 Found 149 hits with Last Name = 'spehar' and Initial = 'gm'
Found 149 hits with Last Name = 'spehar' and Initial = 'gm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256016
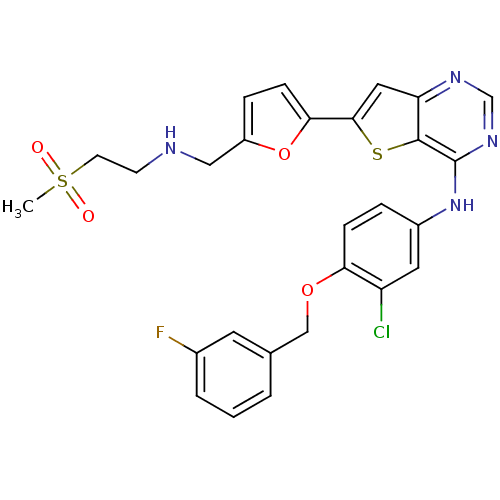
(CHEMBL475768 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)10-9-30-14-20-6-8-24(37-20)25-13-22-26(38-25)27(32-16-31-22)33-19-5-7-23(21(28)12-19)36-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30H,9-10,14-15H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256032
(CHEMBL475594 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN(C)Cc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H22ClFN4O2S/c1-32(2)13-19-7-9-23(34-19)24-12-21-25(35-24)26(30-15-29-21)31-18-6-8-22(20(27)11-18)33-14-16-4-3-5-17(28)10-16/h3-12,15H,13-14H2,1-2H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28175

(5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(trifl...)Show SMILES COc1ccc2ncn(-c3cc(OCc4ccccc4C(F)(F)F)c(s3)C(N)=O)c2c1 Show InChI InChI=1S/C21H16F3N3O3S/c1-29-13-6-7-15-16(8-13)27(11-26-15)18-9-17(19(31-18)20(25)28)30-10-12-4-2-3-5-14(12)21(22,23)24/h2-9,11H,10H2,1H3,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM25120

(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4C(F)(F)F)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C22H18F3N3O4S/c1-30-16-7-14-15(8-17(16)31-2)28(11-27-14)19-9-18(20(33-19)21(26)29)32-10-12-5-3-4-6-13(12)22(23,24)25/h3-9,11H,10H2,1-2H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28200

