

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50098576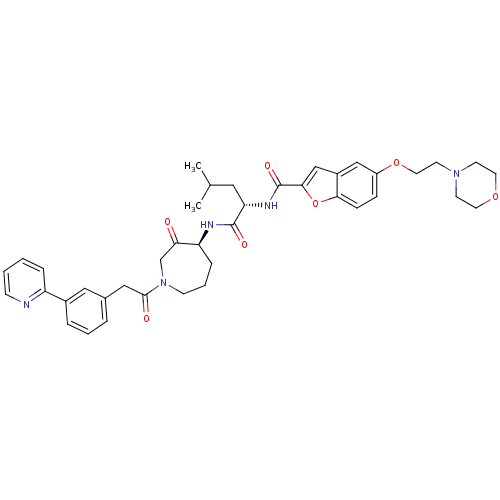 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19770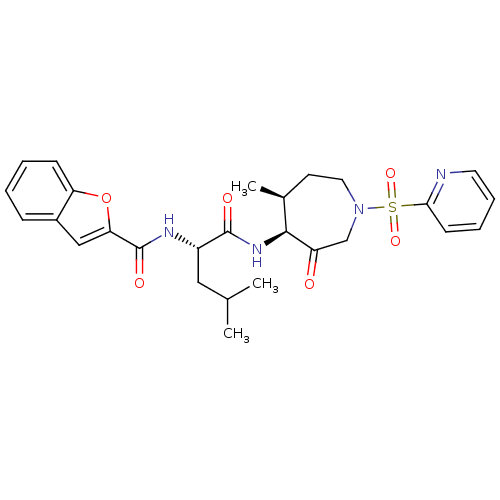 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19770 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17355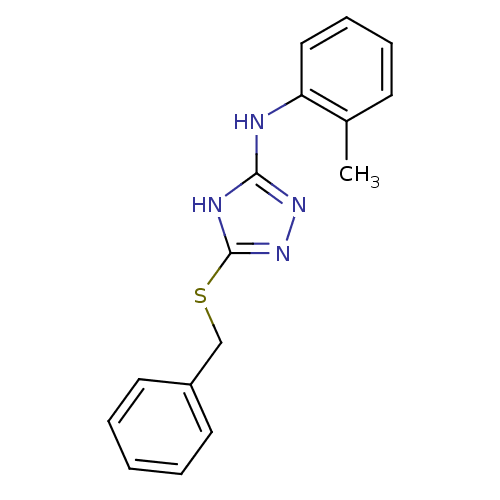 (1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17428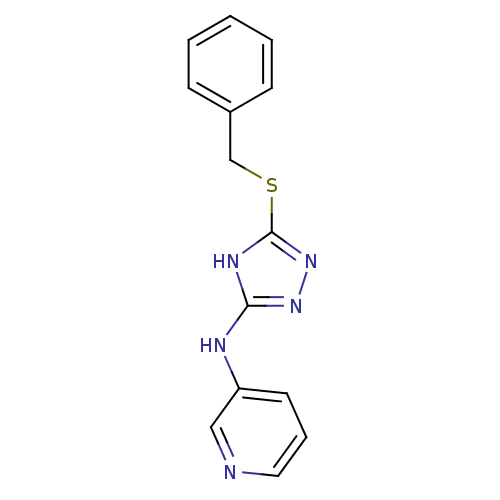 (1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17388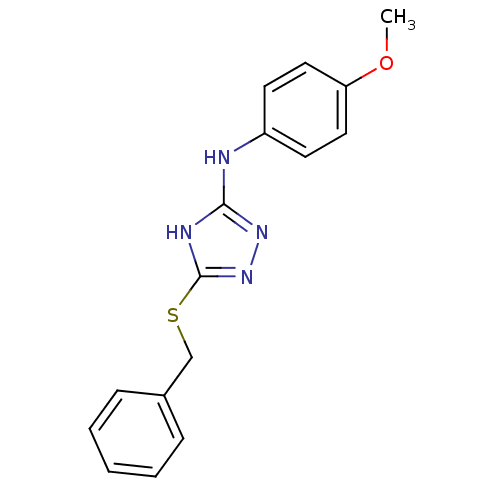 (1,2,4-Triazole Compound, 46 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17365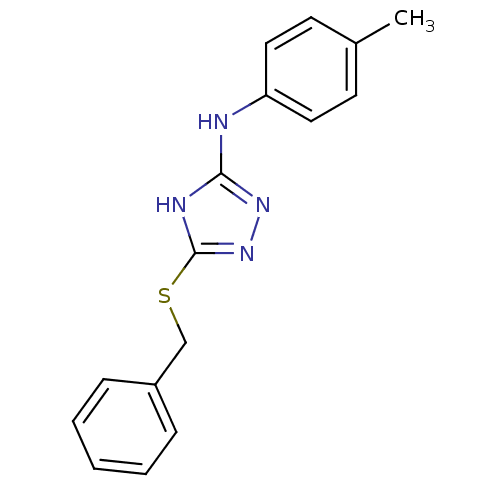 (1,2,4-Triazole Compound, 23 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17390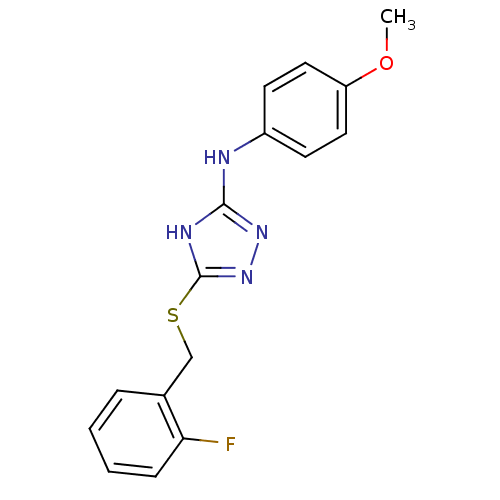 (1,2,4-Triazole Compound, 48 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19775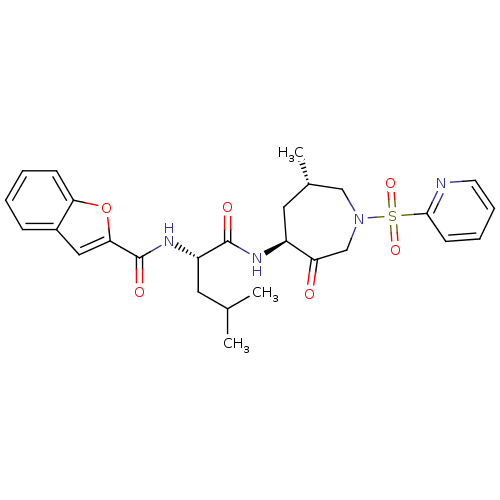 