

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133069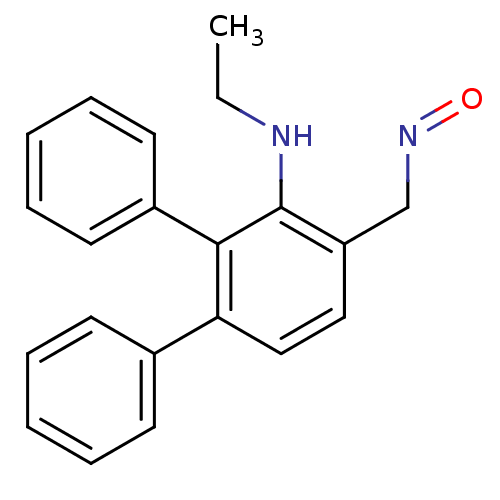 (3'-Ethylamino-[1,1';2',1'']terphenyl-4'-carbaldehy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238727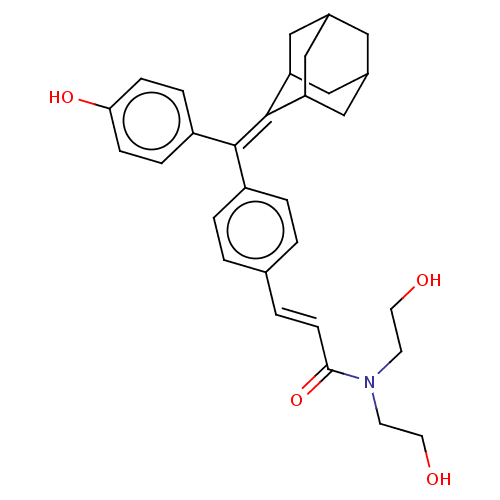 (CHEMBL4065838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133070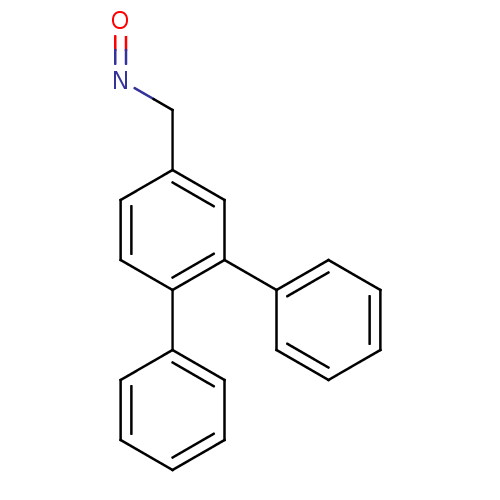 (CHEMBL335465 | [1,1';2',1'']Terphenyl-4'-carbaldeh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238724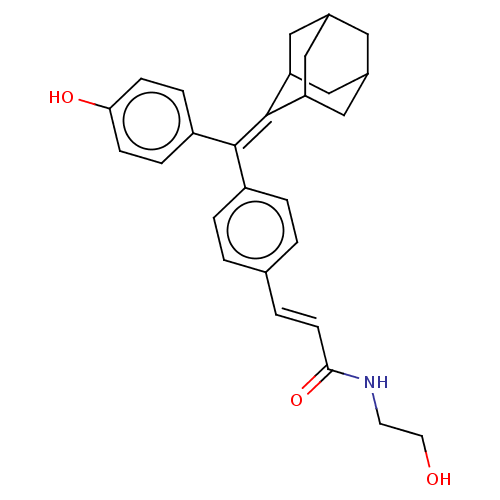 (CHEMBL4060067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238716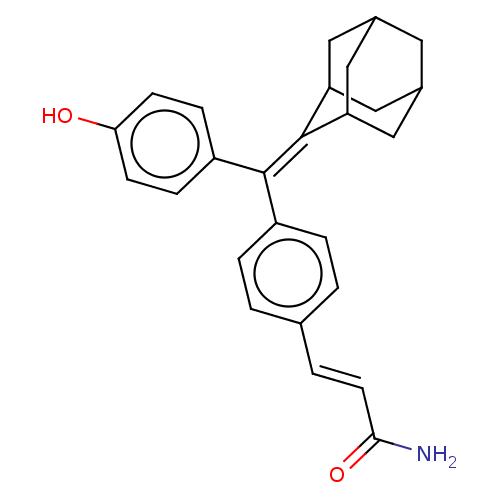 (CHEMBL4079530) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM50364076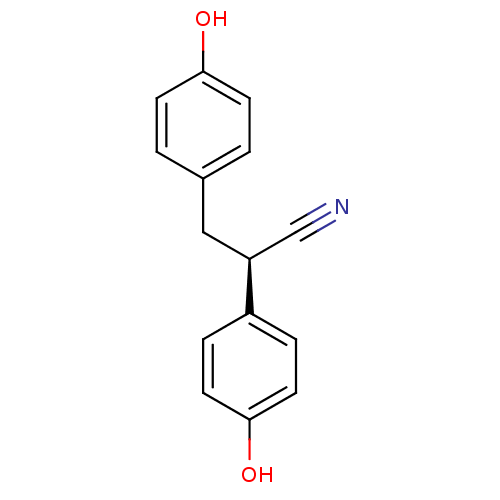 (CHEMBL198159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Displacement of [3H]E2 