 Found 60305 hits with Last Name = 'ke' and Initial = 'd'
Found 60305 hits with Last Name = 'ke' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548
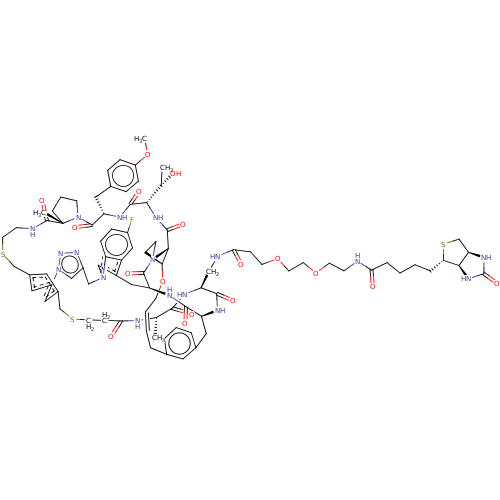
(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547
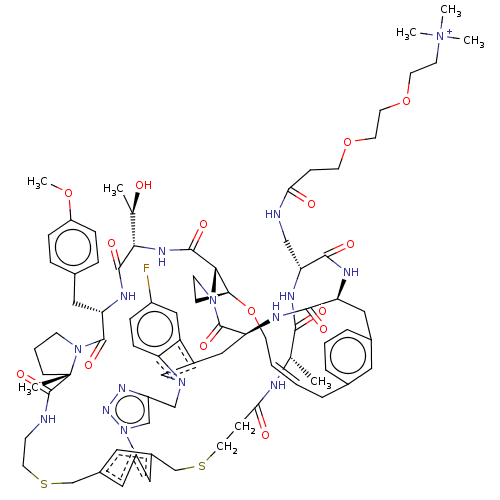
(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM578
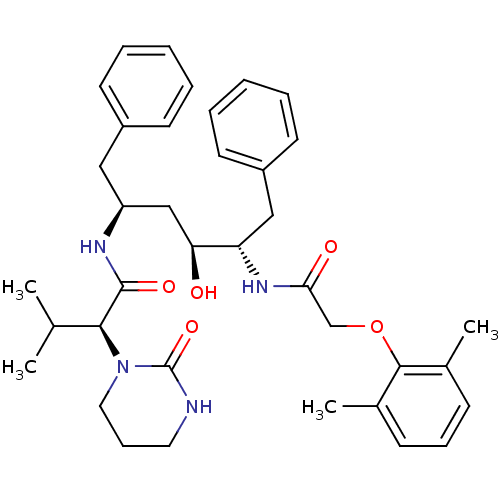
((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50099842

((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...)Show SMILES CC(C)[C@H](N1CCC(=O)NC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H46N4O6/c1-24(2)34(41-19-18-32(43)40-37(41)46)36(45)38-29(20-27-14-7-5-8-15-27)22-31(42)30(21-28-16-9-6-10-17-28)39-33(44)23-47-35-25(3)12-11-13-26(35)4/h5-17,24,29-31,34,42H,18-23H2,1-4H3,(H,38,45)(H,39,44)(H,40,43,46)/t29-,30-,31-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50099843
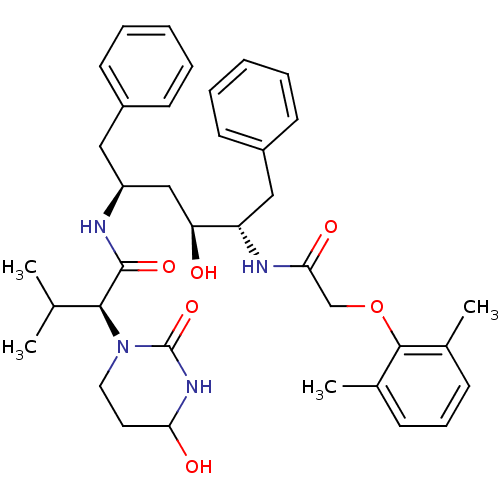
((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...)Show SMILES CC(C)[C@H](N1CCC(O)NC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H48N4O6/c1-24(2)34(41-19-18-32(43)40-37(41)46)36(45)38-29(20-27-14-7-5-8-15-27)22-31(42)30(21-28-16-9-6-10-17-28)39-33(44)23-47-35-25(3)12-11-13-26(35)4/h5-17,24,29-32,34,42-43H,18-23H2,1-4H3,(H,38,45)(H,39,44)(H,40,46)/t29-,30-,31-,32?,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50166288
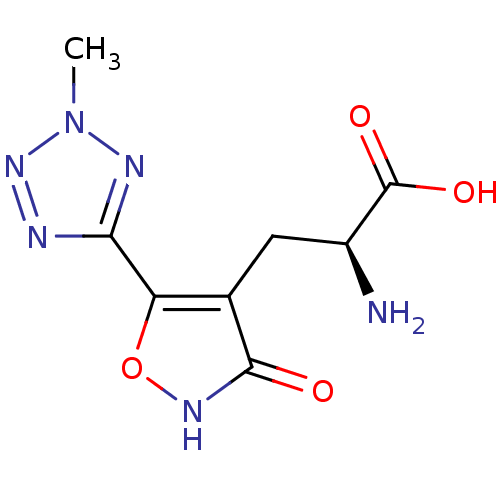
((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...)Show InChI InChI=1S/C8H10N6O4/c1-14-11-6(10-13-14)5-3(7(15)12-18-5)2-4(9)8(16)17/h4H,2,9H2,1H3,(H,12,15)(H,16,17)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR3 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546
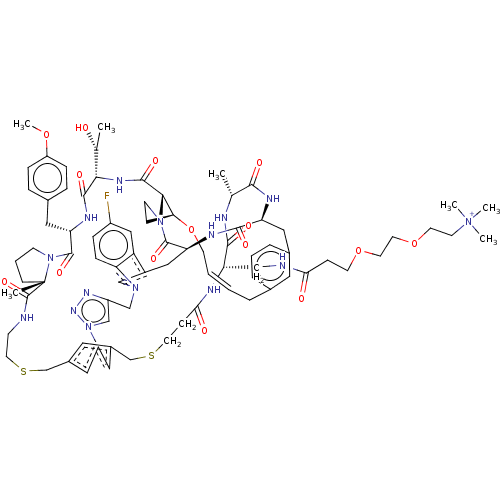
(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930
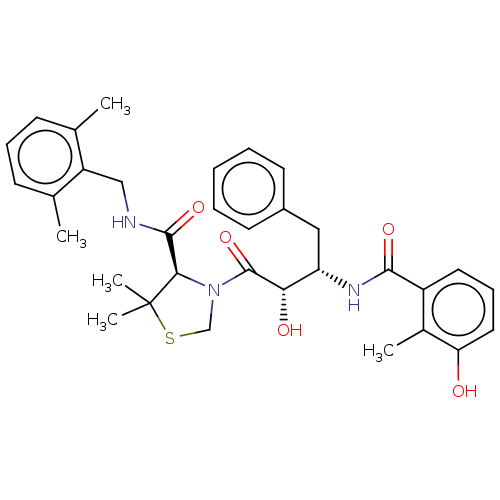