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17362 (1,2,4-Triazole Compound, 20 | 5-[(3-methylbut-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17395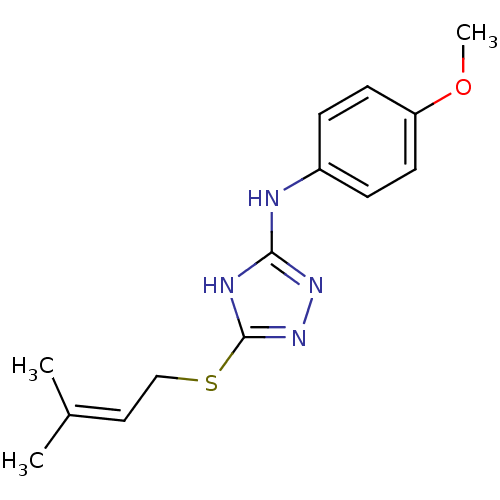 (1,2,4-Triazole Compound, 53 | N-(4-methoxyphenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin K | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17358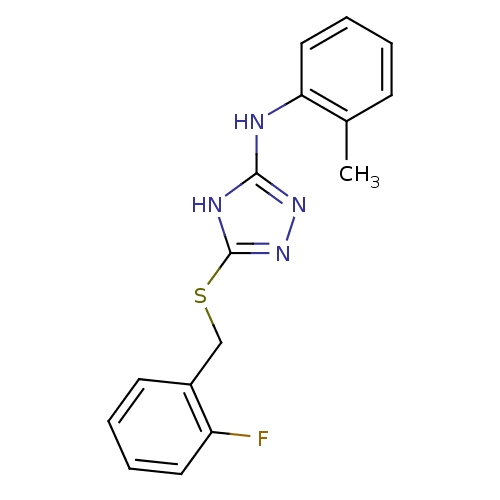 (1,2,4-Triazole Compound, 16 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50084650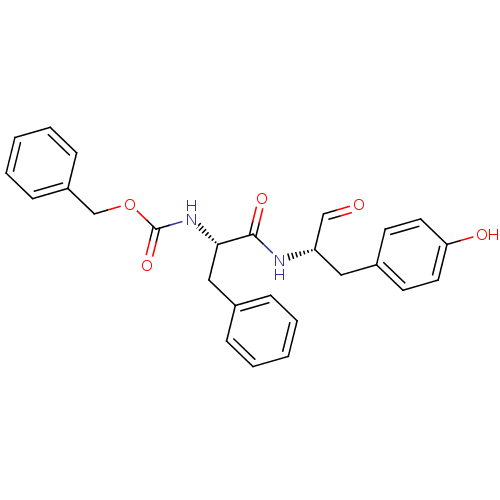 (CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin S | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17430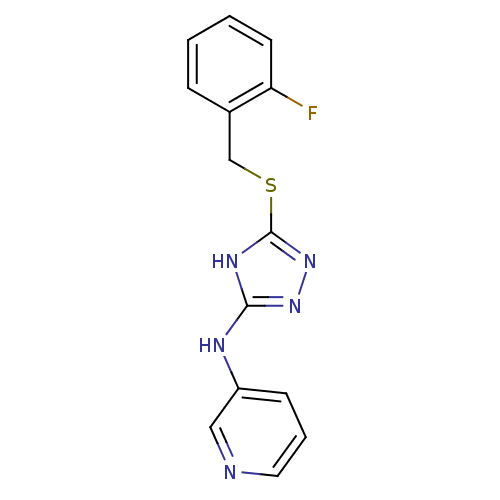 (1,2,4-Triazole Compound, 88 | N-(5-{[(2-fluorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359261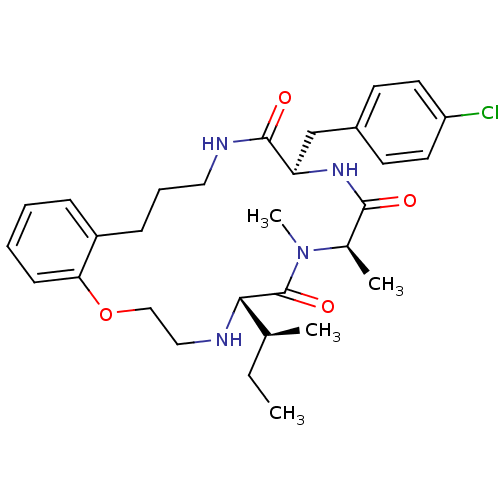 (CHEMBL1923617) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359239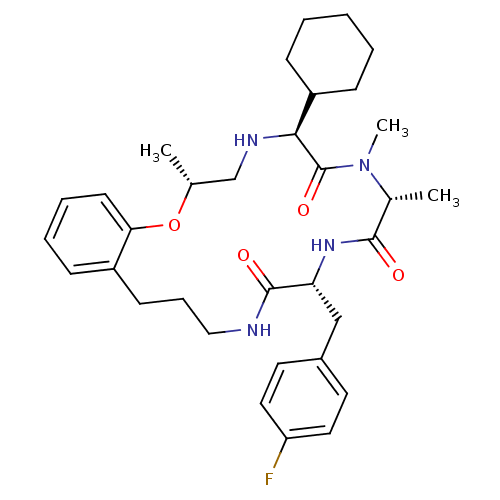 (CHEMBL1923629) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17411 (1,2,4-Triazole Compound, 69 | methyl 4-[(5-{[(2-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50002402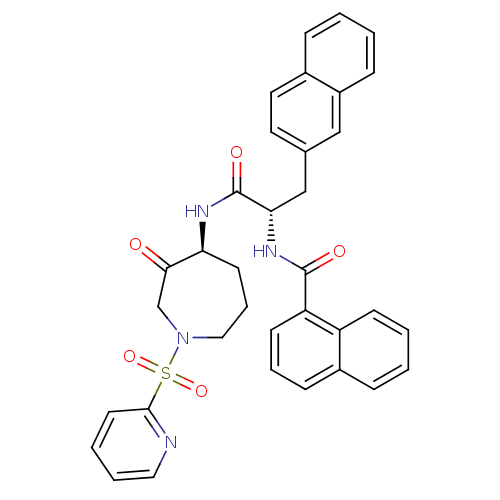 (CHEMBL194643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin L | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17377 (1,2,4-Triazole Compound, 35 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50084650 (CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin L | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50098576 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Human cathepsin L | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17352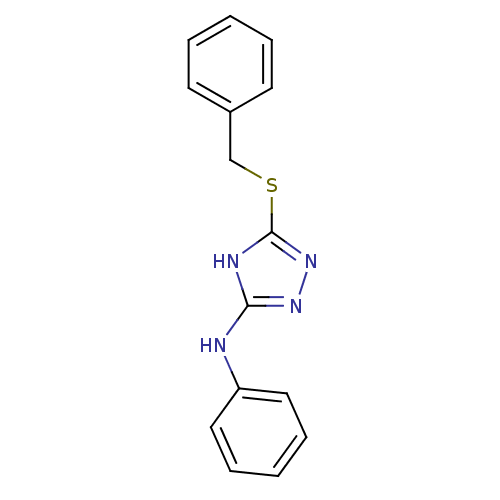 (1,2,4-Triazole Compound, 6 | 5-(benzylsulfanyl)-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17431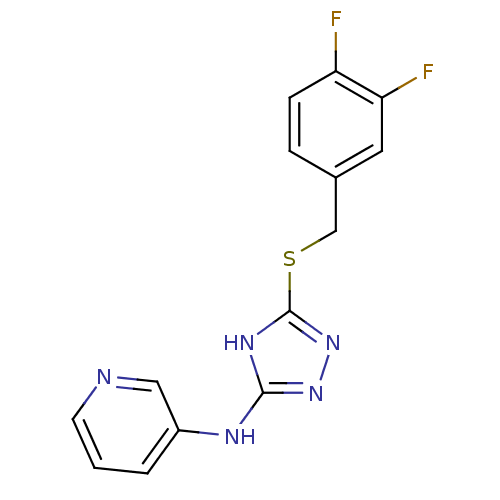 (1,2,4-Triazole Compound, 89 | N-(5-{[(3,4-difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50066647 (CHEMBL113724 | {(S)-3-Methyl-1-[3-oxo-1-(4-phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin K | J Med Chem 41: 3563-7 (1998) Article DOI: 10.1021/jm980295f BindingDB Entry DOI: 10.7270/Q2G15ZZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17416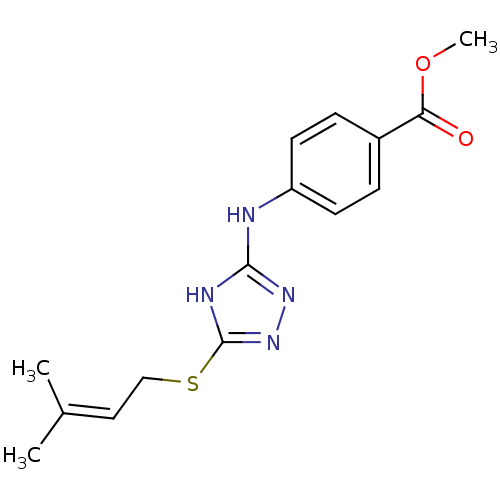 (1,2,4-Triazole Compound, 74 | methyl 4-({5-[(3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17385 (1,2,4-Triazole Compound, 43 | N-(4-chlorophenyl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50002399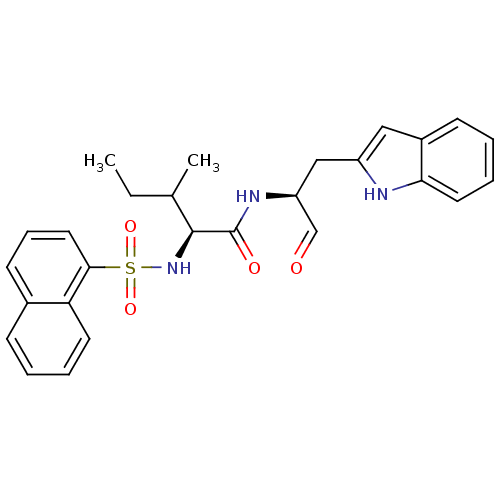 (CHEMBL197958) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin S | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50002374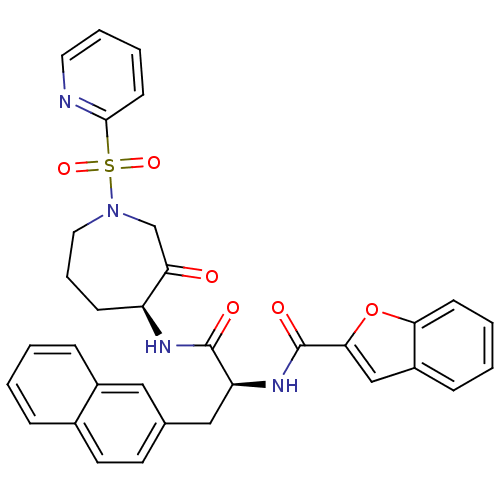 (CHEMBL194861) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin L | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19808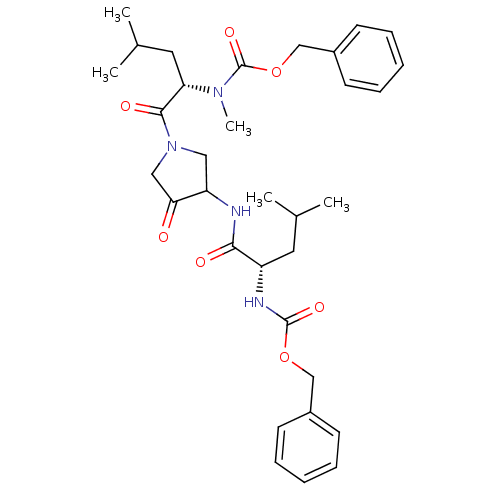 (benzyl N-[(1S)-1-({1-[(2S)-2-{[(benzyloxy)carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... | J