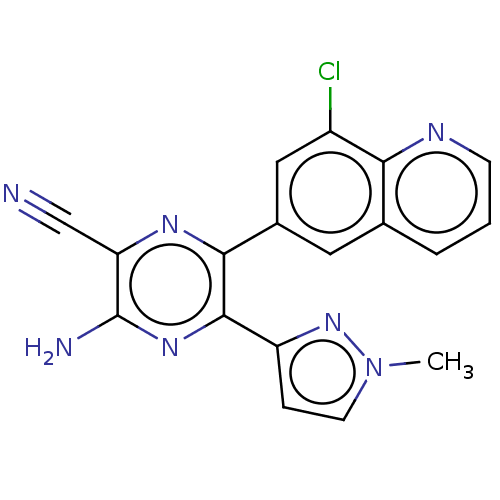 (US11028058, Compound 1.309) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501676 (US11028058, Compound 1.271) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501677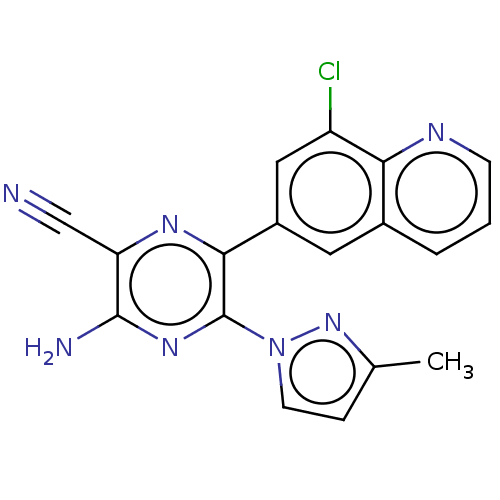 (US11028058, Compound 1.292) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501679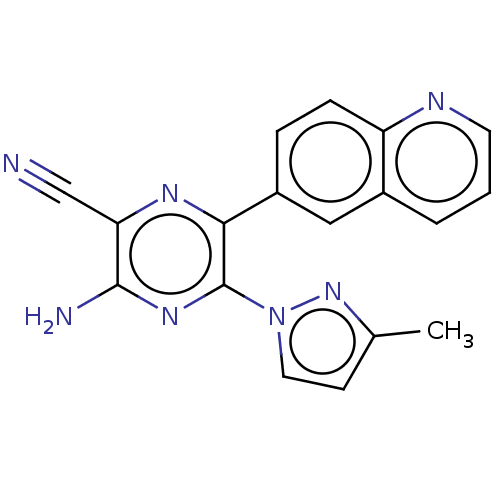 (US11028058, Compound 1.290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501680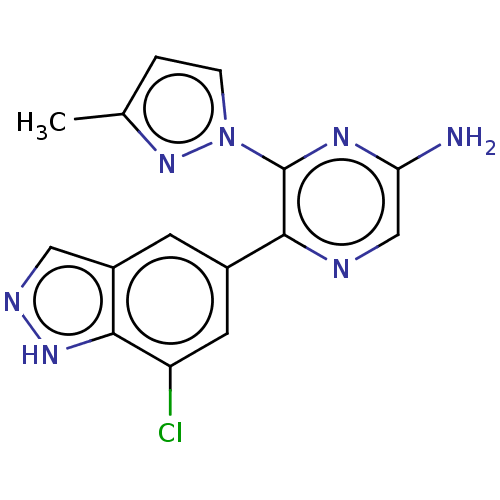 (US11028058, Compound 1.295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501682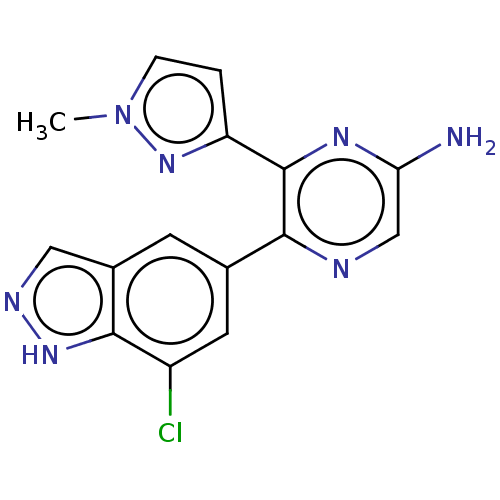 (US11028058, Compound 1.304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501686 (US11028058, Compound 1.318) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501681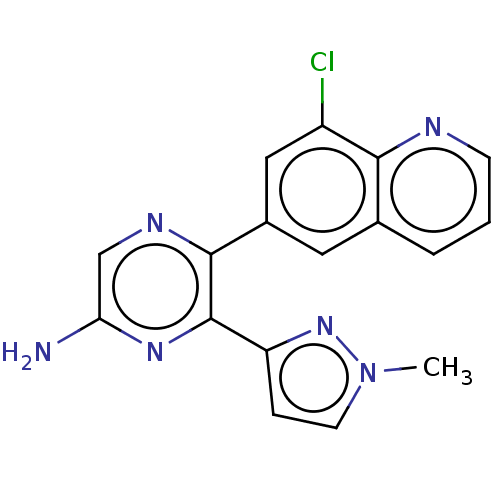 (US11028058, Compound 1.297) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468244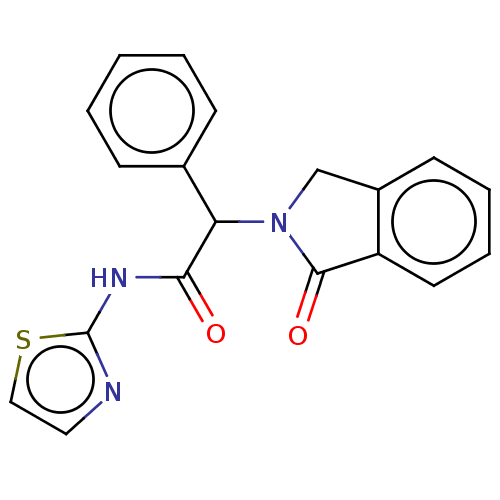 (CHEMBL4284413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of human EGFR L858R mutant expressed in Sf9 cells in presence of 1 mM ATP by HTRF assay | Eur J Med Chem 142: 32-47 (2017) Article DOI: 10.1016/j.ejmech.2017.05.027 BindingDB Entry DOI: 10.7270/Q2T156B8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205634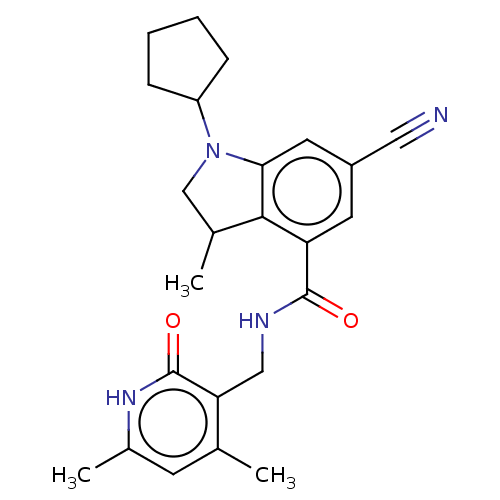 (CHEMBL3933203) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM464678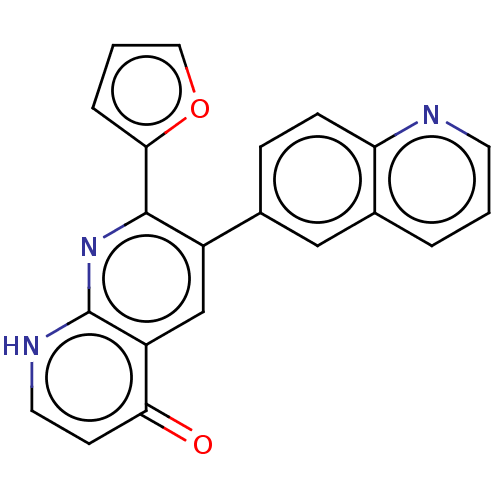 (US10793561, Compound 1.14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the adenosine A2A receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US10793561 (2020) BindingDB Entry DOI: 10.7270/Q2ST7SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501678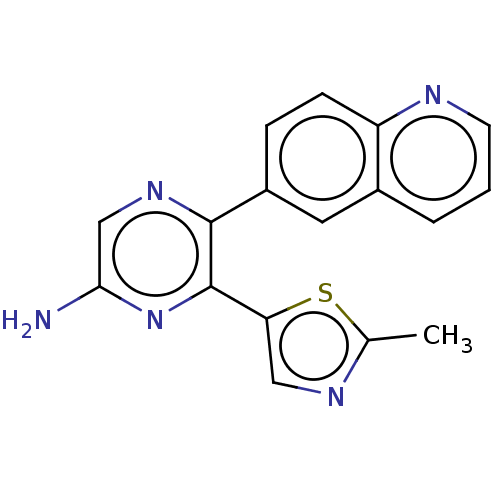 (US11028058, Compound 1.288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55.5 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501675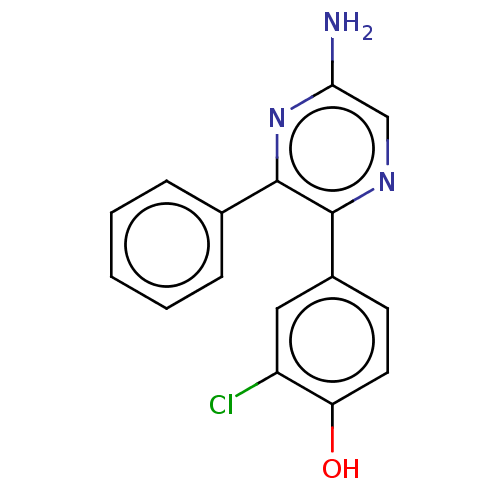 (US11028058, Compound 1.1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of human EGFR preincubated for 5 mins with substrate followed by ATP addition measured after 30 mins by HTRF method | Bioorg Med Chem 25: 2713-2723 (2017) Article DOI: 10.1016/j.bmc.2017.03.039 BindingDB Entry DOI: 10.7270/Q2NP26TD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205628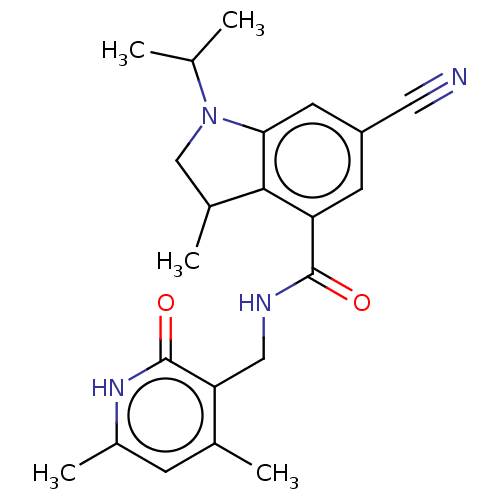 (CHEMBL3906288) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM501685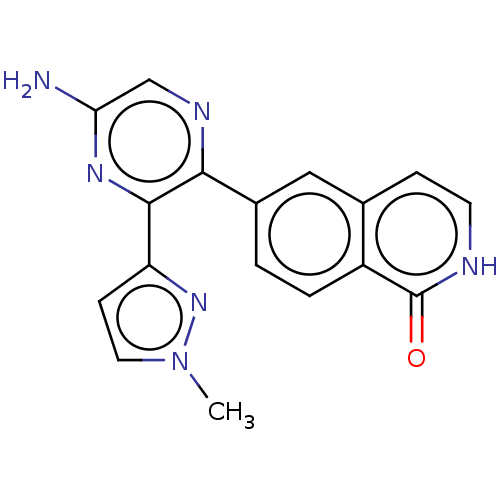 (US11028058, Compound 1.315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the A2A adenosine receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US11028058 (2021) BindingDB Entry DOI: 10.7270/Q23J3H46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM464677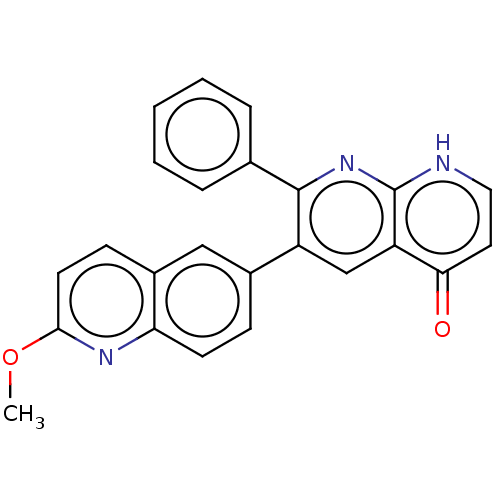 (US10793561, Compound 1.5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