(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4C(F)(F)F)c(s3)C(O)=O)c2cc1OC Show InChI InChI=1S/C22H17F3N2O5S/c1-30-16-7-14-15(8-17(16)31-2)27(11-26-14)19-9-18(20(33-19)21(28)29)32-10-12-5-3-4-6-13(12)22(23,24)25/h3-9,11H,10H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28178
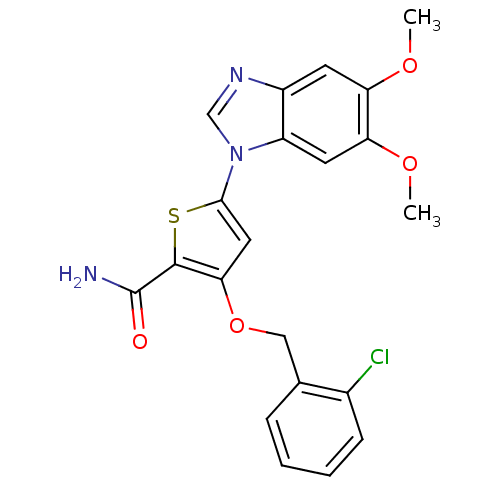
(3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4Cl)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C21H18ClN3O4S/c1-27-16-7-14-15(8-17(16)28-2)25(11-24-14)19-9-18(20(30-19)21(23)26)29-10-12-5-3-4-6-13(12)22/h3-9,11H,10H2,1-2H3,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28194
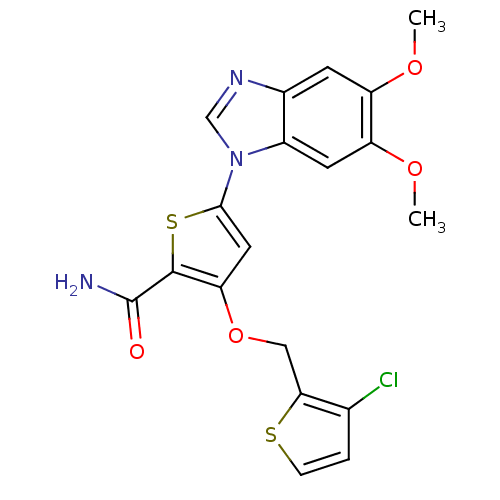
(3-[(3-chlorothiophen-2-yl)methoxy]-5-(5,6-dimethox...)Show SMILES COc1cc2ncn(-c3cc(OCc4sccc4Cl)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C19H16ClN3O4S2/c1-25-13-5-11-12(6-14(13)26-2)23(9-22-11)17-7-15(18(29-17)19(21)24)27-8-16-10(20)3-4-28-16/h3-7,9H,8H2,1-2H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28177
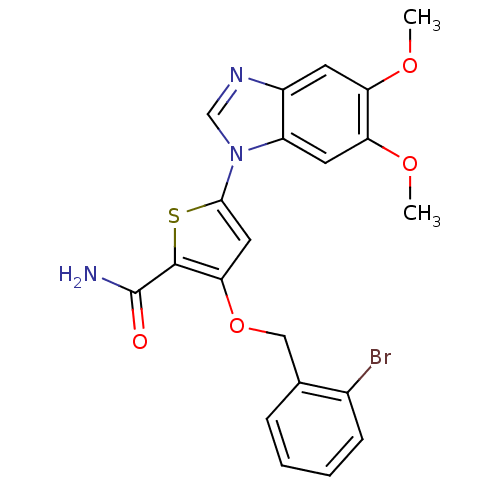
(3-[(2-bromophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,3...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4Br)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C21H18BrN3O4S/c1-27-16-7-14-15(8-17(16)28-2)25(11-24-14)19-9-18(20(30-19)21(23)26)29-10-12-5-3-4-6-13(12)22/h3-9,11H,10H2,1-2H3,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256034