Med Chem 44: 725-36 (2001) Article DOI: 10.1021/jm000320t BindingDB Entry DOI: 10.7270/Q2J67F6C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17380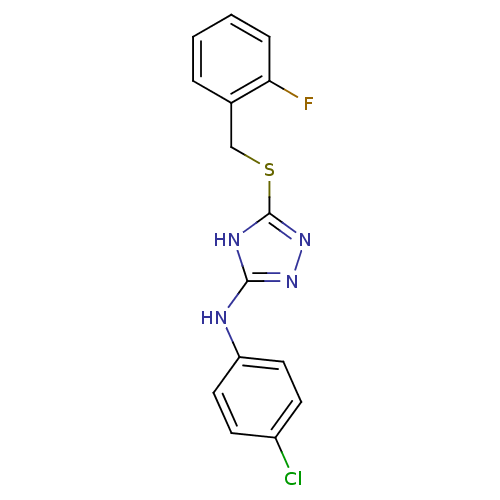 (1,2,4-Triazole Compound, 38 | N-(4-chlorophenyl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19775 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17368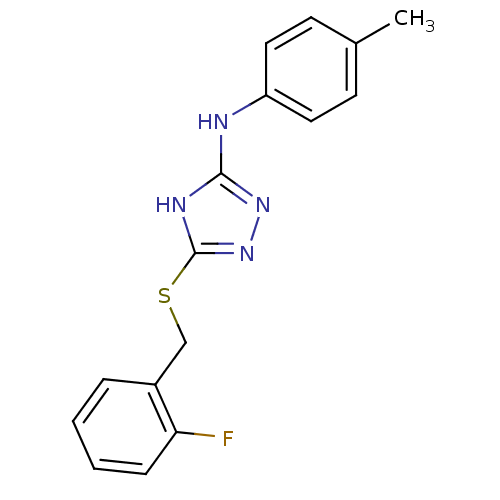 (1,2,4-Triazole Compound, 26 | 5-{[(2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.650 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17408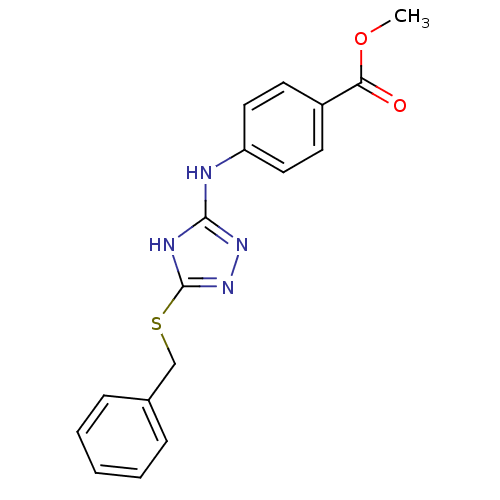 (1,2,4-Triazole Compound, 66 | methyl 4-{[5-(benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81891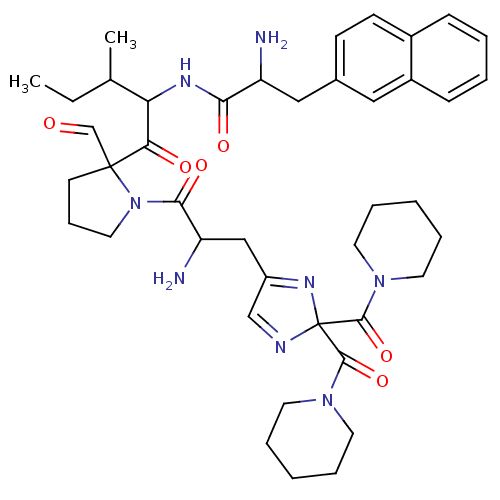 (CAS_188397 | L-366,948 | NSC_188397) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50408519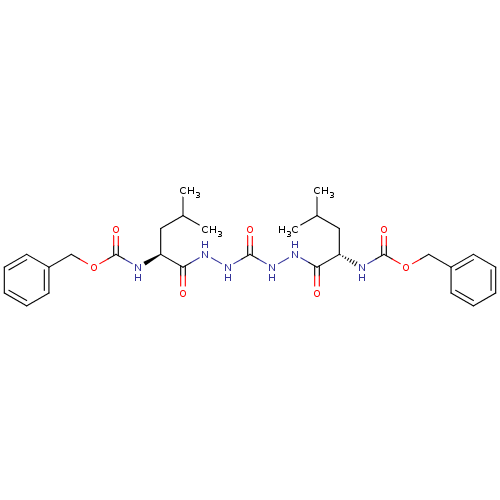 (CHEMBL115357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Relative