(CHEMBL584130 | KNI-814)Show SMILES Cc1cccc(C)c1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C33H39N3O5S/c1-20-11-9-12-21(2)25(20)18-34-31(40)29-33(4,5)42-19-36(29)32(41)28(38)26(17-23-13-7-6-8-14-23)35-30(39)24-15-10-16-27(37)22(24)3/h6-16,26,28-29,37-38H,17-19H2,1-5H3,(H,34,40)(H,35,39)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 4
(Rattus norvegicus) | BDBM50166288

((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...)Show InChI InChI=1S/C8H10N6O4/c1-14-11-6(10-13-14)5-3(7(15)12-18-5)2-4(9)8(16)17/h4H,2,9H2,1H3,(H,12,15)(H,16,17)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR4 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Rattus norvegicus) | BDBM50166288

((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...)Show InChI InChI=1S/C8H10N6O4/c1-14-11-6(10-13-14)5-3(7(15)12-18-5)2-4(9)8(16)17/h4H,2,9H2,1H3,(H,12,15)(H,16,17)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR2 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM200
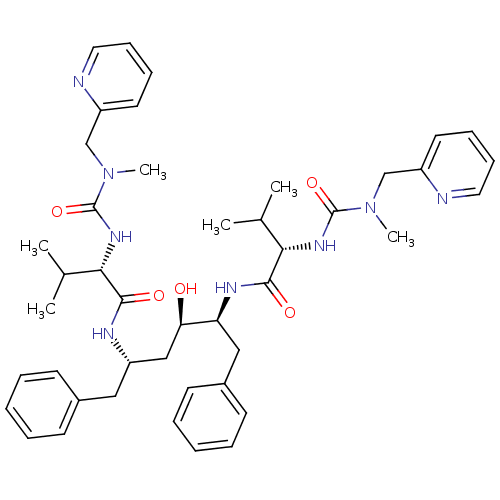
((2S)-N-[(2S,3R,5S)-3-hydroxy-5-[(2S)-3-methyl-2-{[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C)Cc1ccccc1 |r| Show InChI InChI=1S/C44H58N8O5/c1-30(2)39(49-43(56)51(5)28-34-21-13-15-23-45-34)41(54)47-36(25-32-17-9-7-10-18-32)27-38(53)37(26-33-19-11-8-12-20-33)48-42(55)40(31(3)4)50-44(57)52(6)29-35-22-14-16-24-46-35/h7-24,30-31,36-40,53H,25-29H2,1-6H3,(H,47,54)(H,48,55)(H,49,56)(H,50,57)/t36-,37-,38+,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.00400 | -66.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... |
J Am Chem Soc 116: 847-55 (1994)
Article DOI: 10.1021/ja00082a004
BindingDB Entry DOI: 10.7270/Q2KK98Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM194
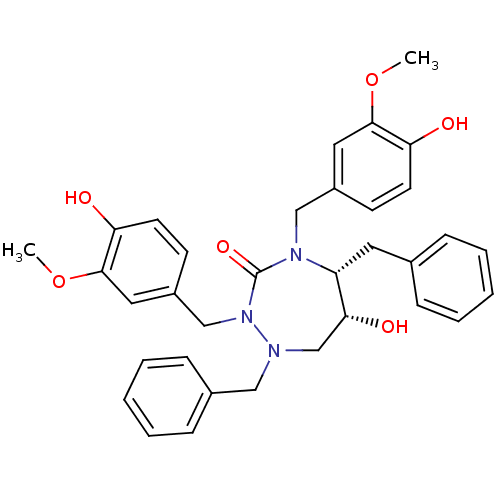
((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxy-...)Show SMILES COc1cc(CN2[C@H](Cc3ccccc3)[C@H](O)CN(Cc3ccccc3)N(Cc3ccc(O)c(OC)c3)C2=O)ccc1O |r| Show InChI InChI=1S/C34H37N3O6/c1-42-32-18-26(13-15-29(32)38)21-36-28(17-24-9-5-3-6-10-24)31(40)23-35(20-25-11-7-4-8-12-25)37(34(36)41)22-27-14-16-30(39)33(19-27)43-2/h3-16,18-19,28,31,38-40H,17,20-23H2,1-2H3/t28-,31-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00500 | -65.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... |