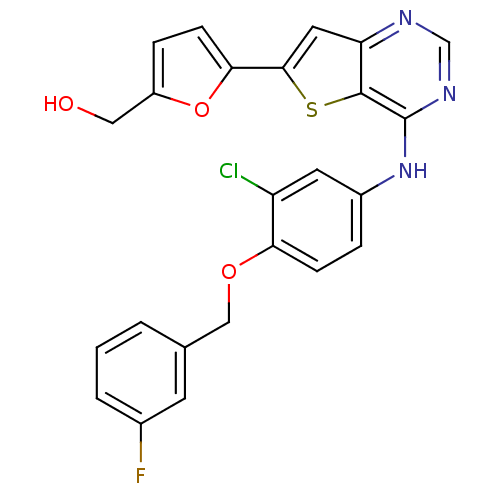
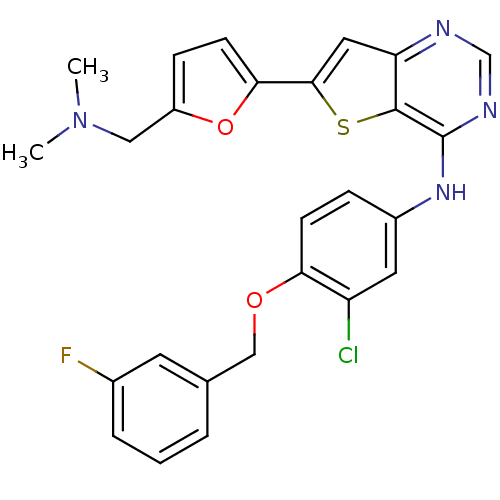
((5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)t...)Show SMILES OCc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C24H17ClFN3O3S/c25-18-9-16(4-6-20(18)31-12-14-2-1-3-15(26)8-14)29-24-23-19(27-13-28-24)10-22(33-23)21-7-5-17(11-30)32-21/h1-10,13,30H,11-12H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256036
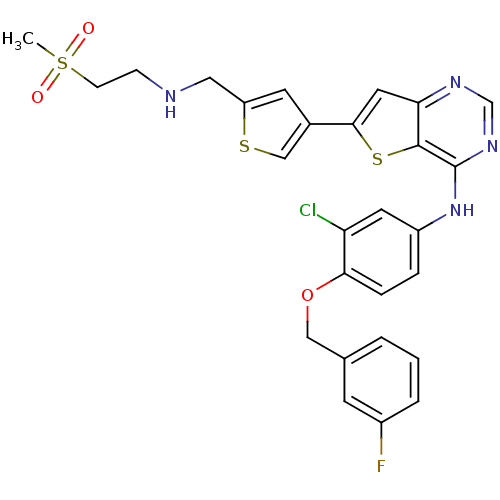
(CHEMBL474431 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1cc(cs1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O3S3/c1-39(34,35)8-7-30-13-21-10-18(15-37-21)25-12-23-26(38-25)27(32-16-31-23)33-20-5-6-24(22(28)11-20)36-14-17-3-2-4-19(29)9-17/h2-6,9-12,15-16,30H,7-8,13-14H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256035
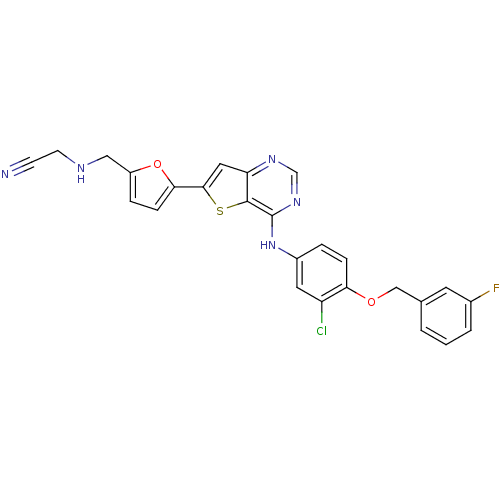
(2-((5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamin...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)-c3ccc(CNCC#N)o3)cc2Cl)c1 Show InChI InChI=1S/C26H19ClFN5O2S/c27-20-11-18(4-6-22(20)34-14-16-2-1-3-17(28)10-16)33-26-25-21(31-15-32-26)12-24(36-25)23-7-5-19(35-23)13-30-9-8-29/h1-7,10-12,15,30H,9,13-14H2,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256017
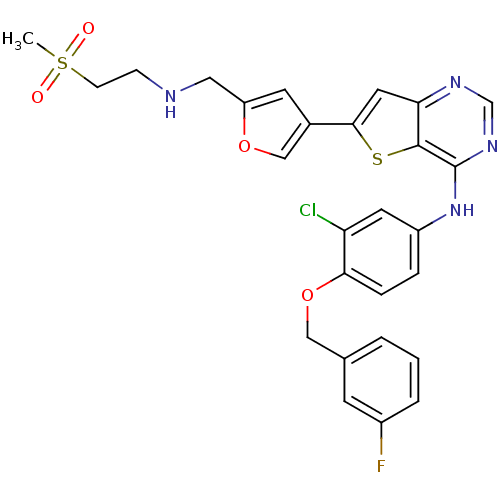