NUVATION BIO INC. US Patent | Assay Description For the adenosine A2A receptor radioligand binding assay, the following modifications were made to the general protocol. GF/C filters (Perkin Elmer, ... | US Patent US10793561 (2020) BindingDB Entry DOI: 10.7270/Q2ST7SX9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205630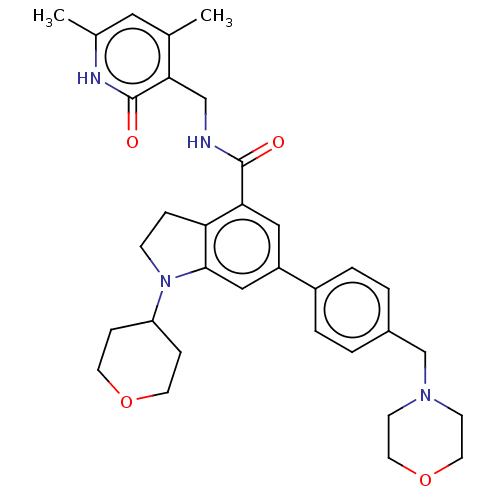 (CHEMBL3915266) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50245703 (CHEMBL4103440) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of human EGFR preincubated for 5 mins with substrate followed by ATP addition measured after 30 mins by HTRF method | Bioorg Med Chem 25: 2713-2723 (2017) Article DOI: 10.1016/j.bmc.2017.03.039 BindingDB Entry DOI: 10.7270/Q2NP26TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205635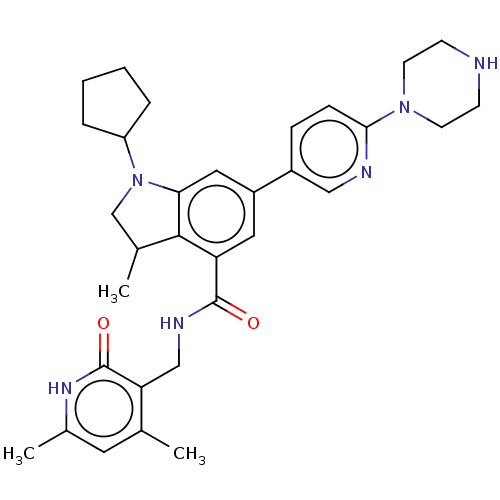 (CHEMBL3898133) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205635 (CHEMBL3898133) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50245700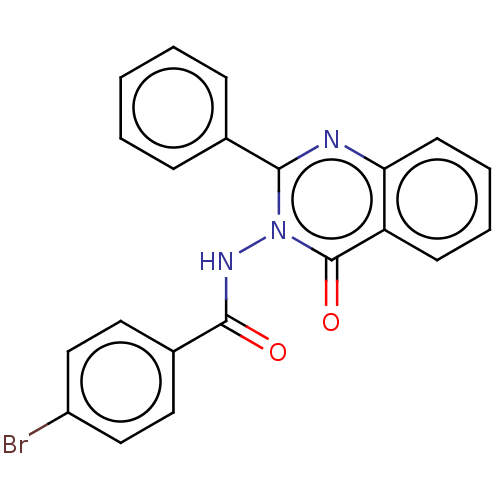 (CHEMBL4077349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of human EGFR preincubated for 5 mins with substrate followed by ATP addition measured