from rat ERbeta1 after 90 mins | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50133070 (CHEMBL335465 | [1,1';2',1'']Terphenyl-4'-carbaldeh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161235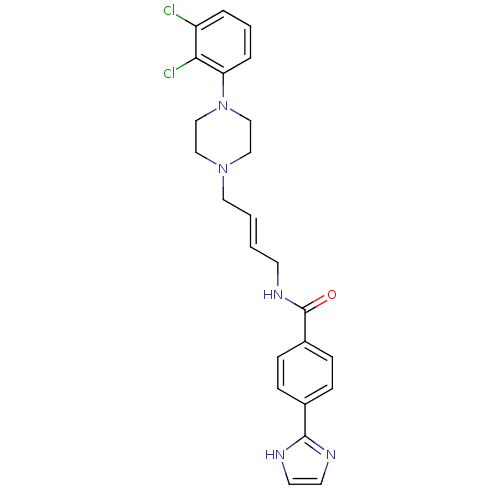 (CHEMBL179351 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238726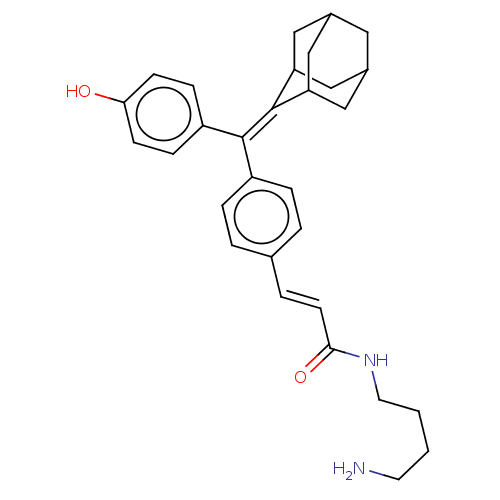 (CHEMBL4083780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238743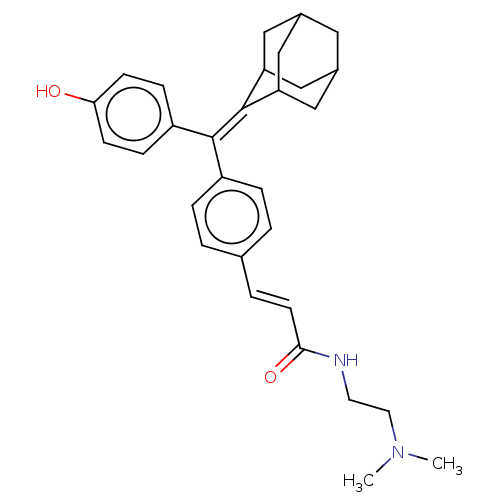 (CHEMBL4070669) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238720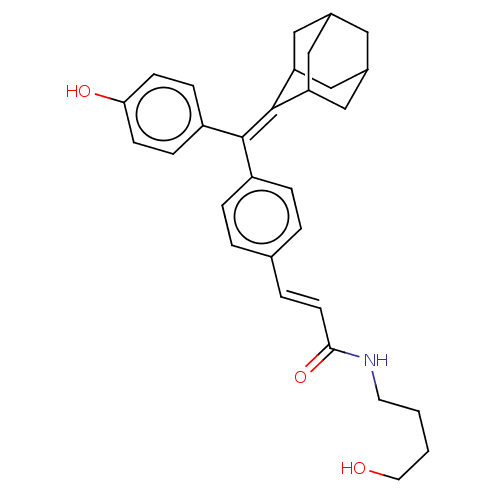 (CHEMBL4102857) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238733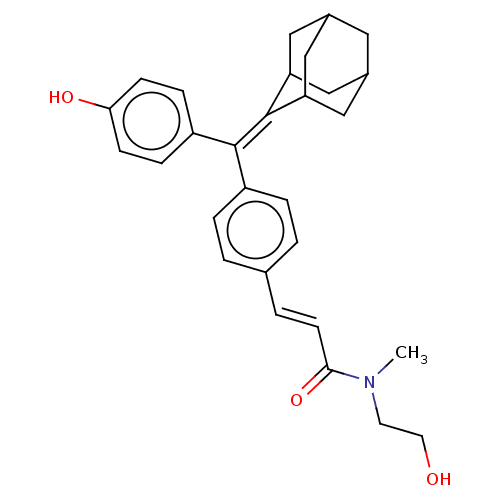 (CHEMBL4099302) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238742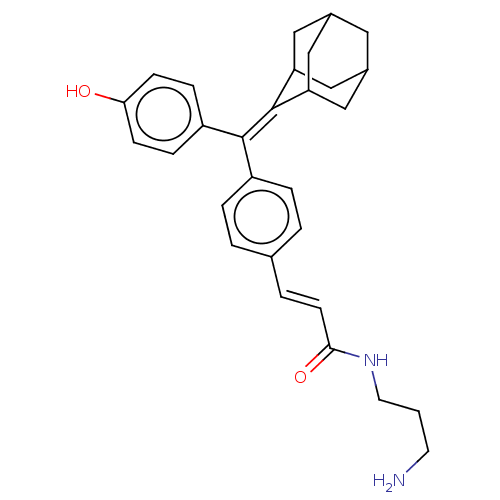 (CHEMBL4062045) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238739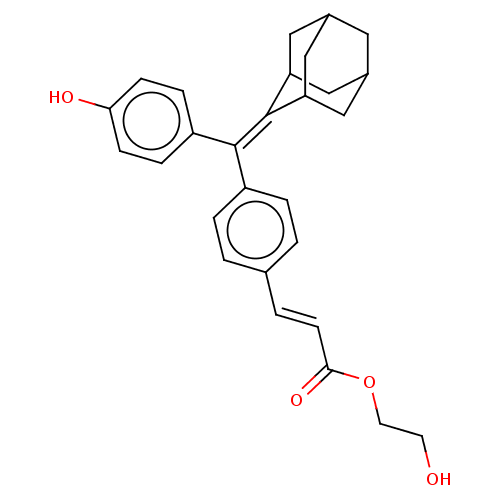 (CHEMBL4079644) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated against Histamine N-methyl-transferase | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50346462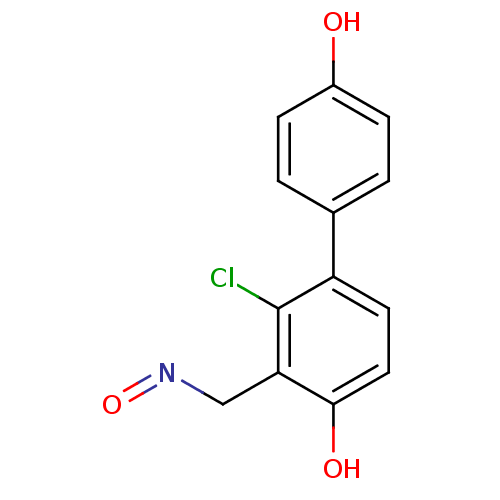 ((E)-2-chloro-4,4'-dihydroxybiphenyl-3-carbaldehyde...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human full-length ERbeta receptor by competitive radiometric binding assay | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238717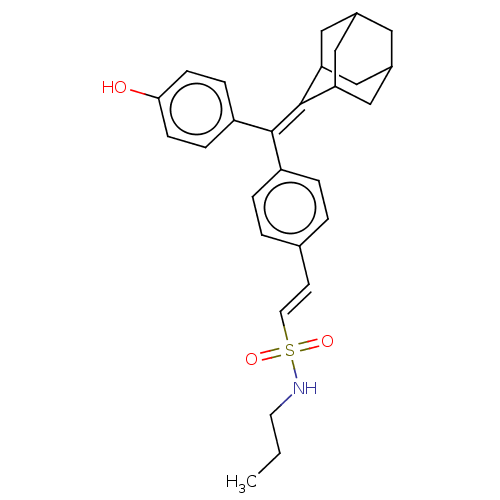 (CHEMBL4097562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161217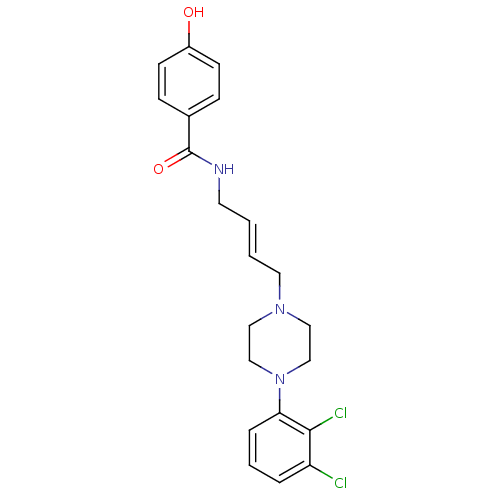 (CHEMBL195057 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238737 (CHEMBL4083658) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238738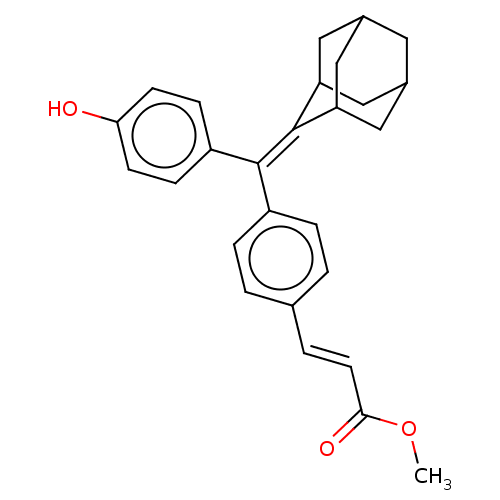 (CHEMBL4060817) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238740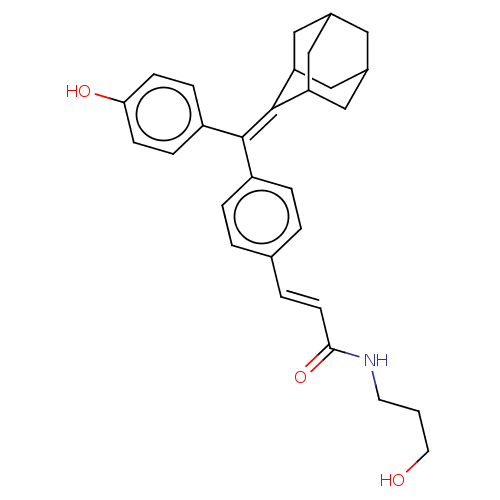 (CHEMBL4078577) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238728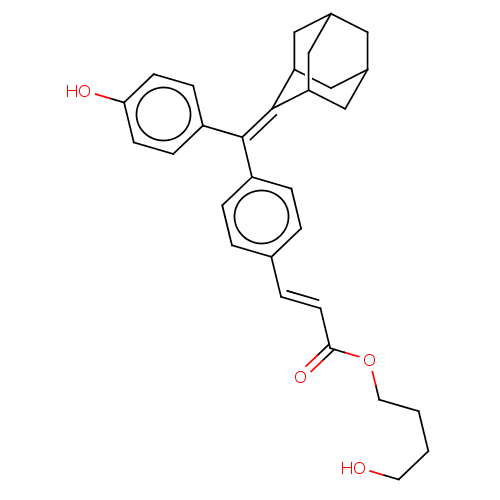 (CHEMBL4089824) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50133069 (3'-Ethylamino-[1,1';2',1'']terphenyl-4'-carbaldehy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa Curated by ChEMBL | Assay Description Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol | J Med Chem 46: 4032-42 (2003) Article DOI: 10.1021/jm0308390 BindingDB Entry DOI: 10.7270/Q27W6BKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129426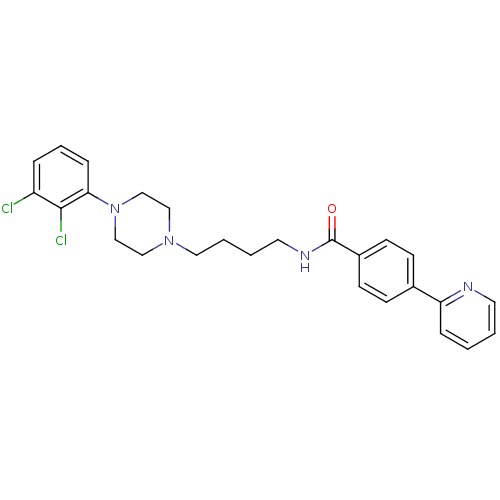 (CHEMBL69451 | N-(4-(4-(2,3-dichlorophenyl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161238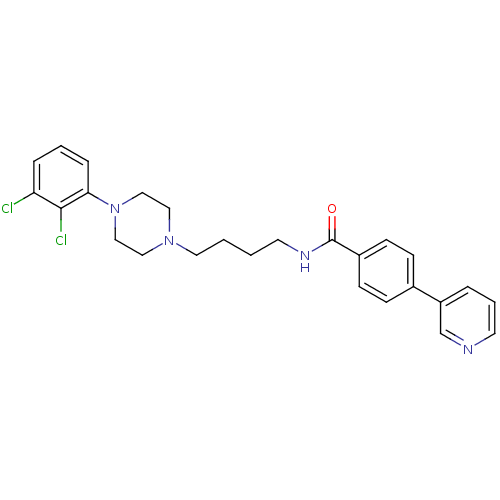 (CHEMBL179960 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238719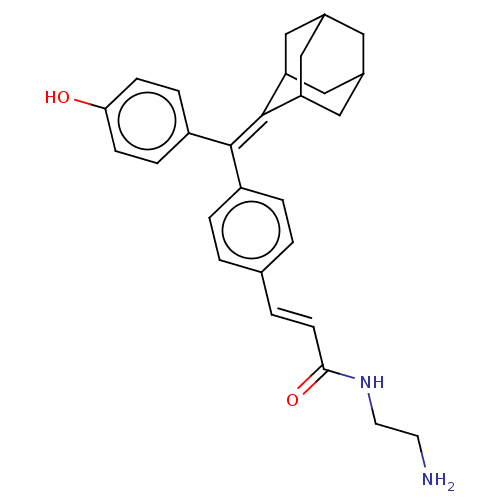 (CHEMBL4073395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50346463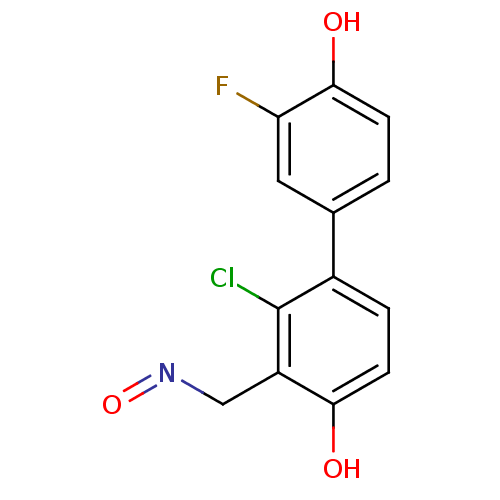 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human full-length ERbeta receptor by competitive radiometric binding assay | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161218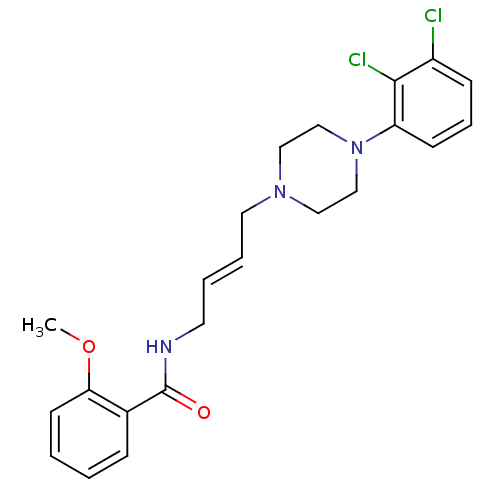 (CHEMBL414839 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50125339 (3-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin II receptor, type 1 in rat aortic smooth muscle cells using 0.2 nM [125I]-labeled Sar-Ile-angiotensin II | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238735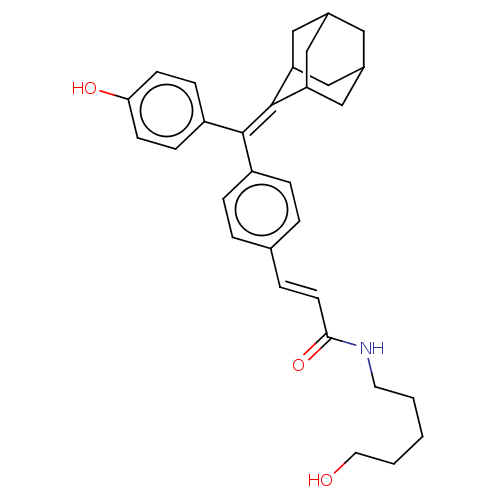 (CHEMBL4100247) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161214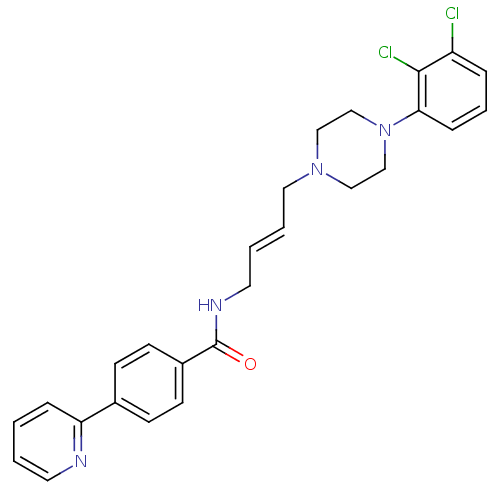 (CHEMBL180010 | N-(4-(4-(2,3-dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161221 (CHEMBL194493 | N-{(E)-4-[4-(2,3-Dichloro-phenyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238725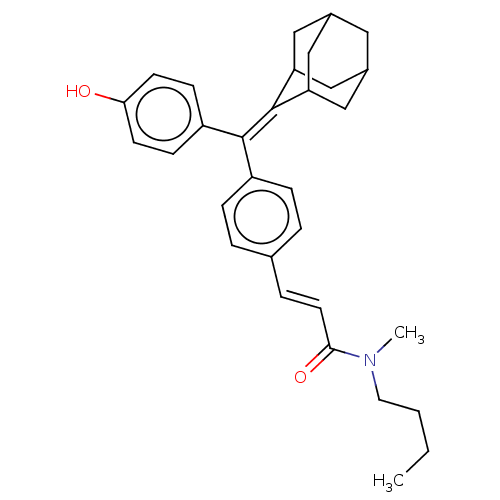 (CHEMBL4081283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238729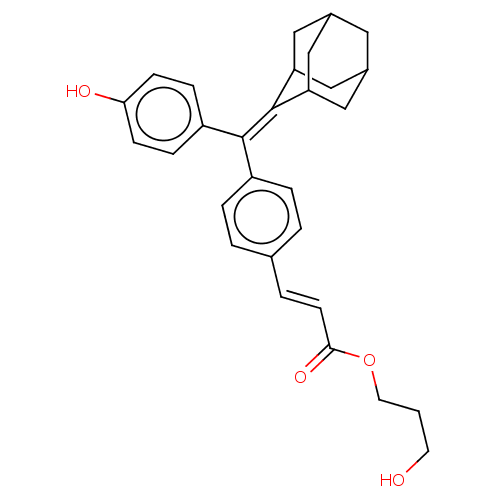 (CHEMBL4098232) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238715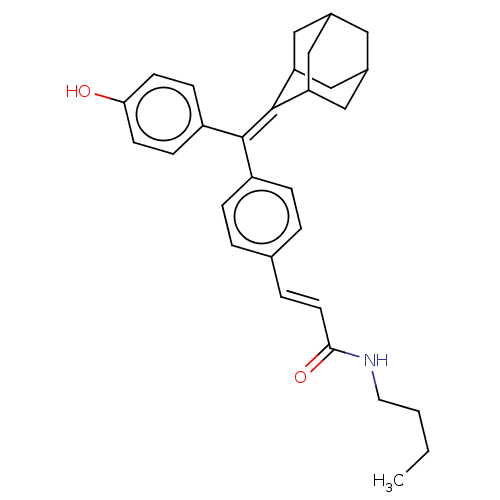 (CHEMBL4098470) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]BMS-725519 from human CB1 receptor expressed in CHO cells after 90 mins by scintillation counting | Bioorg Med Chem Lett 21: 6856-60 (2011) Article