(CHEMBL475769 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1cc(co1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)8-7-30-13-21-10-18(15-36-21)25-12-23-26(38-25)27(32-16-31-23)33-20-5-6-24(22(28)11-20)37-14-17-3-2-4-19(29)9-17/h2-6,9-12,15-16,30H,7-8,13-14H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28169
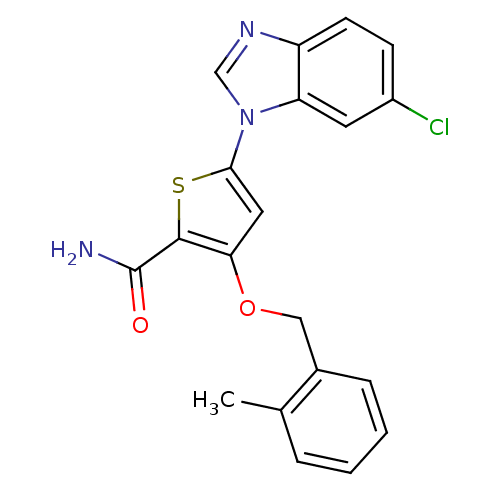
(5-(6-chloro-1H-1,3-benzodiazol-1-yl)-3-[(2-methylp...)Show SMILES Cc1ccccc1COc1cc(sc1C(N)=O)-n1cnc2ccc(Cl)cc12 Show InChI InChI=1S/C20H16ClN3O2S/c1-12-4-2-3-5-13(12)10-26-17-9-18(27-19(17)20(22)25)24-11-23-15-7-6-14(21)8-16(15)24/h2-9,11H,10H2,1H3,(H2,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256038
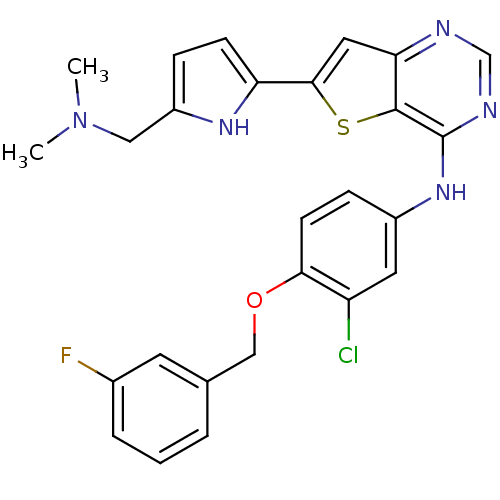
(CHEMBL473437 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN(C)Cc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H23ClFN5OS/c1-33(2)13-19-6-8-21(31-19)24-12-22-25(35-24)26(30-15-29-22)32-18-7-9-23(20(27)11-18)34-14-16-4-3-5-17(28)10-16/h3-12,15,31H,13-14H2,1-2H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28182
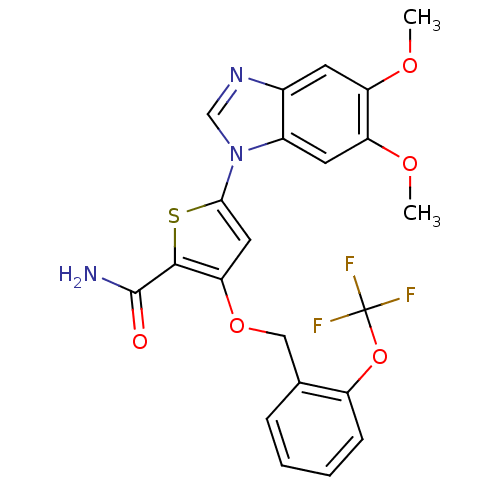
(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4OC(F)(F)F)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C22H18F3N3O5S/c1-30-16-7-13-14(8-17(16)31-2)28(11-27-13)19-9-18(20(34-19)21(26)29)32-10-12-5-3-4-6-15(12)33-22(23,24)25/h3-9,11H,10H2,1-2H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256033
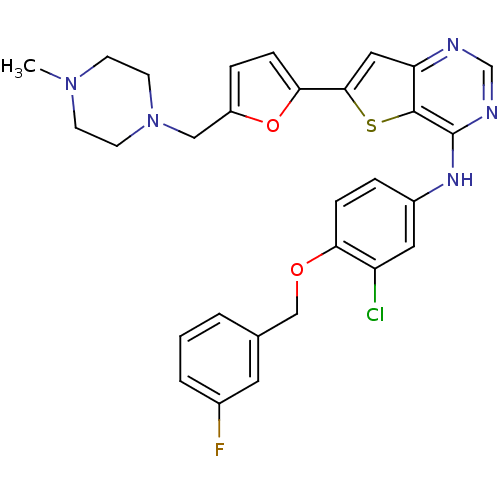