J Med Chem 39: 392-7 (1996)
Article DOI: 10.1021/jm9507183
BindingDB Entry DOI: 10.7270/Q2V40SC6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50166288

((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...)Show InChI InChI=1S/C8H10N6O4/c1-14-11-6(10-13-14)5-3(7(15)12-18-5)2-4(9)8(16)17/h4H,2,9H2,1H3,(H,12,15)(H,16,17)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR1 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545
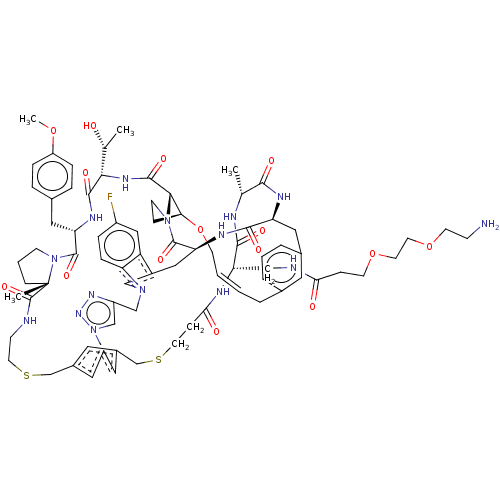
(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544
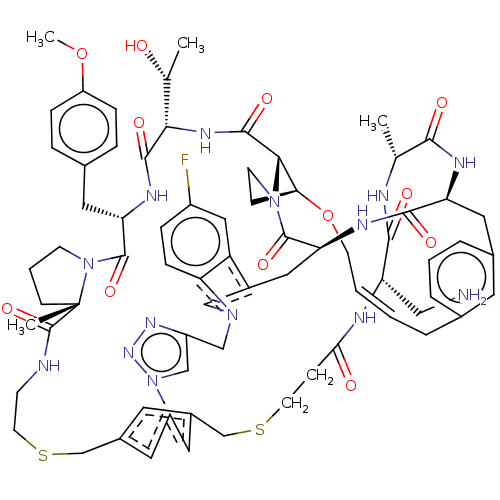
(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50157823
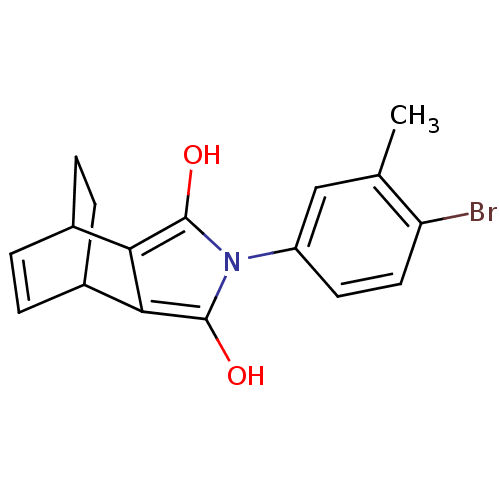
((2R,6S)-4-(4-Bromo-3-methyl-phenyl)-4-aza-tricyclo...)Show SMILES Cc1cc(ccc1Br)-n1c(O)c2C3CCC(C=C3)c2c1O |c:17,THB:9:11:16.17:13.14| Show InChI InChI=1S/C17H16BrNO2/c1-9-8-12(6-7-13(9)18)19-16(20)14-10-2-3-11(5-4-10)15(14)17(19)21/h2-3,6-8,10-11,20-21H,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for mutant T877A Androgen receptor in human LNCaP cells |
Bioorg Med Chem Lett 15: 271-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.085
BindingDB Entry DOI: 10.7270/Q2N58KVH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50150028

((5R,6R)-1-Benzoyl-5-benzyl-6-hydroxy-2,4-bis-(4-hy...)Show SMILES O[C@@H]1CN(N(Cc2ccc(O)cc2)C(=O)N(Cc2ccc(O)cc2)[C@@H]1Cc1ccccc1)C(=O)c1ccccc1 Show InChI InChI=1S/C32H31N3O5/c36-27-15-11-24(12-16-27)20-33-29(19-23-7-3-1-4-8-23)30(38)22-34(31(39)26-9-5-2-6-10-26)35(32(33)40)21-25-13-17-28(37)18-14-25/h1-18,29-30,36-38H,19-22H2/t29-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for inhibition of HIV protease |
Bioorg Med Chem Lett 14: 4075-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.036
BindingDB Entry DOI: 10.7270/Q25B01ZK |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50184069
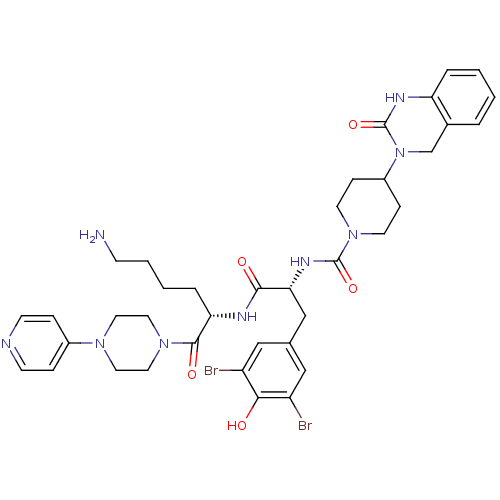
(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor |
Bioorg Med Chem Lett 23: 3157-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.012
BindingDB Entry DOI: 10.7270/Q2348MSQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50184069

(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells |
ACS Med Chem Lett 3: 337-341 (2012)
Article DOI: 10.1021/ml300021s
BindingDB Entry DOI: 10.7270/Q26D5V2R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM198
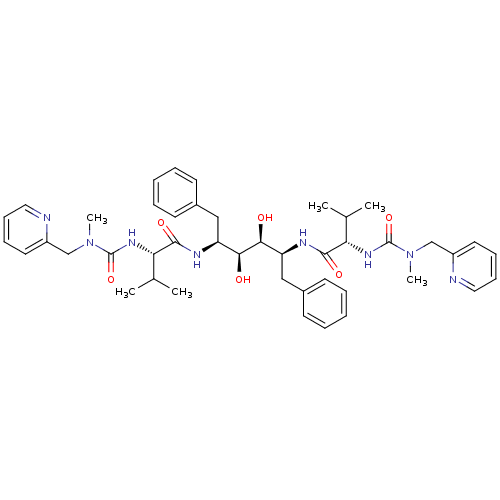
((2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36-,37-,38-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.0110 | -63.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... |
J Am Chem Soc 116: 847-55 (1994)
Article DOI: 10.1021/ja00082a004
BindingDB Entry DOI: 10.7270/Q2KK98Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM199
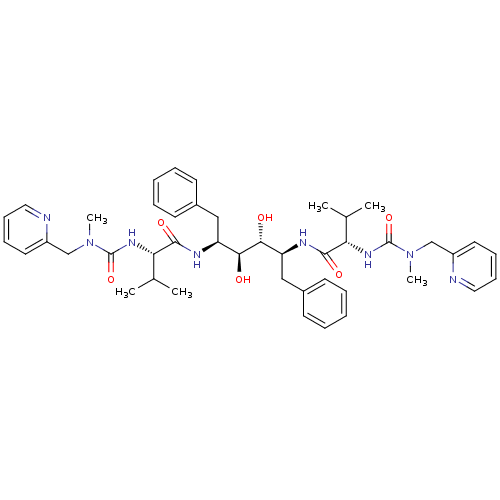
((2S)-N-[(2S,3R,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36-,37-,38-,39-,40+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.0120 | -63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... |
J Am Chem Soc 116: 847-55 (1994)
Article DOI: 10.1021/ja00082a004
BindingDB Entry DOI: 10.7270/Q2KK98Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50268484
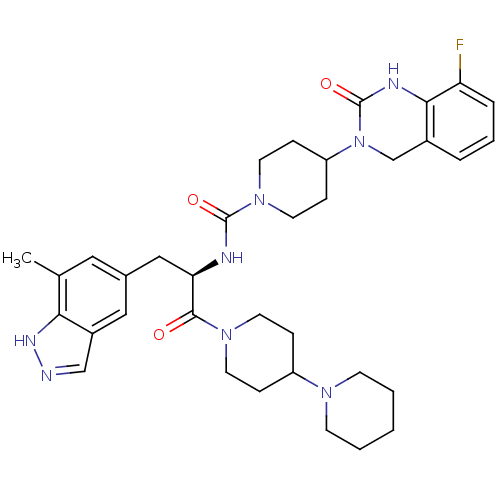
((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3cccc(F)c3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C35H45FN8O3/c1-23-18-24(19-26-21-37-40-31(23)26)20-30(33(45)42-14-8-27(9-15-42)41-12-3-2-4-13-41)38-34(46)43-16-10-28(11-17-43)44-22-25-6-5-7-29(36)32(25)39-35(44)47/h5-7,18-19,21,27-28,30H,2-4,8-17,20,22H2,1H3,(H,37,40)(H,38,46)(H,39,47)/t30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor |
Bioorg Med Chem Lett 23: 3157-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.012
BindingDB Entry DOI: 10.7270/Q2348MSQ |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118030
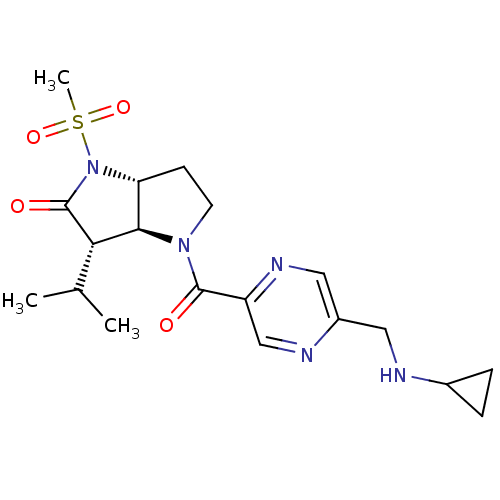
