

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM13863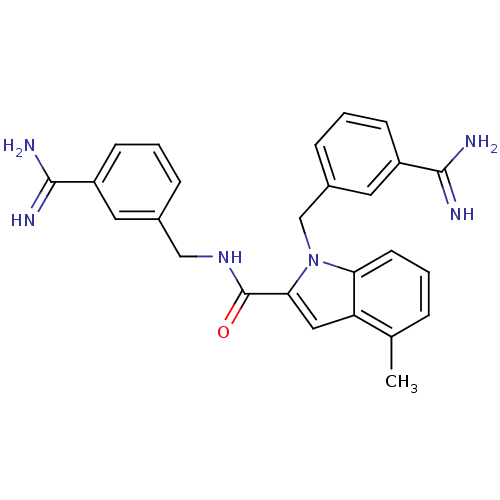 (3-amidinobenzylindole carboxamide 47 | N,1-bis[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13861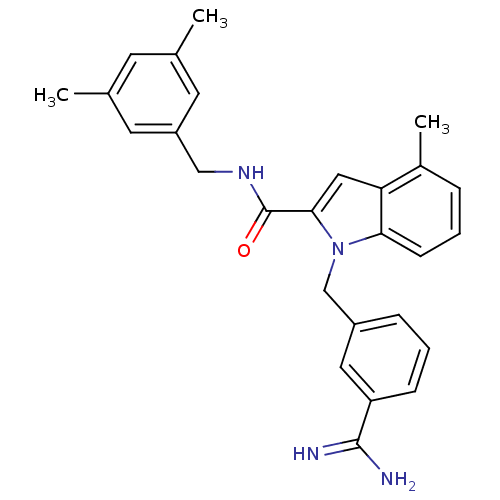 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13825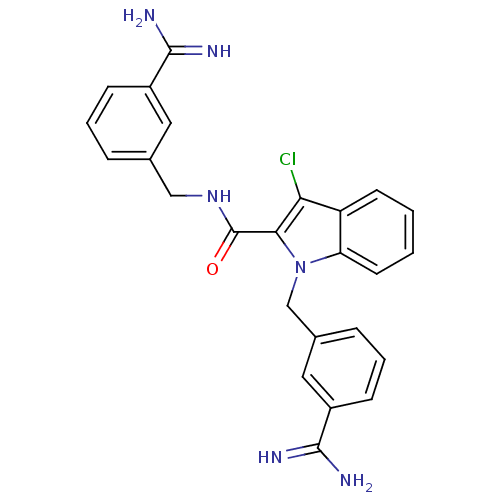 (3-amidinobenzylindole carboxamide 9 | N,1-bis[(3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13866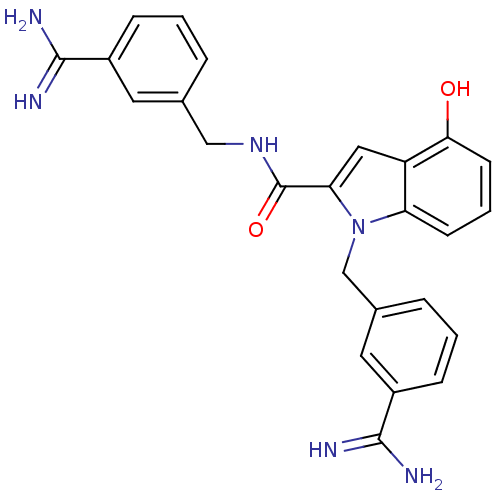 (3-amidinobenzylindole carboxamide 50 | CHEMBL30744...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13662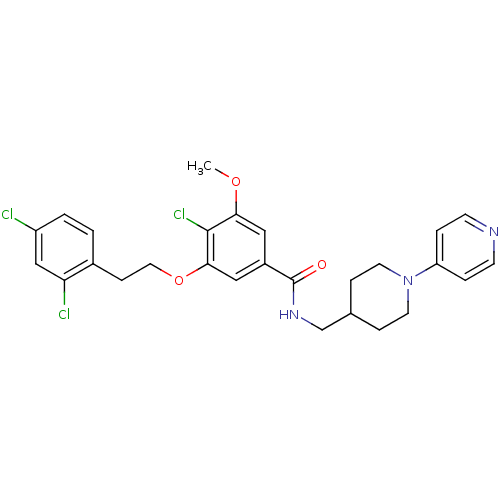 (3-Oxybenzamide 48 | 4-chloro-3-[2-(2,4-dichlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13862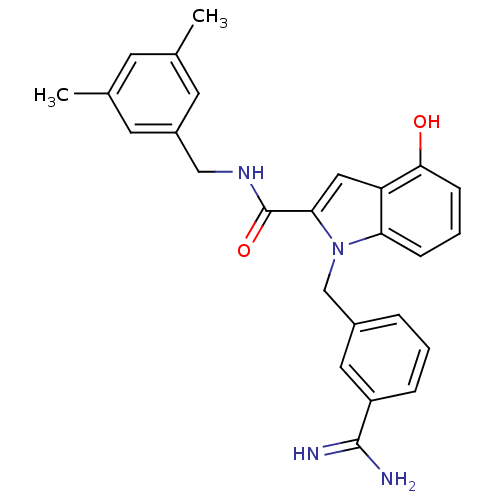 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13663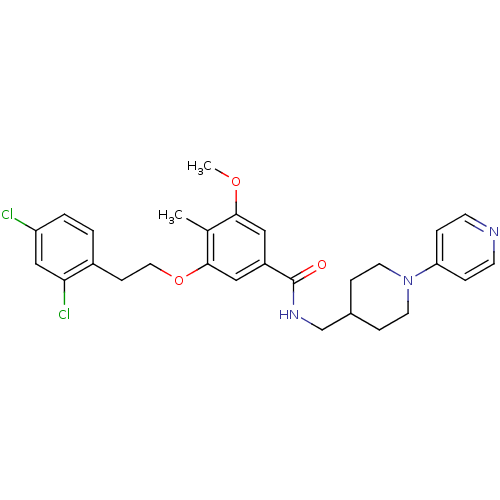 (3-Oxybenzamide 49 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13820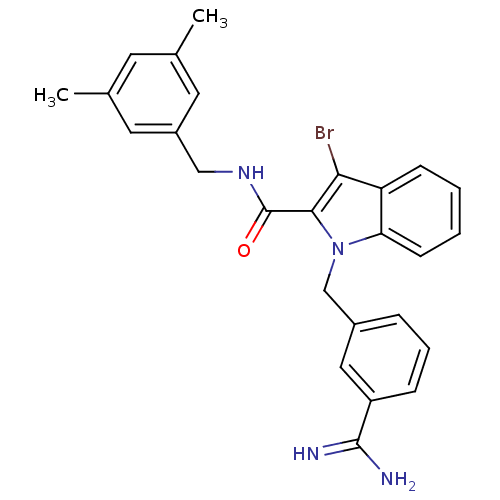 (3-amidinobenzylindole carboxamide 4 | 3-bromo-1-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13668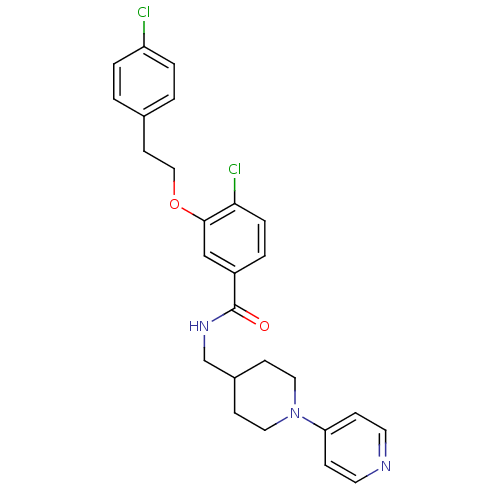 (3-Oxybenzamide 54 | 4-chloro-3-[2-(4-chlorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13880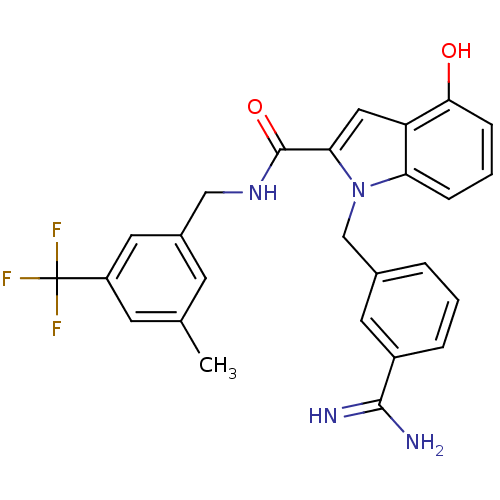 (1-[(3-carbamimidoylphenyl)methyl]-4-hydroxy-N-{[3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13898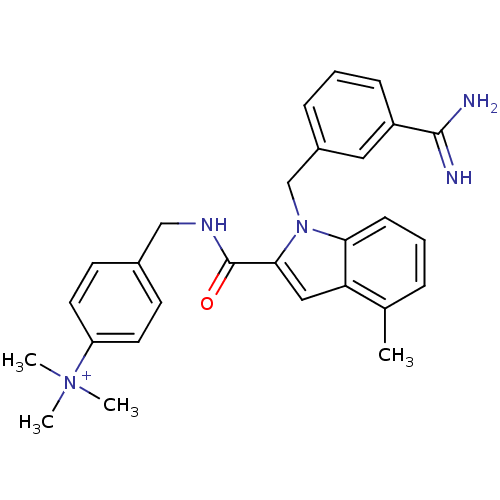 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13664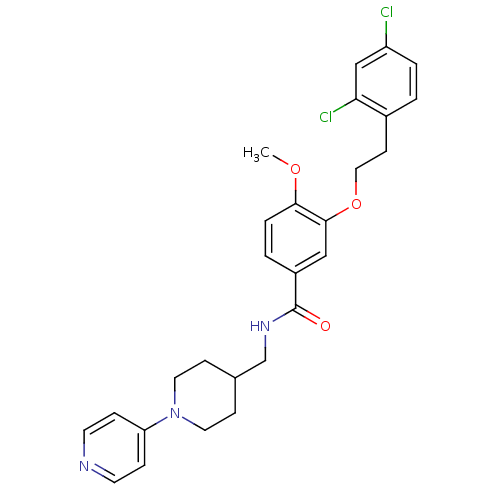 (3-Oxybenzamide 50 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13869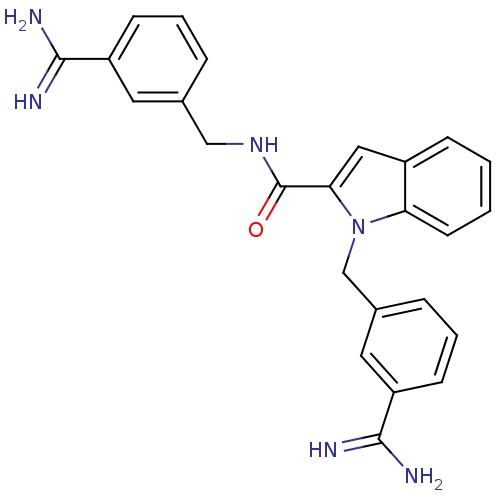 (3-amidinobenzylindole carboxamide 53 | N,1-bis[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13615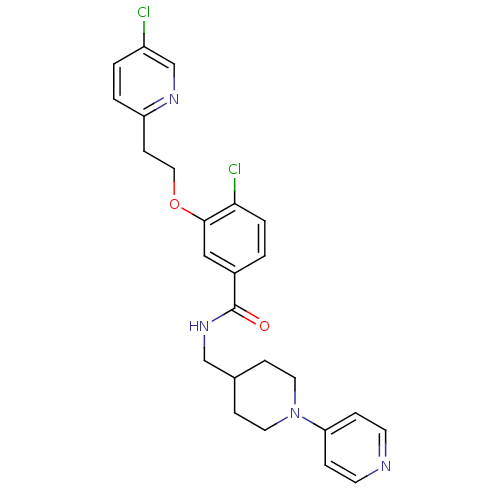 (3-Oxybenzamide 1 | 4-chloro-3-[2-(5-chloropyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13659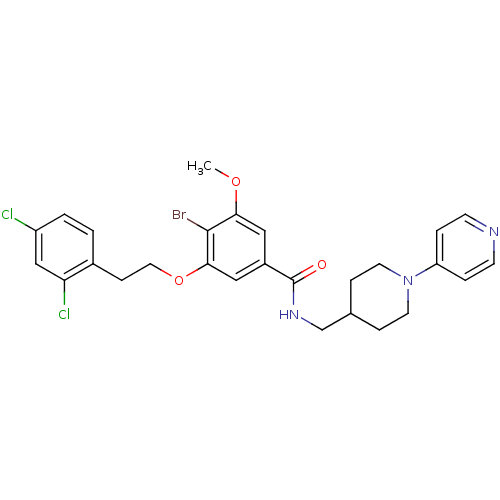 (3-Oxybenzamide 45 | 4-bromo-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13885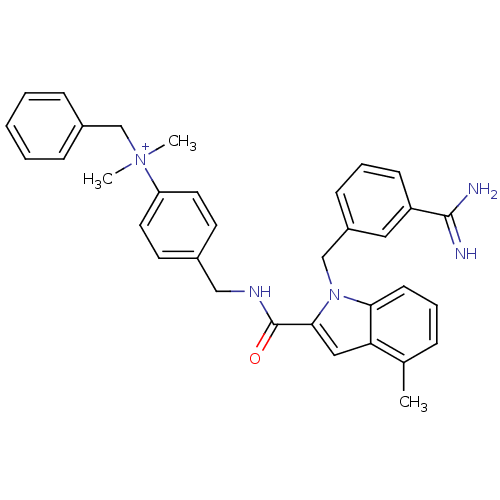 (2,2,2-trifluoroacetate; N-benzyl-4-[({1-[(3-carbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12402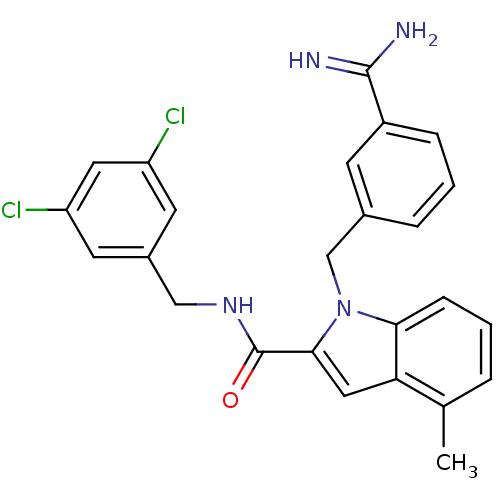 (1-[(3-carbamimidoylphenyl)methyl]-N-[(3,5-dichloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13837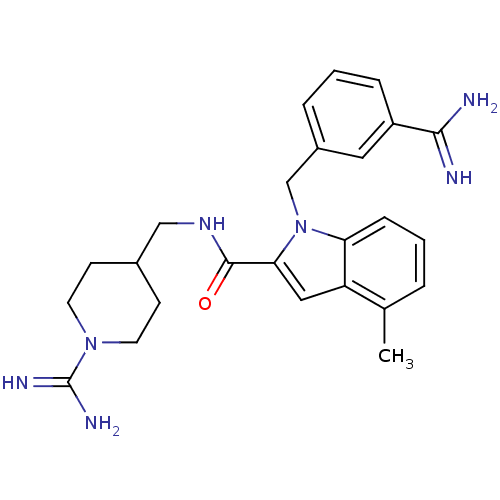 (1-[(3-carbamimidoylphenyl)methyl]-N-[(1-carbamimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13822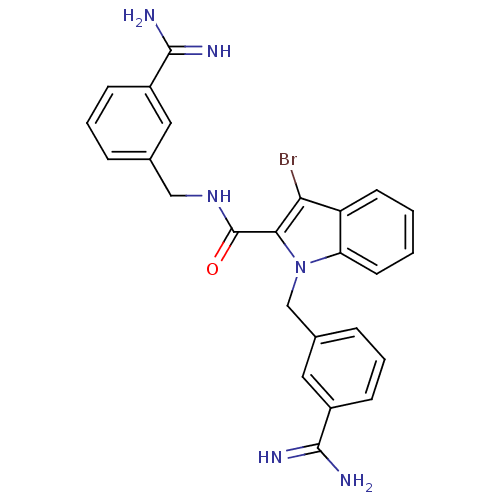 (3-amidinobenzylindole carboxamide 6 | 3-bromo-N,1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13897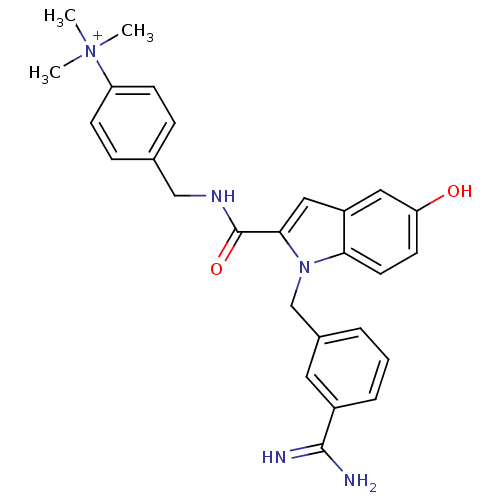 