binding affinity was measured for Cathepsin K | J Med Chem 41: 3923-7 (1998) Article DOI: 10.1021/jm980474x BindingDB Entry DOI: 10.7270/Q2Q81F87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50084650 (CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin K | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17354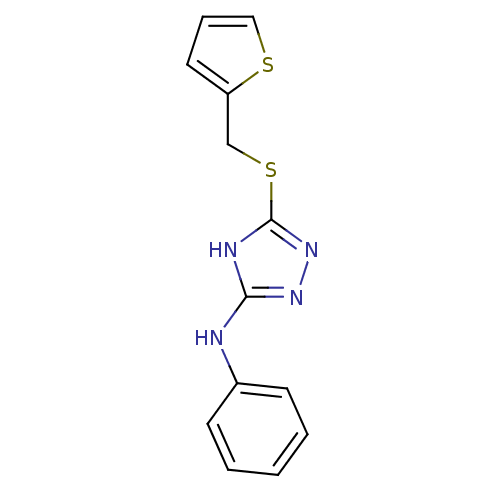 (1,2,4-Triazole Compound, 12 | N-phenyl-5-[(thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.75 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17418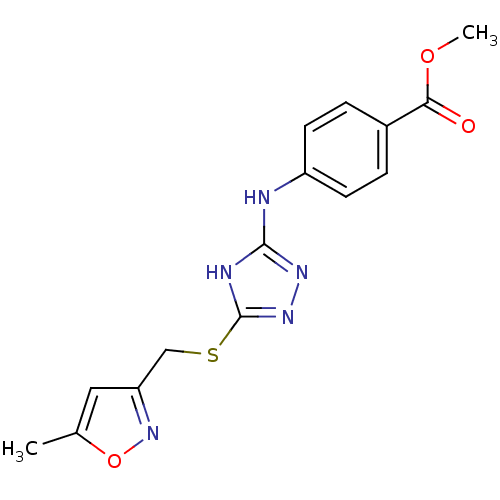 (1,2,4-Triazole Compound, 76 | methyl 4-[(5-{[(5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 83: 4918-22 (1986) Article DOI: 10.1073/pnas.83.13.4918 BindingDB Entry DOI: 10.7270/Q2445K05 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50408522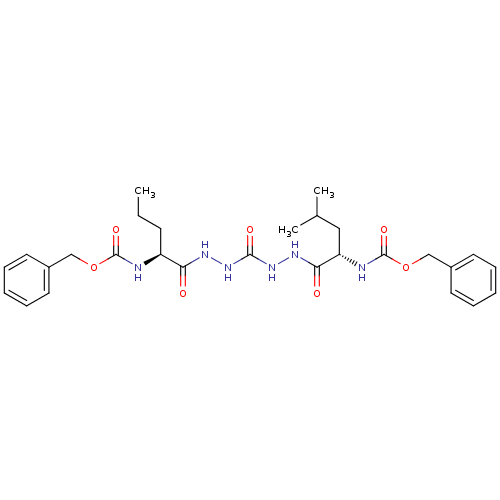 (CHEMBL126820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Relative