(4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)c2cnc(CNC3CC3)cn2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H27N5O4S/c1-11(2)16-17-15(24(19(16)26)29(3,27)28)6-7-23(17)18(25)14-10-21-13(9-22-14)8-20-12-4-5-12/h9-12,15-17,20H,4-8H2,1-3H3/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50576175
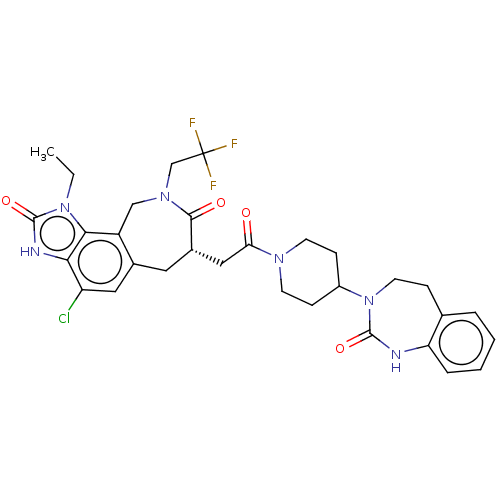
(CHEMBL4859941)Show SMILES CCn1c2c3CN(CC(F)(F)F)C(=O)[C@H](CC(=O)N4CCC(CC4)N4CCc5ccccc5NC4=O)Cc3cc(Cl)c2[nH]c1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128077
BindingDB Entry DOI: 10.7270/Q23N276F |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50576173

(CHEMBL4848032)Show SMILES Cn1c2c3CN(CC(F)(F)F)C(=O)[C@H](CC(=O)N4CCC(CC4)N4CCc5ccccc5NC4=O)Cc3cc(Cl)c2[nH]c1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128077
BindingDB Entry DOI: 10.7270/Q23N276F |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant of ritonavir towards HIV protease was determined |
Bioorg Med Chem Lett 7: 699-704 (1997)
Article DOI: 10.1016/S0960-894X(97)00080-2
BindingDB Entry DOI: 10.7270/Q23N23C1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor 2
(Rattus norvegicus) | BDBM50002370
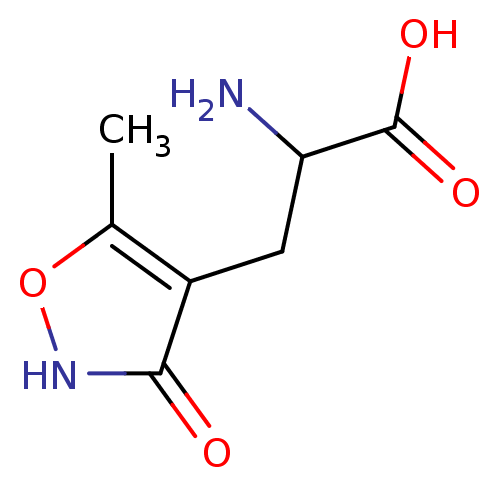
((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR2 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50576172
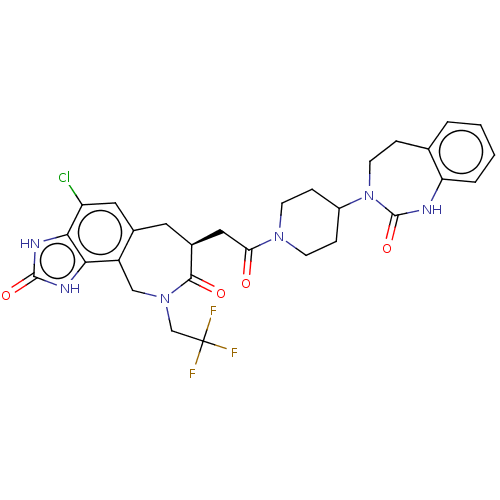
(CHEMBL4848486)Show SMILES FC(F)(F)CN1Cc2c(C[C@@H](CC(=O)N3CCC(CC3)N3CCc4ccccc4NC3=O)C1=O)cc(Cl)c1[nH]c(=O)[nH]c21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128077
BindingDB Entry DOI: 10.7270/Q23N276F |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9192
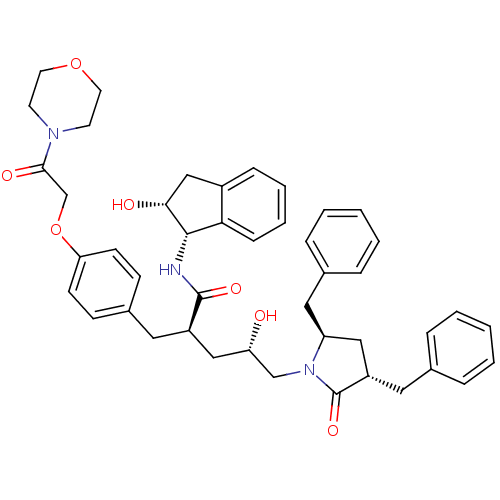
((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES O[C@@H](C[C@@H](Cc1ccc(OCC(=O)N2CCOCC2)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C45H51N3O7/c49-38(29-48-37(25-32-11-5-2-6-12-32)26-36(45(48)53)24-31-9-3-1-4-10-31)27-35(44(52)46-43-40-14-8-7-13-34(40)28-41(43)50)23-33-15-17-39(18-16-33)55-30-42(51)47-19-21-54-22-20-47/h1-18,35-38,41,43,49-50H,19-30H2,(H,46,52)/t35-,36+,37+,38+,41-,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | -61.1 | 63 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9195