DOI: 10.1016/j.bmcl.2011.09.016 BindingDB Entry DOI: 10.7270/Q2WS8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238714 (CHEMBL4081969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161230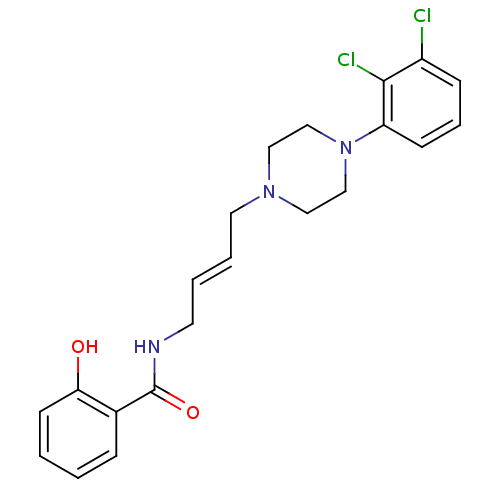 (CHEMBL372022 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129433 (CHEMBL71327 | N-{(E)-4-[4-(2,3-Dichloro-phenyl)-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119390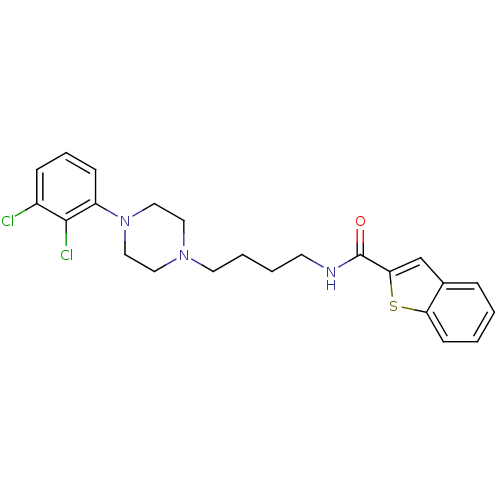 (Benzo[b]thiophene-2-carboxylic acid {4-[4-(2,3-dic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50119384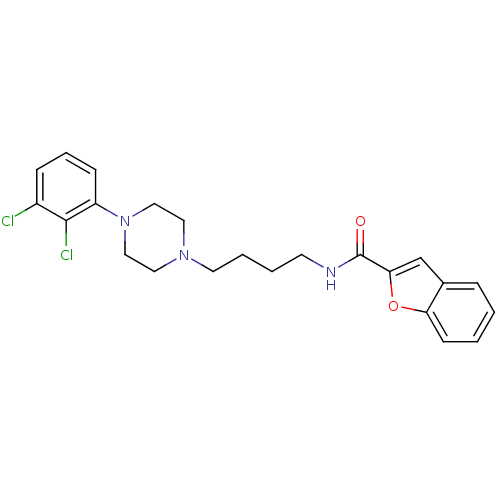 (Benzofuran-2-carboxylic acid {4-[4-(2,3-dichloro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161222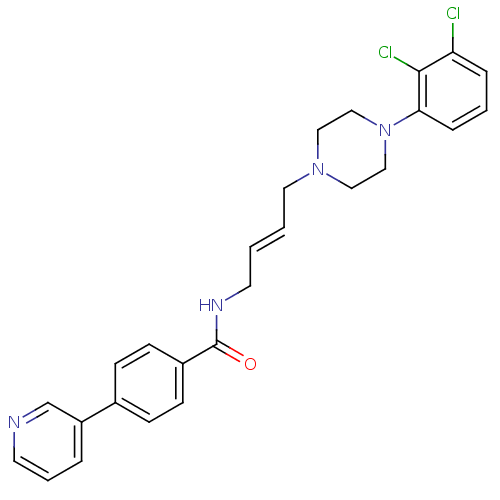 (CHEMBL414838 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161234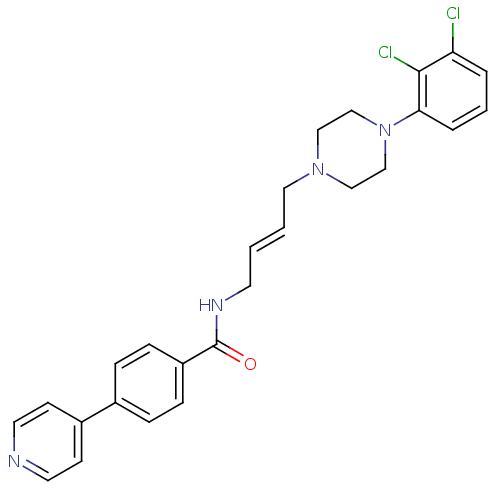 (CHEMBL425143 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50161239 (CHEMBL366900 | N-{4-[4-(2,3-Dichloro-phenyl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity for human dopamine D3 receptor | J Med Chem 48: 839-48 (2005) Article DOI: 10.1021/jm049465g BindingDB Entry DOI: 10.7270/Q27H1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50125343 (2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human ETA receptor expressed in CHO-K1 cells in the presence of 0.05 nM [125I]-labeled endothelin 1 | Bioorg Med Chem Lett 13: 1093-6 (2003) BindingDB Entry DOI: 10.7270/Q2NG4PZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238736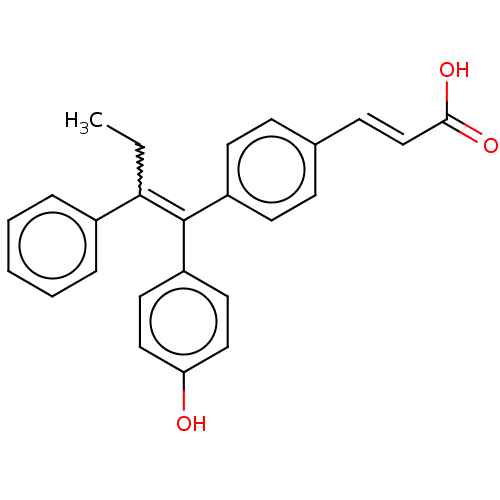 (CHEMBL1091535 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1714 total ) | Next | Last >> |