

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445346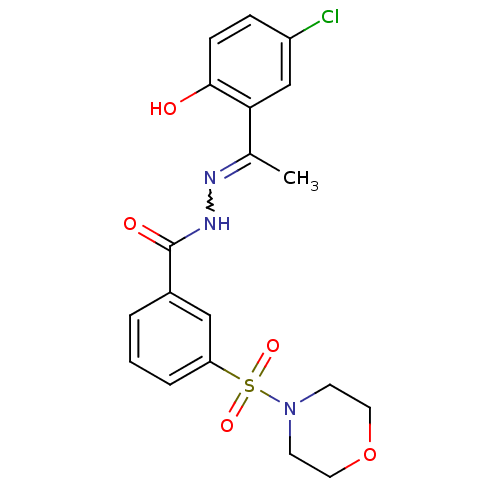 (CHEMBL3104250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 1 ... | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445346 (CHEMBL3104250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 10... | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445346 (CHEMBL3104250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 3 ... | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445346 (CHEMBL3104250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 30... | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445346 (CHEMBL3104250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant full-length His6-tagged LSD1 (unknown origin) using dimethylated K4N-terminal H3 peptide as substrate at 10... | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM28031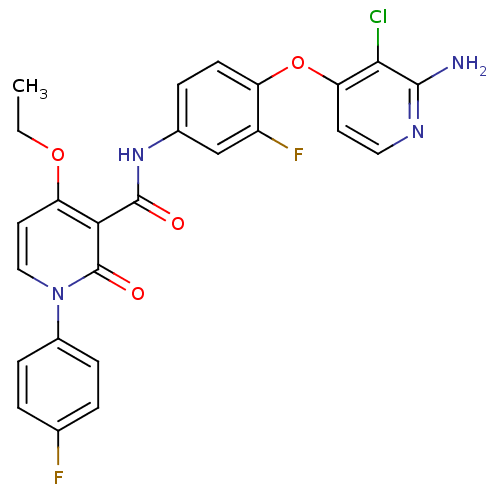 (BMS-777607 | N-{4-[(2-amino-3-chloropyridin-4-yl)o...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50382425 (CHEMBL2022968) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aurora A | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM214693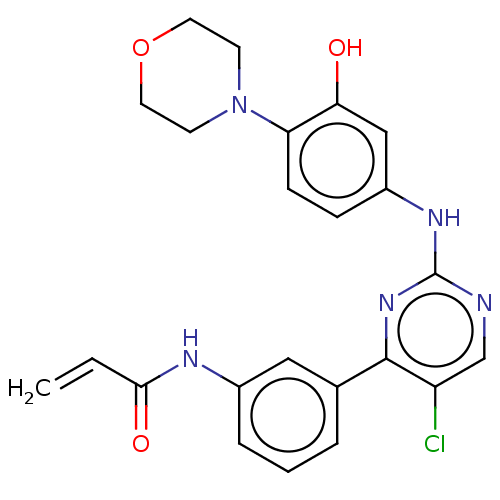 (US9296703, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US9296703 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM27917 (4-cyano-N-[4-(4-methylpiperazin-1-yl)-2-(4-methylp...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM17004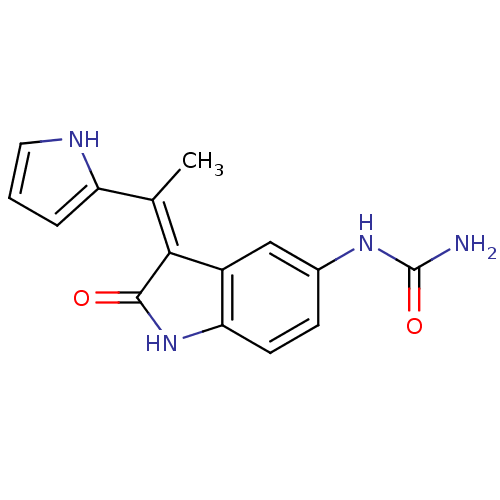 (BX-517 | Indolinone based inhibitor, 4i | [(3Z)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of PDK1 (unknown origin) after 1 hr in presence of Ser/Thr-07 by fluorometric assay | Bioorg Med Chem Lett 27: 5473-5480 (2017) Article DOI: 10.1016/j.bmcl.2017.10.041 BindingDB Entry DOI: 10.7270/Q2KD21JC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50382425 (CHEMBL2022968) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aurora B | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151608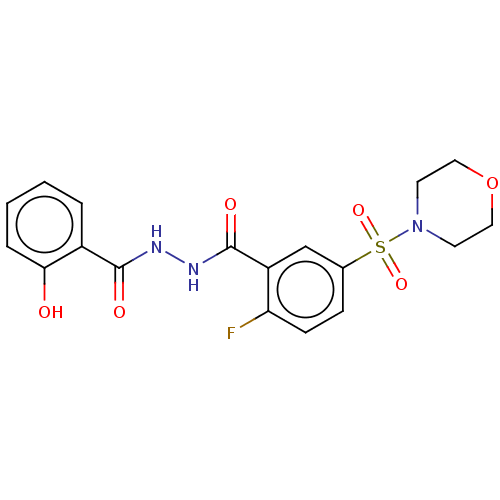 (US8987335, 9 | US9555024, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US8987335 (2015) BindingDB Entry DOI: 10.7270/Q2GQ6WGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151611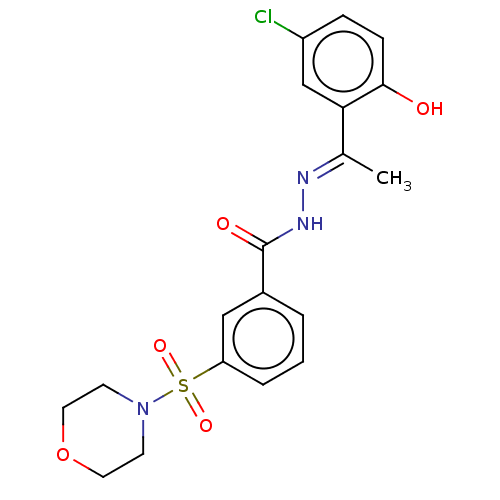 (SP-2509 | US11433053, Example HCI-2509 | US8987335...