after 30 mins by HTRF method | Bioorg Med Chem 25: 2713-2723 (2017) Article DOI: 10.1016/j.bmc.2017.03.039 BindingDB Entry DOI: 10.7270/Q2NP26TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205636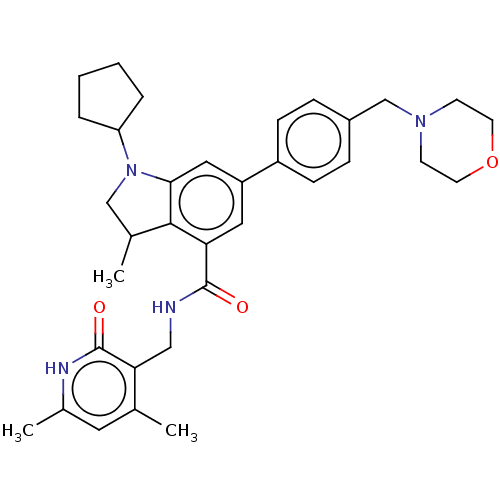 (CHEMBL3923183) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205636 (CHEMBL3923183) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50245705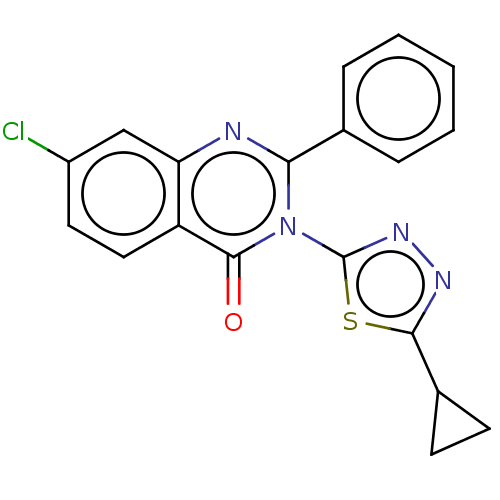 (CHEMBL4079183) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of human EGFR preincubated for 5 mins with substrate followed by ATP addition measured after 30 mins by HTRF method | Bioorg Med Chem 25: 2713-2723 (2017) Article DOI: 10.1016/j.bmc.2017.03.039 BindingDB Entry DOI: 10.7270/Q2NP26TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205627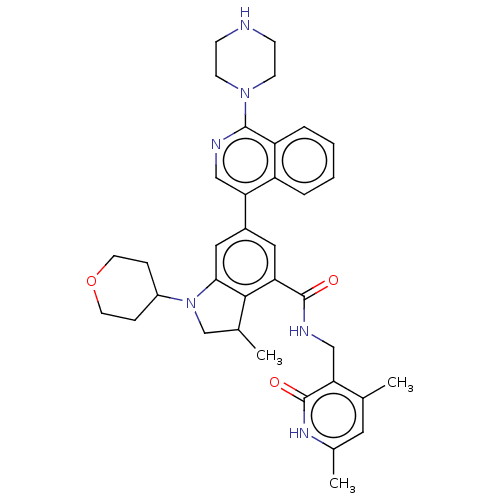 (CHEMBL3914517) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205627 (CHEMBL3914517) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205640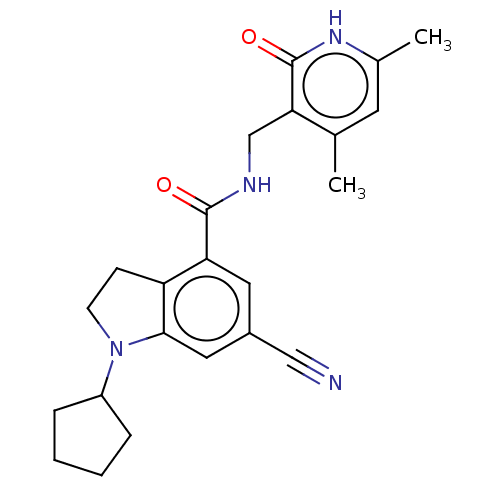 (CHEMBL3901407) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 571 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205632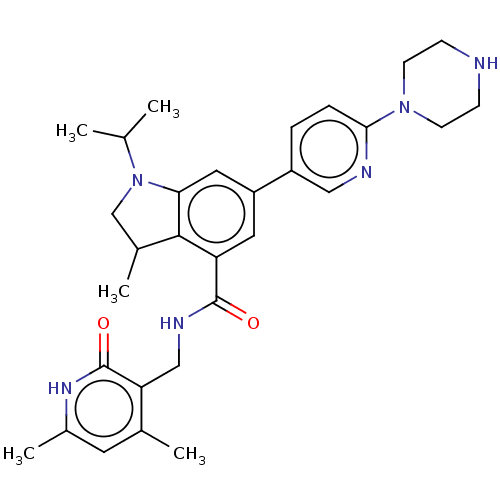 (CHEMBL3901570) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205632 (CHEMBL3901570) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205635 (CHEMBL3898133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205627 (CHEMBL3914517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50245702 (CHEMBL4060821) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of human EGFR preincubated for 5 mins with substrate followed by ATP addition measured after 30 mins by HTRF method | Bioorg Med Chem 25: 2713-2723 (2017) Article DOI: 10.1016/j.bmc.2017.03.039 BindingDB Entry DOI: 10.7270/Q2NP26TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205632 (CHEMBL3901570) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205626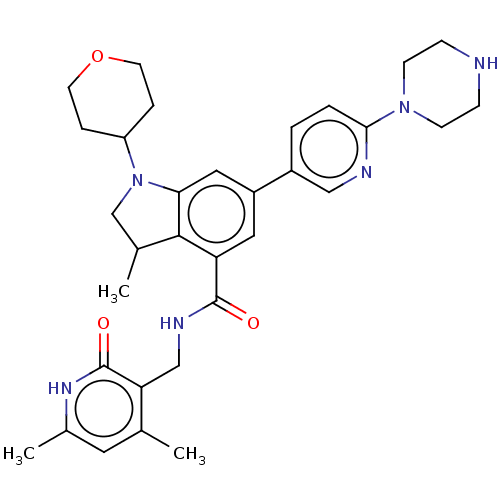 (CHEMBL3900506) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50245707 (CHEMBL4074248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of human EGFR preincubated for 5 mins with substrate followed by ATP addition measured after 30 mins by HTRF method | Bioorg Med Chem 25: 2713-2723 (2017) Article DOI: 10.1016/j.bmc.2017.03.039 BindingDB Entry DOI: 10.7270/Q2NP26TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205626 (CHEMBL3900506) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205626 (CHEMBL3900506) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205633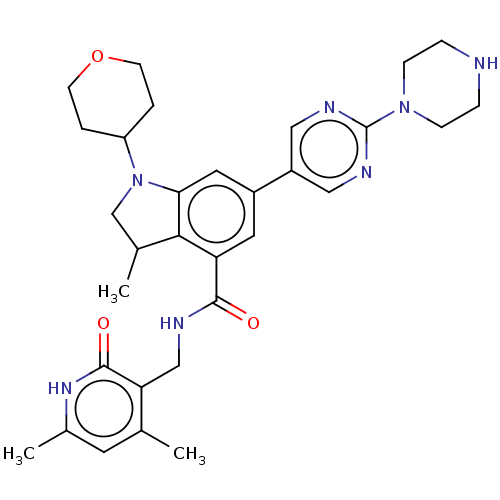 (CHEMBL3909213) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205633 (CHEMBL3909213) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205636 (CHEMBL3923183) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205629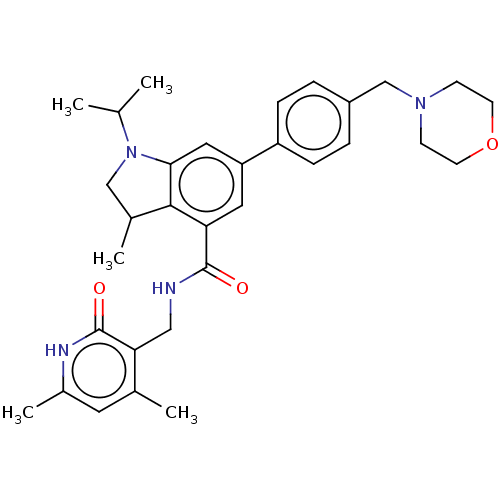 (CHEMBL3905192) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50245701 (CHEMBL4083467) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of human EGFR preincubated for 5 mins with substrate followed by ATP addition measured after 30 mins by HTRF method | Bioorg Med Chem 25: 2713-2723 (2017) Article DOI: 10.1016/j.bmc.2017.03.039 BindingDB Entry DOI: 10.7270/Q2NP26TD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205639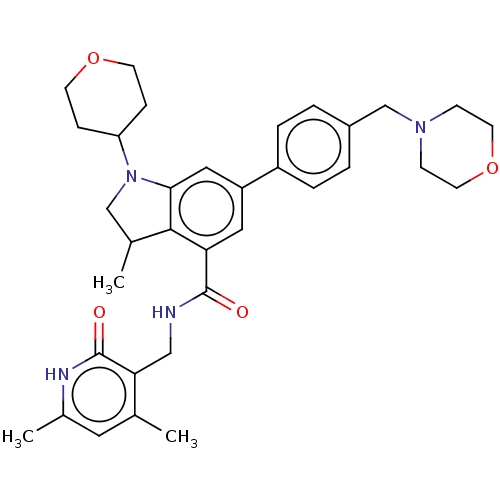 (CHEMBL3892623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205629 (CHEMBL3905192) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205629 (CHEMBL3905192) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205634 (CHEMBL3933203) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205639 (CHEMBL3892623) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50205639 (CHEMBL3892623) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50205628 (CHEMBL3906288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis | Bioorg Med Chem Lett 27: 217-222 (2017) Article DOI: 10.1016/j.bmcl.2016.11.080 BindingDB Entry DOI: 10.7270/Q2S46TZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 121 total ) | Next | Last >> |