((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES COCCOCCOc1ccc(C[C@H](C[C@H](O)CN2[C@@H](Cc3ccccc3)C[C@H](Cc3ccccc3)C2=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C44H52N2O7/c1-51-20-21-52-22-23-53-39-18-16-33(17-19-39)24-35(43(49)45-42-40-15-9-8-14-34(40)29-41(42)48)28-38(47)30-46-37(26-32-12-6-3-7-13-32)27-36(44(46)50)25-31-10-4-2-5-11-31/h2-19,35-38,41-42,47-48H,20-30H2,1H3,(H,45,49)/t35-,36+,37+,38+,41-,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | -61.1 | 93 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9190
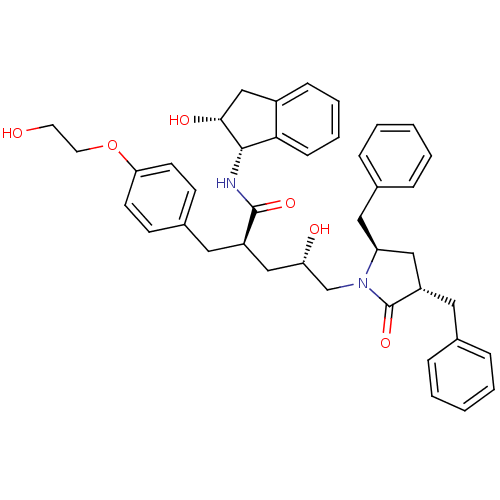
((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES OCCOc1ccc(C[C@H](C[C@H](O)CN2[C@@H](Cc3ccccc3)C[C@H](Cc3ccccc3)C2=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C41H46N2O6/c44-19-20-49-36-17-15-30(16-18-36)21-32(40(47)42-39-37-14-8-7-13-31(37)26-38(39)46)25-35(45)27-43-34(23-29-11-5-2-6-12-29)24-33(41(43)48)22-28-9-3-1-4-10-28/h1-18,32-35,38-39,44-46H,19-27H2,(H,42,47)/t32-,33+,34+,35+,38-,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | -61.1 | 94 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified HIV protease |
Bioorg Med Chem Lett 5: 2725-2728 (1995)
Article DOI: 10.1016/0960-894X(95)00462-3
BindingDB Entry DOI: 10.7270/Q26973JN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50576174
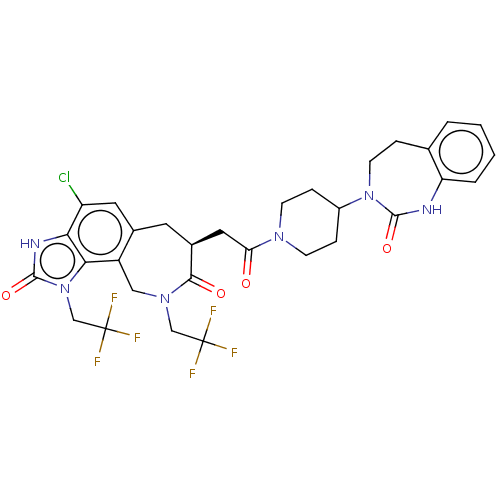
(CHEMBL4857649)Show SMILES FC(F)(F)CN1Cc2c(C[C@@H](CC(=O)N3CCC(CC3)N3CCc4ccccc4NC3=O)C1=O)cc(Cl)c1[nH]c(=O)n(CC(F)(F)F)c21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128077
BindingDB Entry DOI: 10.7270/Q23N276F |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50448785
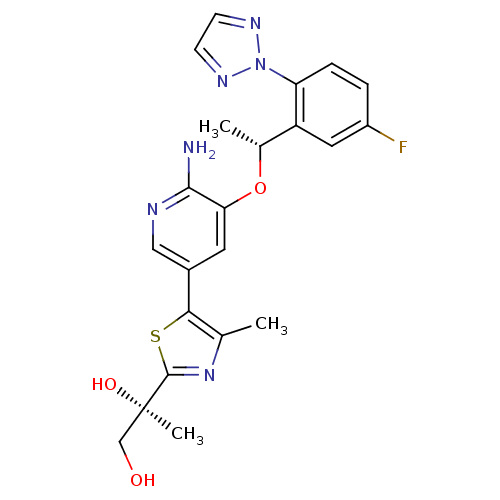
(CHEMBL3128069)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1sc(nc1C)[C@](C)(O)CO)c1cc(F)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H23FN6O3S/c1-12-19(33-21(28-12)22(3,31)11-30)14-8-18(20(24)25-10-14)32-13(2)16-9-15(23)4-5-17(16)29-26-6-7-27-29/h4-10,13,30-31H,11H2,1-3H3,(H2,24,25)/t13-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay |
J Med Chem 57: 1170-87 (2014)
Article DOI: 10.1021/jm401805h
BindingDB Entry DOI: 10.7270/Q29C6ZX5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373939
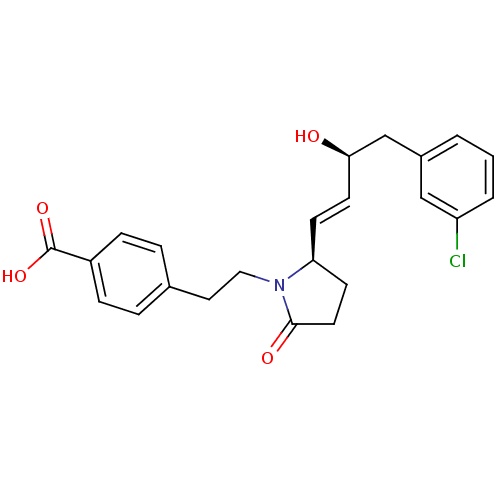
(CHEMBL258332)Show SMILES O[C@@H](Cc1cccc(Cl)c1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24ClNO4/c24-19-3-1-2-17(14-19)15-21(26)10-8-20-9-11-22(27)25(20)13-12-16-4-6-18(7-5-16)23(28)29/h1-8,10,14,20-21,26H,9,11-13,15H2,(H,28,29)/b10-8+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50356282
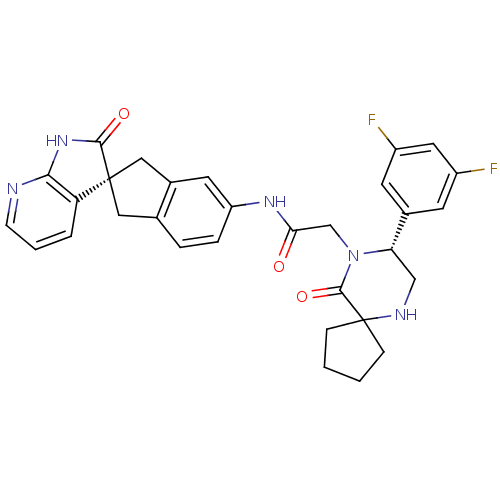
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human CGRP receptor |
Bioorg Med Chem Lett 23: 3157-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.012
BindingDB Entry DOI: 10.7270/Q2348MSQ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50002370

((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR3 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Rattus norvegicus (Rat)) | BDBM50002370

((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from rat recombinant GluR1 expressed in Sf9 cells |
J Med Chem 50: 2408-14 (2007)
Article DOI: 10.1021/jm061439q
BindingDB Entry DOI: 10.7270/Q2DZ0809 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50436107

(CHEMBL2397415)Show SMILES CN1CCC(CC1)N1CCN(CC1)C(=O)[C@@H](Cc1cc(C)c2[nH]ncc2c1)NC(=O)N1CCC(CC1)c1cc2ccccc2[nH]c1=O |r| Show InChI InChI=1S/C36H46N8O3/c1-24-19-25(20-28-23-37-40-33(24)28)21-32(35(46)43-17-15-42(16-18-43)29-9-11-41(2)12-10-29)39-36(47)44-13-7-26(8-14-44)30-22-27-5-3-4-6-31(27)38-34(30)45/h3-6,19-20,22-23,26,29,32H,7-18,21H2,1-2H3,(H,37,40)(H,38,45)(H,39,47)/t32-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cell membranes after 2 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 23: 3157-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.012
BindingDB Entry DOI: 10.7270/Q2348MSQ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384817