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US8987335 (2015) BindingDB Entry DOI: 10.7270/Q2GQ6WGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151617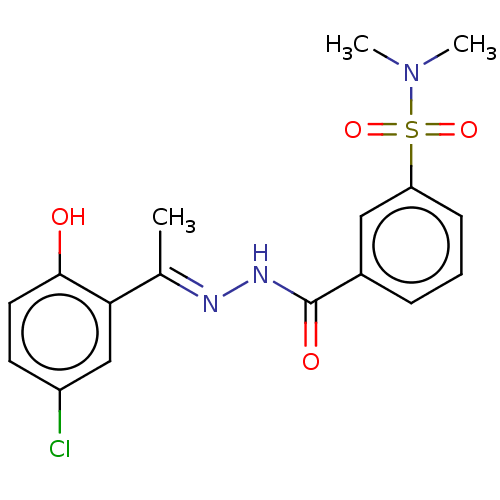 (US8987335, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US8987335 (2015) BindingDB Entry DOI: 10.7270/Q2GQ6WGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445346 (CHEMBL3104250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of LSD1 (unknown origin) using dimethyl K4 peptide as substrate assessed as resorufin level by spectrophotometric analysis | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151608 (US8987335, 9 | US9555024, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US9555024 (2017) BindingDB Entry DOI: 10.7270/Q21R6SH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151611 (SP-2509 | US11433053, Example HCI-2509 | US8987335...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US9555024 (2017) BindingDB Entry DOI: 10.7270/Q21R6SH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151617 (US8987335, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US9555024 (2017) BindingDB Entry DOI: 10.7270/Q21R6SH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50382441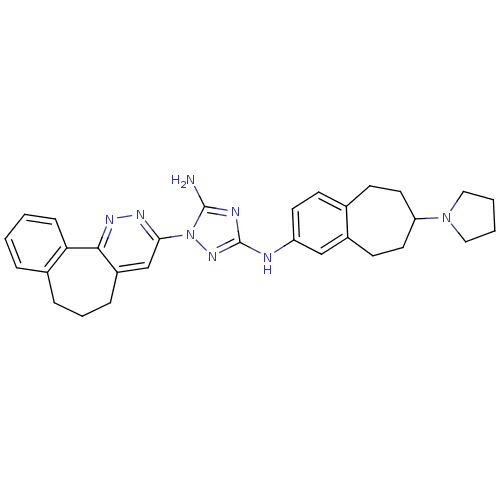 (CHEMBL2023349) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM28031 (BMS-777607 | N-{4-[(2-amino-3-chloropyridin-4-yl)o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer kinase | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445347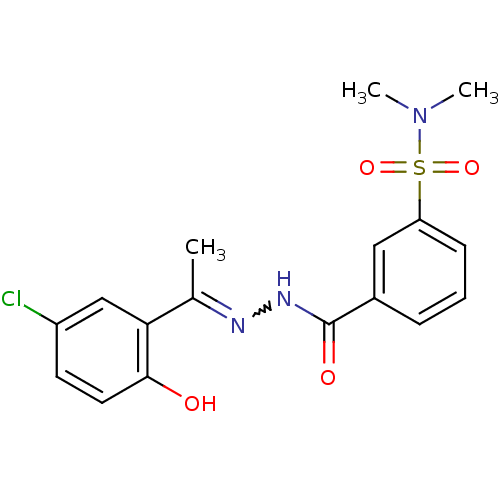 (CHEMBL3104252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of LSD1 (unknown origin) using dimethyl K4 peptide as substrate assessed as resorufin level by spectrophotometric analysis | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50382443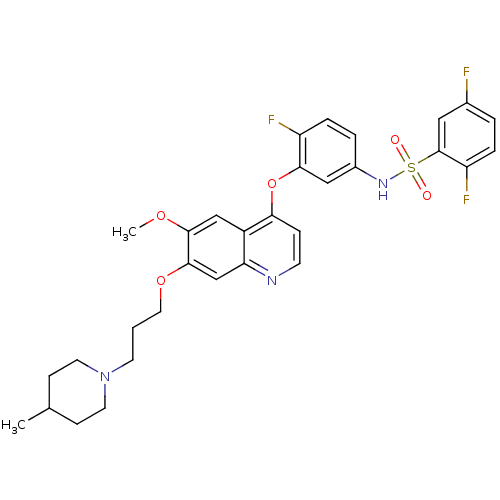 (CHEMBL2023351) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50382414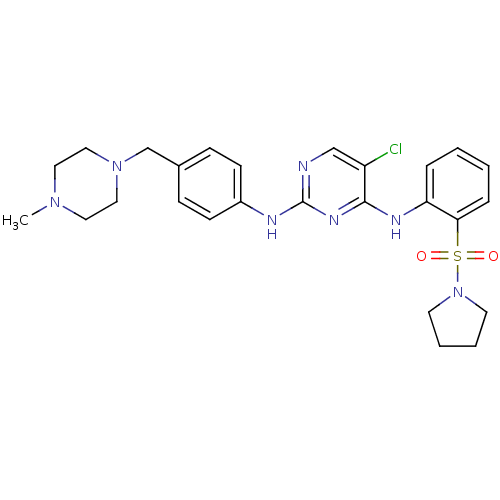 (CHEMBL2022975) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445348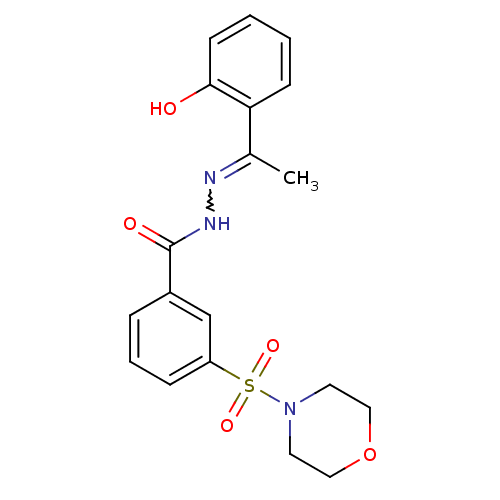 (CHEMBL3104350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of LSD1 (unknown origin) using dimethyl K4 peptide as substrate assessed as resorufin level by spectrophotometric analysis | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445349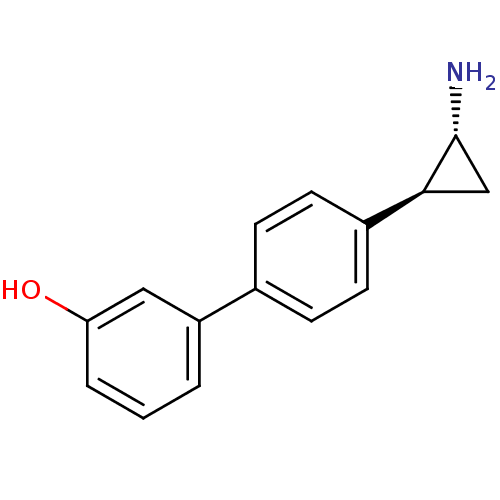 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human recombinant LSD1 using dimethylated H3K4 peptide as