binding affinity was measured for Cathepsin K | J Med Chem 41: 3923-7 (1998) Article DOI: 10.1021/jm980474x BindingDB Entry DOI: 10.7270/Q2Q81F87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81891 (CAS_188397 | L-366,948 | NSC_188397) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 256: 304-8 (1991) BindingDB Entry DOI: 10.7270/Q2028Q1G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50002399 (CHEMBL197958) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin L | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50205990 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity against oxytocin receptor in rat uterus | J Med Chem 33: 2321-3 (1990) BindingDB Entry DOI: 10.7270/Q2F76D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50002366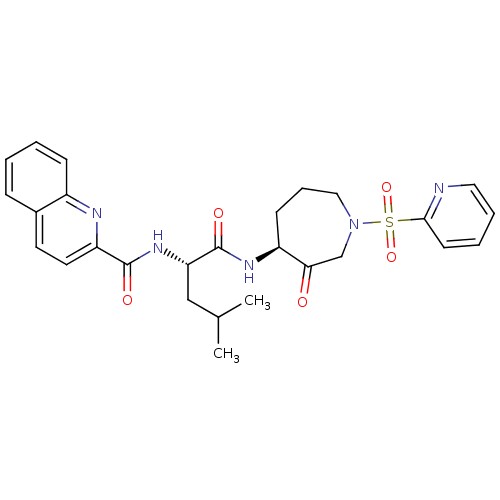 (CHEMBL433985) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin K | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1887 total ) | Next | Last >> |