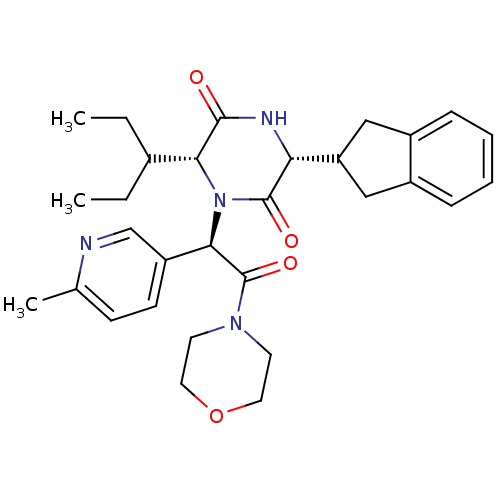
(CHEMBL2037514)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-4-20(5-2)26-28(35)32-25(24-16-21-8-6-7-9-22(21)17-24)29(36)34(26)27(23-11-10-19(3)31-18-23)30(37)33-12-14-38-15-13-33/h6-11,18,20,24-27H,4-5,12-17H2,1-3H3,(H,32,35)/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50576177
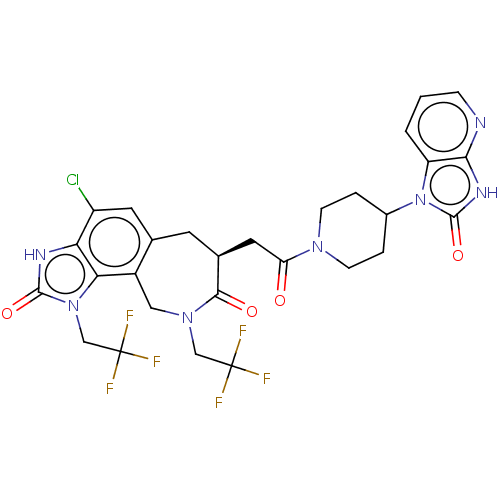
(CHEMBL4875327)Show SMILES FC(F)(F)CN1Cc2c(C[C@@H](CC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C1=O)cc(Cl)c1[nH]c(=O)n(CC(F)(F)F)c21 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128077
BindingDB Entry DOI: 10.7270/Q23N276F |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50400098
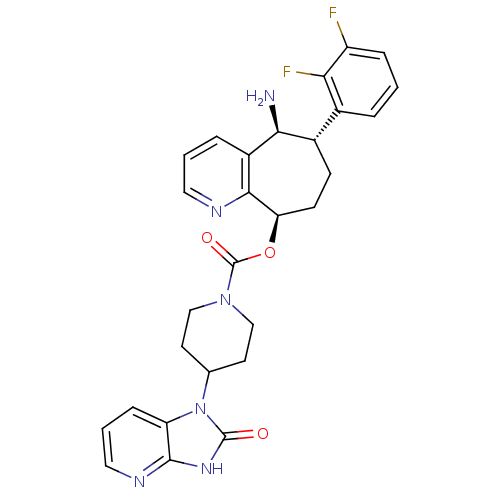
(CHEMBL2178422)Show SMILES N[C@H]1[C@@H](CC[C@@H](OC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)c2ncccc12)c1cccc(F)c1F |r| Show InChI InChI=1S/C28H28F2N6O3/c29-20-6-1-4-17(23(20)30)18-8-9-22(25-19(24(18)31)5-2-12-32-25)39-28(38)35-14-10-16(11-15-35)36-21-7-3-13-33-26(21)34-27(36)37/h1-7,12-13,16,18,22,24H,8-11,14-15,31H2,(H,33,34,37)/t18-,22+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis |
J Med Chem 55: 10644-51 (2012)
Article DOI: 10.1021/jm3013147
BindingDB Entry DOI: 10.7270/Q2M046M8 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50167072
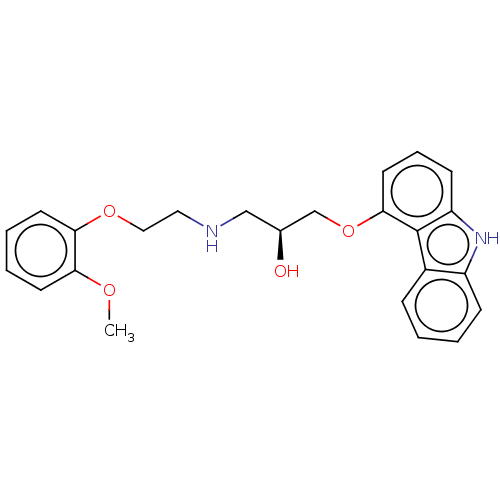
(CHEMBL3799125)Show SMILES COc1ccccc1OCCNC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C24H26N2O4/c1-28-21-10-4-5-11-22(21)29-14-13-25-15-17(27)16-30-23-12-6-9-20-24(23)18-7-2-3-8-19(18)26-20/h2-12,17,25-27H,13-16H2,1H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg (FAU)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP-12177 from human beta2-adrenergic receptor expressed in CHO cells after 60 mins by radioligand competition binding assay |
J Med Chem 62: 5111-5131 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00349
BindingDB Entry DOI: 10.7270/Q2DZ0CRD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data