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13652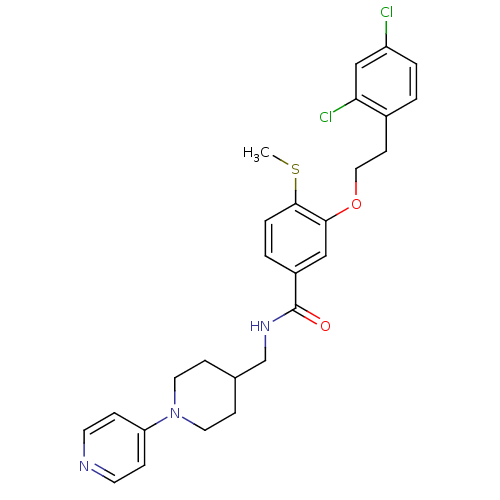 (3-Oxybenzamide 38 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13645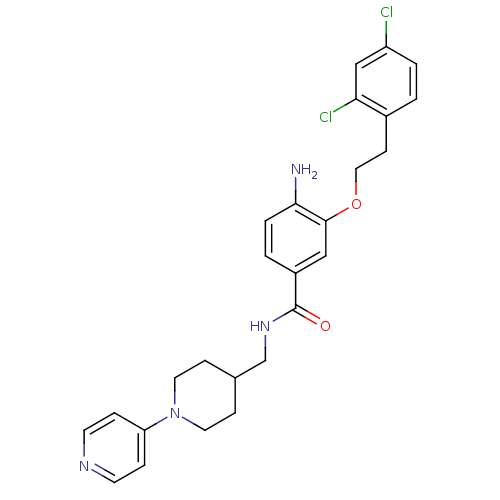 (3-Oxybenzamide 31 | 4-amino-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13619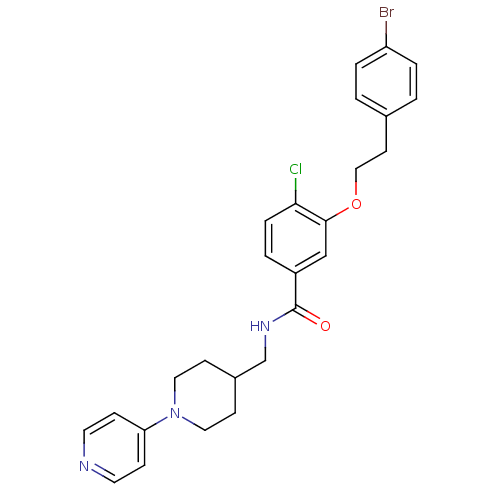 (3-Oxybenzamide 5 | 3-[2-(4-bromophenyl)ethoxy]-4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13618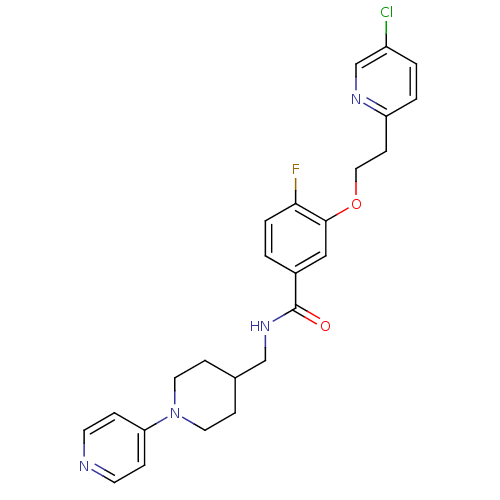 (3-Oxybenzamide 4 | 3-[2-(5-chloropyridin-2-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13642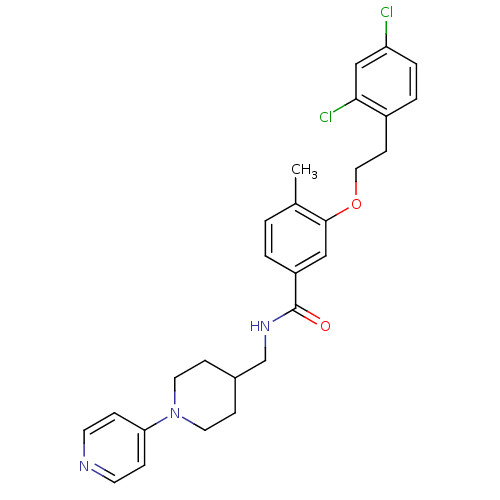 (3-Oxybenzamide 28 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13874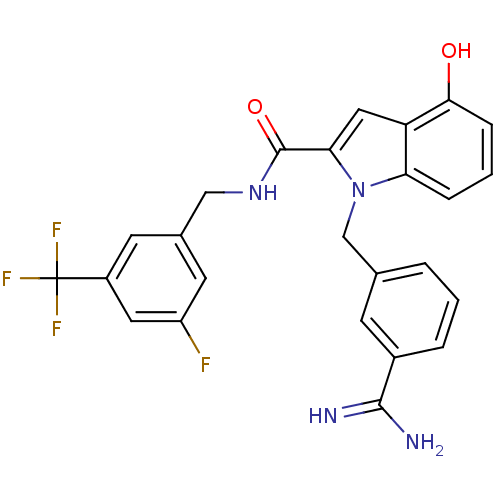 (1-[(3-carbamimidoylphenyl)methyl]-N-{[3-fluoro-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13658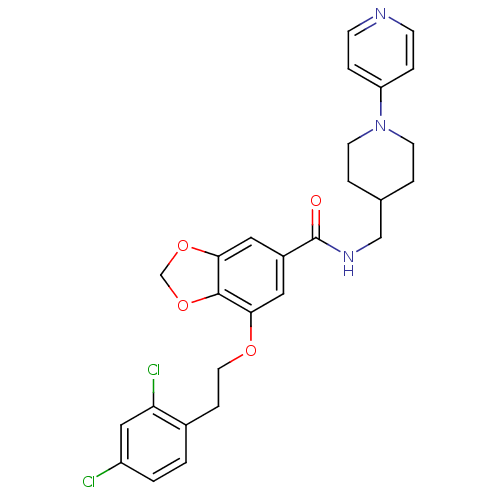 (3-Oxybenzamide 44 | 7-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13617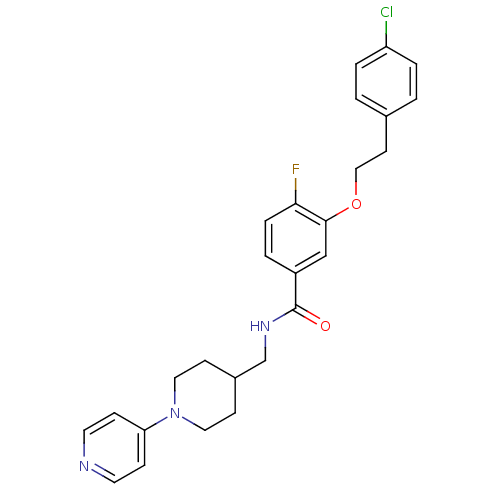 (3-Oxybenzamide 3 | 3-[2-(4-chlorophenyl)ethoxy]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13826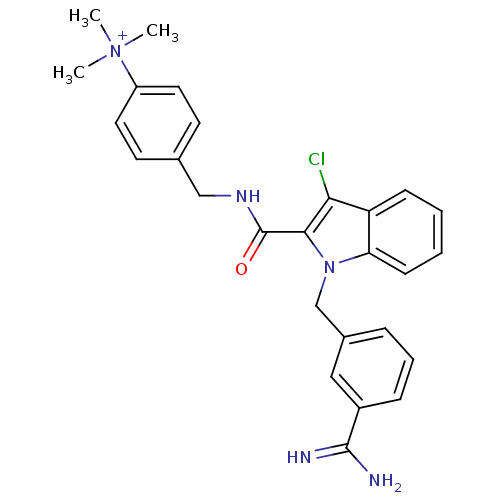 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13667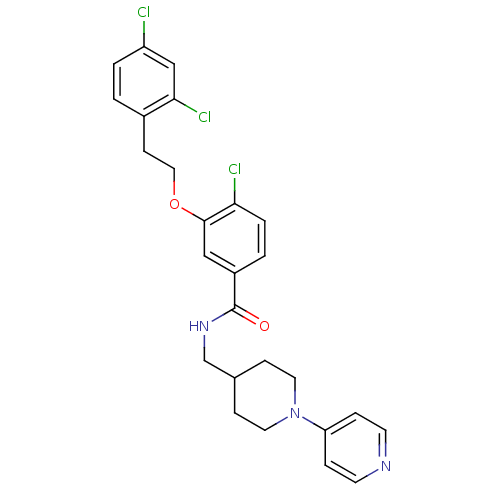 (3-Oxybenzamide 53 | 4-chloro-3-[2-(2,4-dichlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13665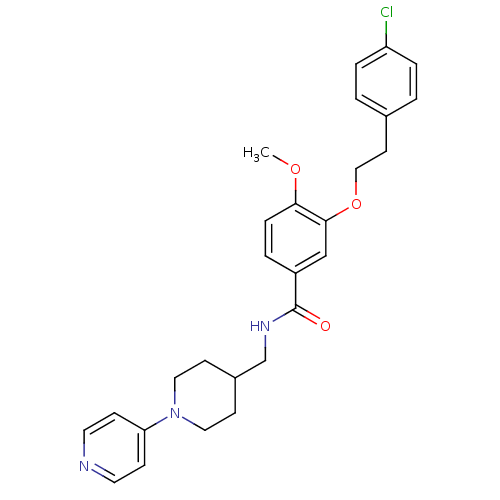 (3-Oxybenzamide 51 | 3-[2-(4-chlorophenyl)ethoxy]-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13877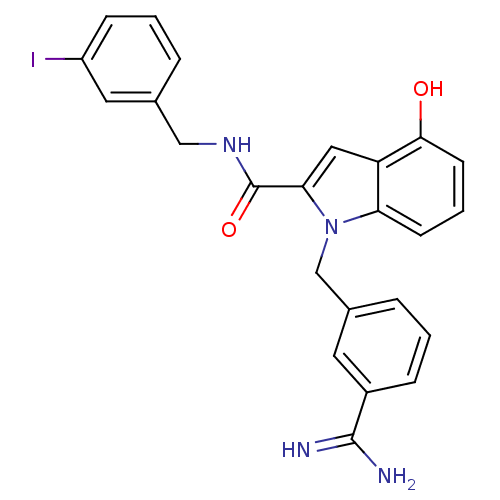 (1-[(3-carbamimidoylphenyl)methyl]-4-hydroxy-N-[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13644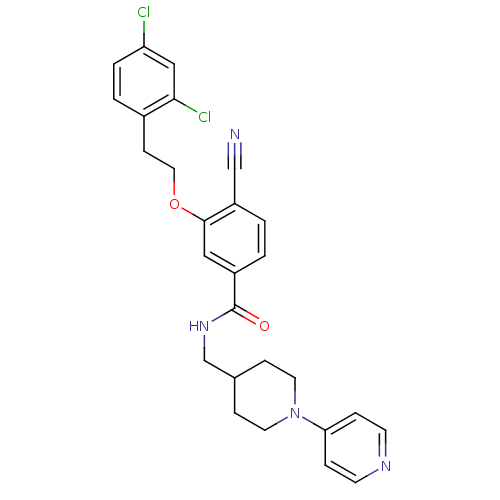 (3-Oxybenzamide 30 | 4-cyano-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13646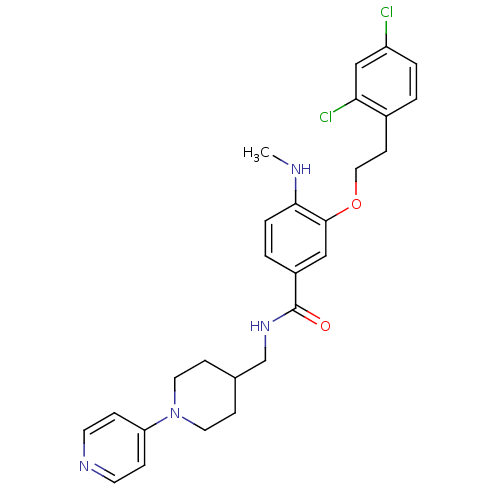 (3-Oxybenzamide 32 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13616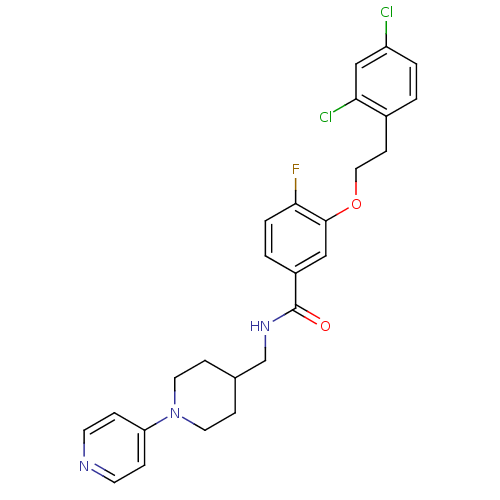 (3-Oxybenzamide 2 | 3-[2-(2,4-dichlorophenyl)ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13821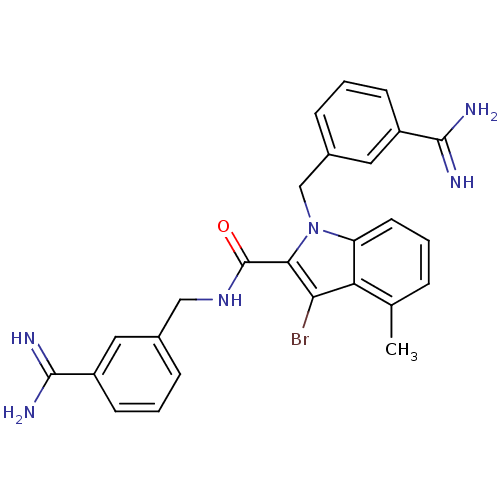 (3-amidinobenzylindole carboxamide 5 | 3-bromo-N,1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13838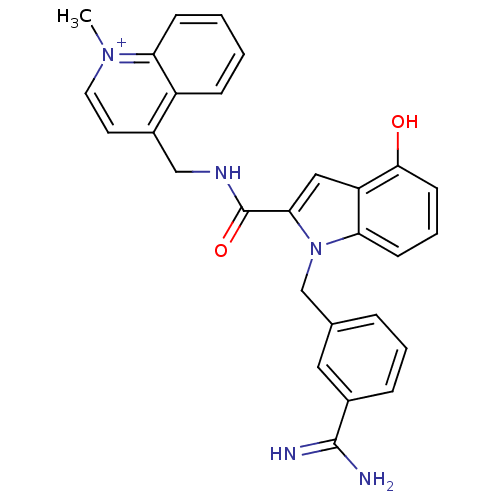 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13656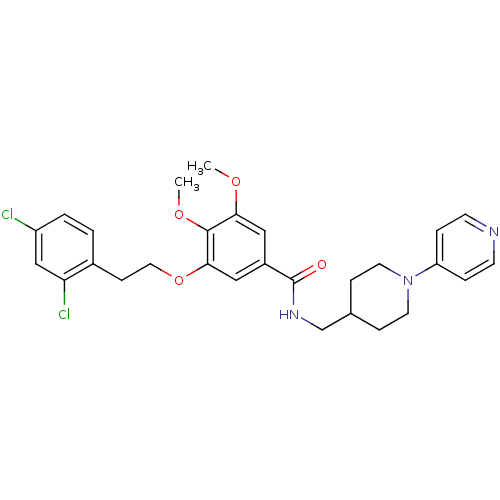 (3-Oxybenzamide 42 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13865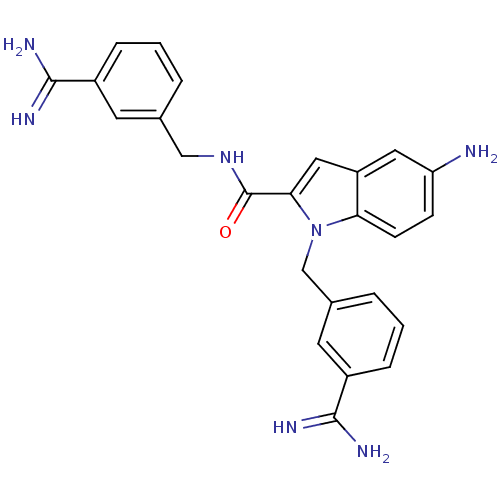 (3-amidinobenzylindole carboxamide 49 | 5-amino-N,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13651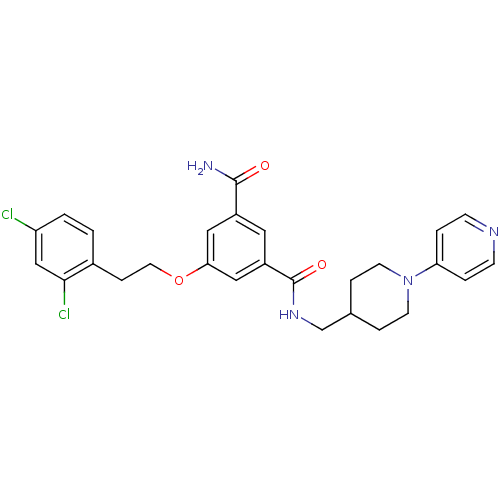 (3-Oxybenzamide 37 | 5-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13666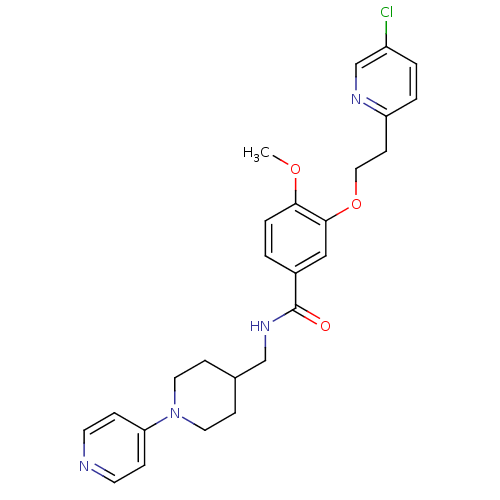 (3-Oxybenzamide 52 | 3-[2-(5-chloropyridin-2-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13673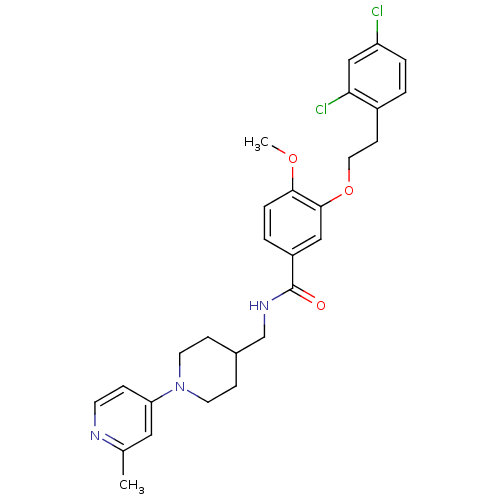 (3-Oxybenzamide 59 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13864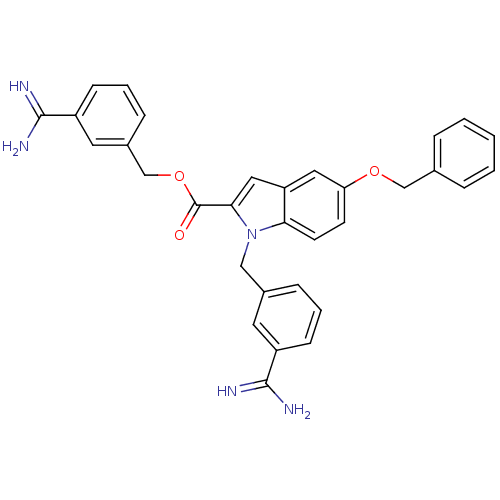 ((3-carbamimidoylphenyl)methyl 5-(benzyloxy)-1-[(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13653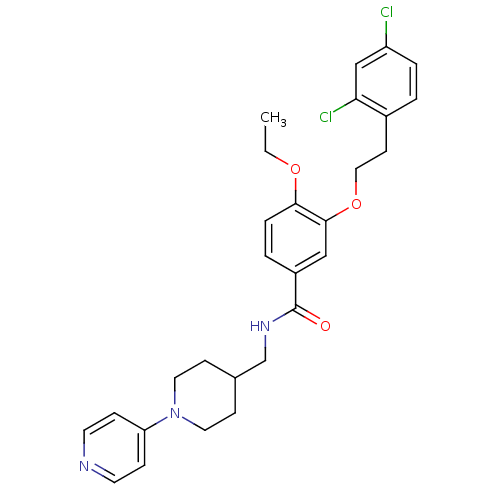 (3-Oxybenzamide 39 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13893 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13895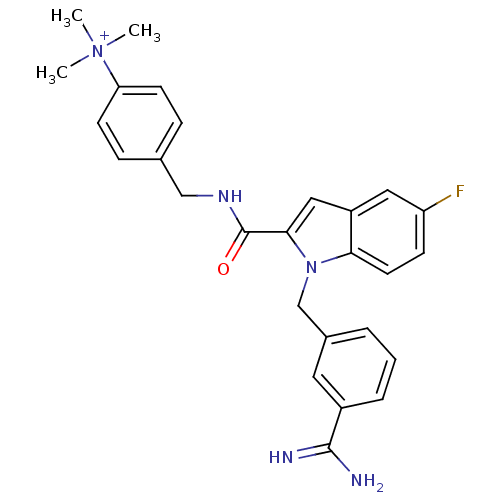 (2,2,2-trifluoroacetate; 4-[({1-[(3-carbamimidoylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13840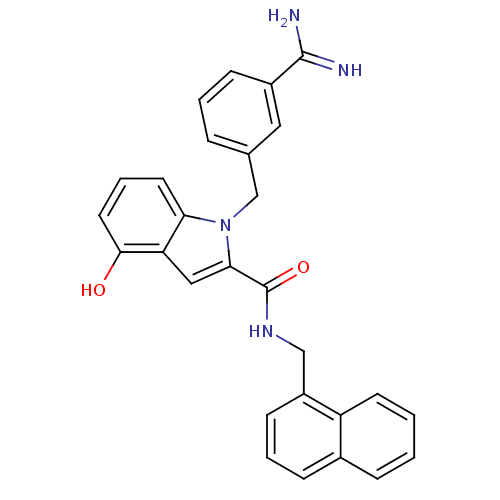 (1-[(3-carbamimidoylphenyl)methyl]-4-hydroxy-N-(nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... | J Med Chem 45: 2749-69 (2002) Article DOI: 10.1021/jm0111346 BindingDB Entry DOI: 10.7270/Q27H1GTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13629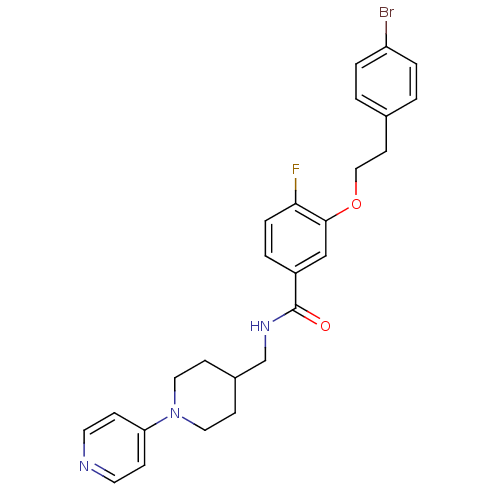 (3-Oxybenzamide 15 | 3-[2-(4-bromophenyl)ethoxy]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13647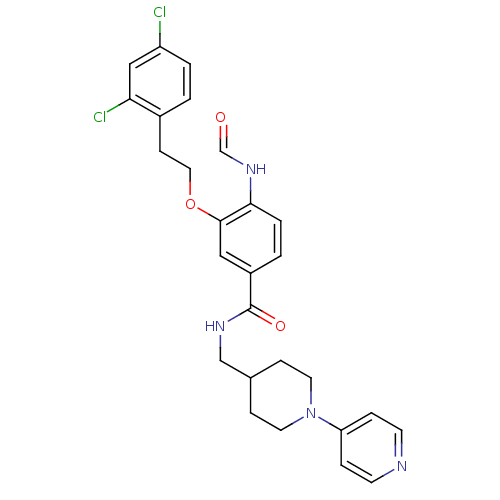 (3-Oxybenzamide 33 | 3-[2-(2,4-dichlorophenyl)ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13640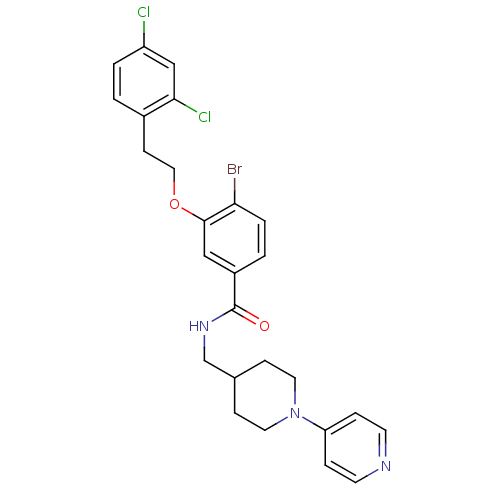 (3-Oxybenzamide 26 | 4-bromo-3-[2-(2,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 14: 2801-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.059 BindingDB Entry DOI: 10.7270/Q2XS5WW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 218 total ) | Next | Last >> |