substrate after 1 hr | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445366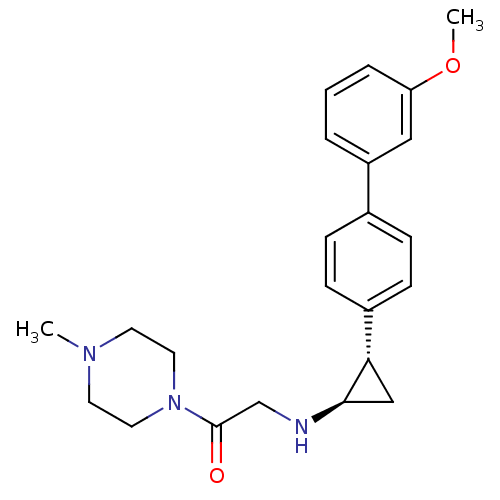 (CHEMBL3104342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human recombinant LSD1 using dimethylated H3K4 peptide as substrate after 1 hr | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50382425 (CHEMBL2022968) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151623 (US8987335, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US8987335 (2015) BindingDB Entry DOI: 10.7270/Q2GQ6WGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151623 (US8987335, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US9555024 (2017) BindingDB Entry DOI: 10.7270/Q21R6SH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50382442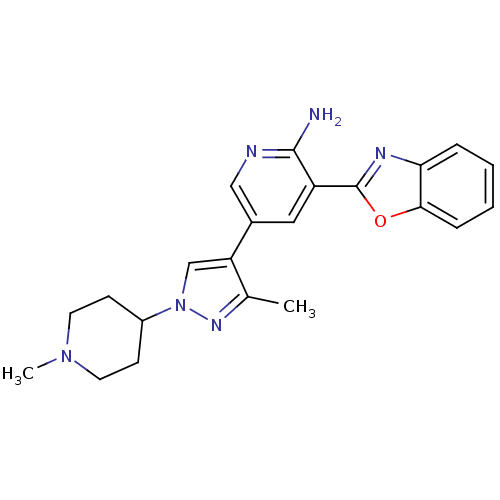 (CHEMBL2023350) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50382431 (CHEMBL2022974) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120748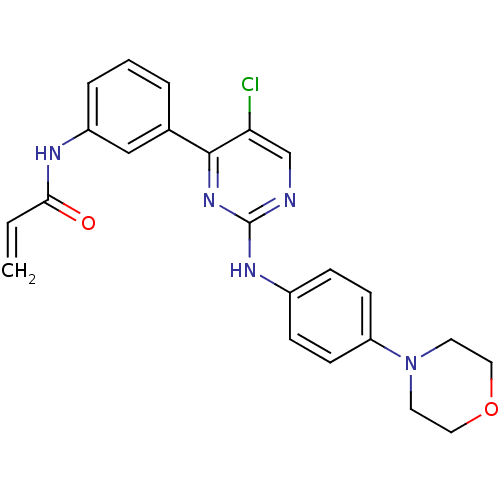 (US8703767, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the ADP generation assay described herein. Test compounds were diluted to desired concentratio... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50382433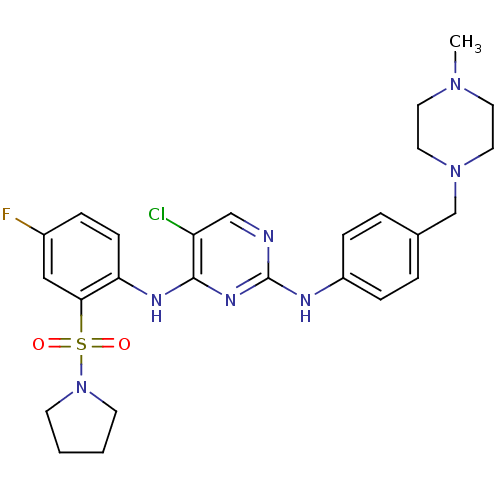 (CHEMBL2022977) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged AXL kinase using fluorescein-labelled poly-GT as substrate after 60 mins by TR-FRET analysis | ACS Med Chem Lett 2: 907-912 (2011) Article DOI: 10.1021/ml200198x BindingDB Entry DOI: 10.7270/Q2DZ099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120741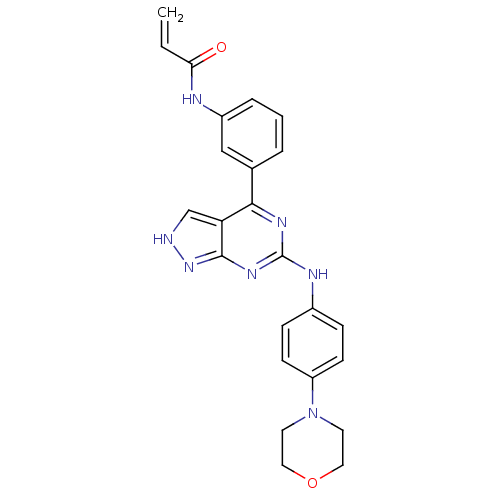 (US8703767, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description Activity of compounds was routinely confirmed using a secondary assay as described herein. The secondary assay was a time resolved-FRET kinase assay.... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM120748 (US8703767, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | 25 |
University of Utah Research Foundation US Patent | Assay Description Activity of compounds was routinely confirmed using a secondary assay as described herein. The secondary assay was a time resolved-FRET kinase assay.... | US Patent US8703767 (2014) BindingDB Entry DOI: 10.7270/Q23X85BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151624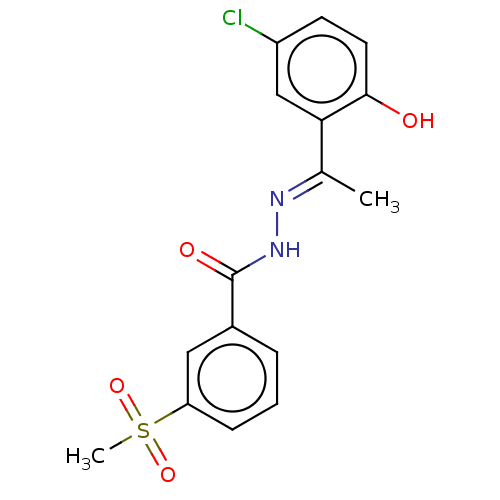 (US8987335, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US8987335 (2015) BindingDB Entry DOI: 10.7270/Q2GQ6WGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM151624 (US8987335, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US9555024 (2017) BindingDB Entry DOI: 10.7270/Q21R6SH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593123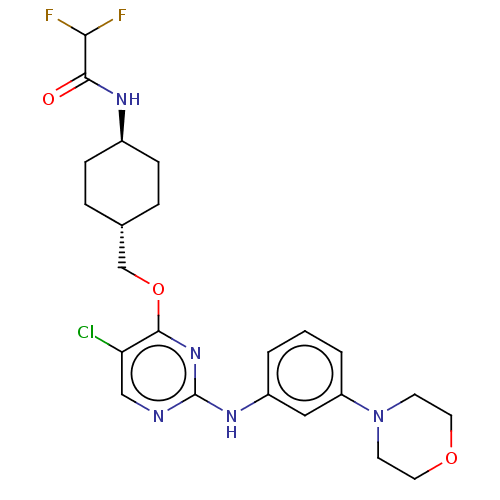 (N-((1R,4R)-4-((5-chloro-2-((3- morpholinophenyl)am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593124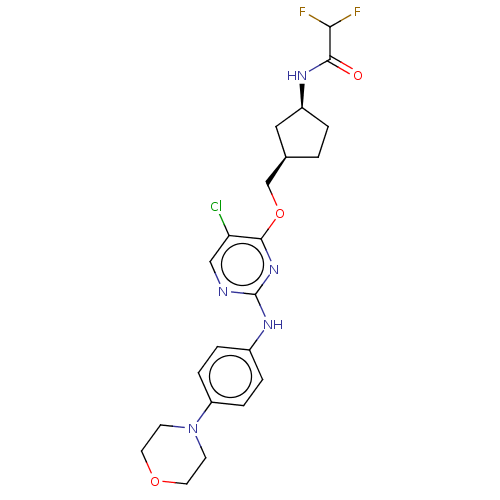 (N-((1S,3R)-3-(((5-chloro-2-((4- morpholinophenyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593125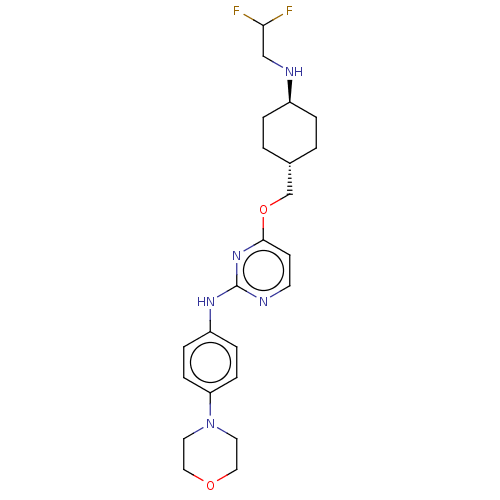 (4-(((1R,4R)-4-((2,2- difluoroethyl)amino)cyclohexy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593001 (4-(((1S,4S)-4- aminocyclohexyl)methoxy)-N-(4- morp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593003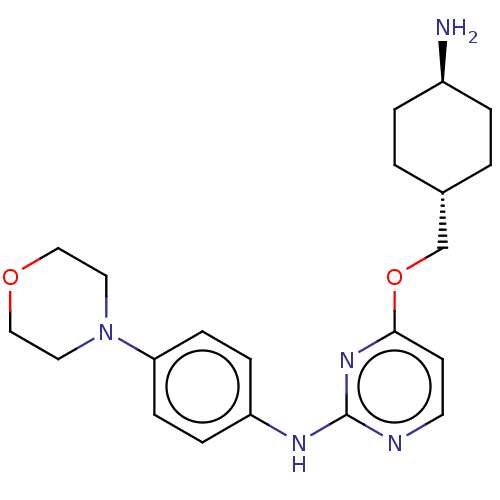 (4-(((1R,4R)-4- aminocyclohexyl)methoxy)-N-(4- morp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593024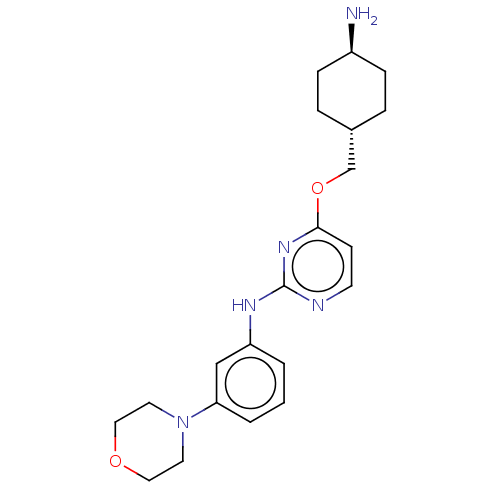 (4-(((1R,4R)-4- aminocyclohexyl)methoxy)-N-(3- morp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593026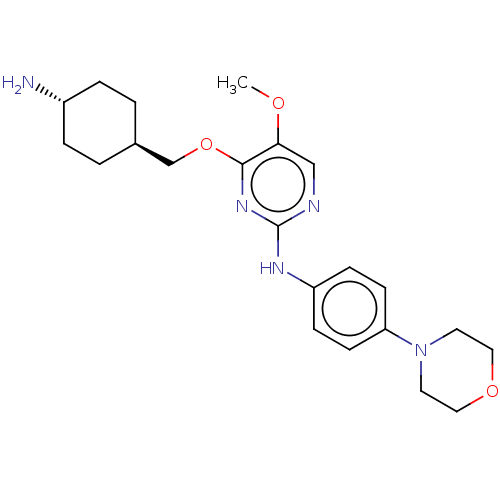 (4-(((1R,4R)-4- aminocyclohexyl)methoxy)-5- methoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593027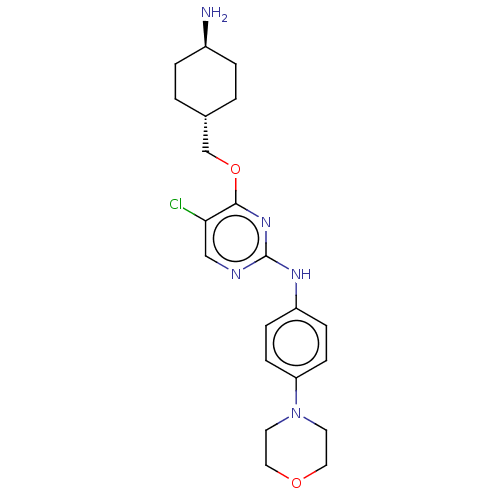 (4-(((1R,4R)-4- aminocyclohexyl)methoxy)-5- chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593028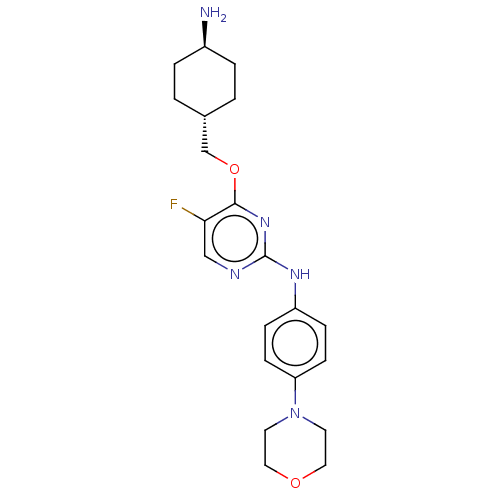 (4-(((1R,4R)-4- aminocyclohexyl)methoxy)-5- fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593030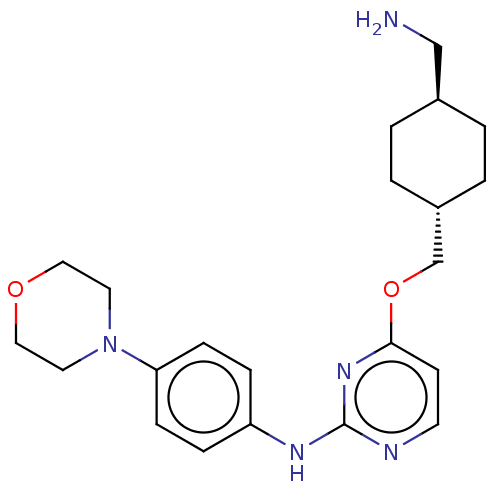 (4-(((1R,4R)-4- (aminomethyl)cyclohexyl)methoxy)- N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593031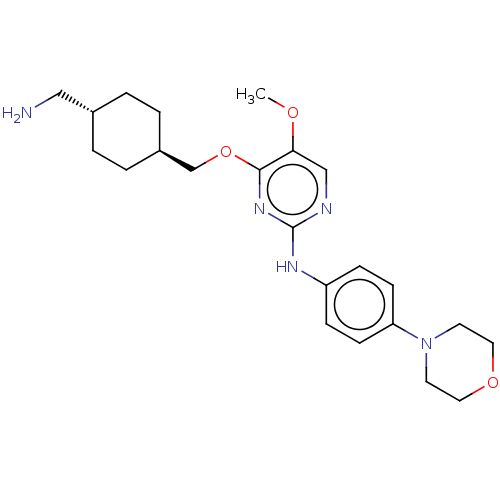 (4-(((1R,4R)-4- (aminomethyl)cyclohexyl)methoxy)- 5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593032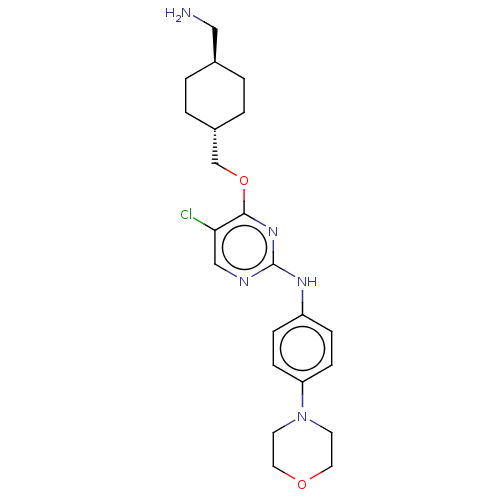 (4-(((1R,4R)-4- (aminomethyl)cyclohexyl)methoxy)- 5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 [970-2527] (Homo sapiens (Human)) | BDBM593167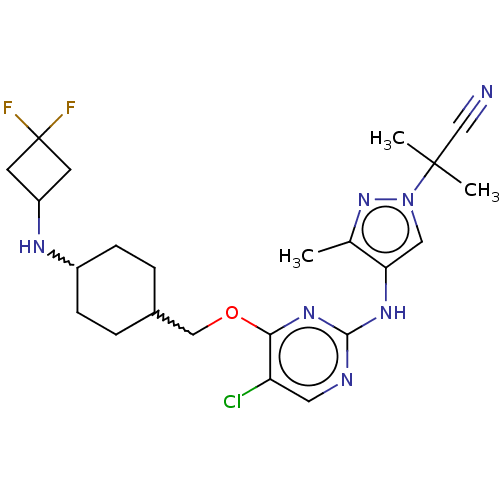 (2-(4-((5-chloro-4-((4-((3,3- difluorocyclobutyl)am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino... | Citation and Details BindingDB Entry DOI: 10.7270/Q2183BFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 635 total ) | Next | Last >> |