(CHEMBL475761 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN1CCN(Cc2ccc(o2)-c2cc3ncnc(Nc4ccc(OCc5cccc(F)c5)c(Cl)c4)c3s2)CC1 Show InChI InChI=1S/C29H27ClFN5O2S/c1-35-9-11-36(12-10-35)16-22-6-8-26(38-22)27-15-24-28(39-27)29(33-18-32-24)34-21-5-7-25(23(30)14-21)37-17-19-3-2-4-20(31)13-19/h2-8,13-15,18H,9-12,16-17H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256039
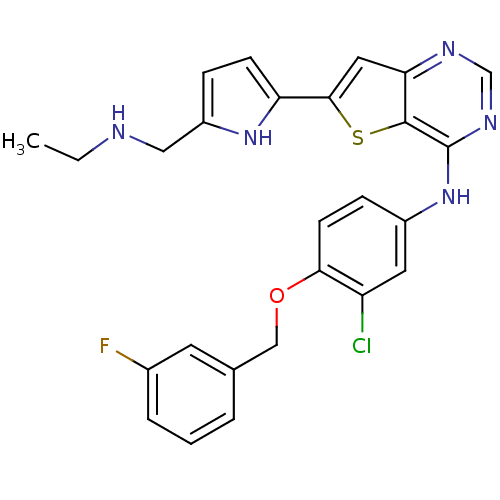
(CHEMBL473438 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CCNCc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H23ClFN5OS/c1-2-29-13-19-6-8-21(32-19)24-12-22-25(35-24)26(31-15-30-22)33-18-7-9-23(20(27)11-18)34-14-16-4-3-5-17(28)10-16/h3-12,15,29,32H,2,13-14H2,1H3,(H,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28202
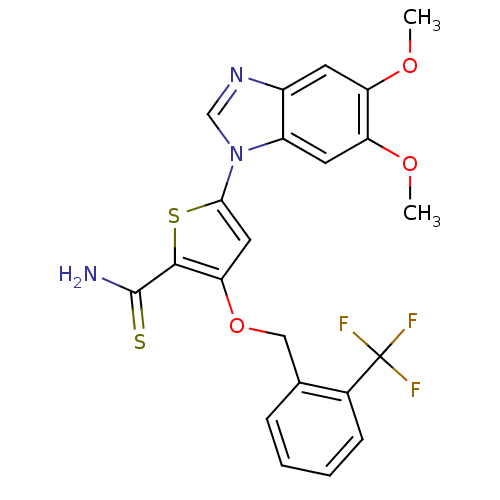
(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4C(F)(F)F)c(s3)C(N)=S)c2cc1OC Show InChI InChI=1S/C22H18F3N3O3S2/c1-29-16-7-14-15(8-17(16)30-2)28(11-27-14)19-9-18(20(33-19)21(26)32)31-10-12-5-3-4-6-13(12)22(23,24)25/h3-9,11H,10H2,1-2H3,(H2,26,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28179
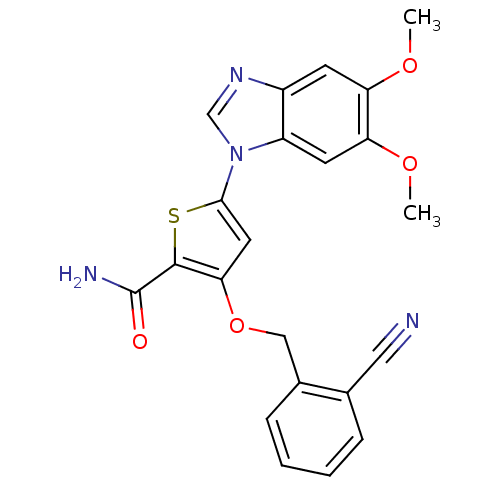
(3-[(2-cyanophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,3...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4C#N)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C22H18N4O4S/c1-28-17-7-15-16(8-18(17)29-2)26(12-25-15)20-9-19(21(31-20)22(24)27)30-11-14-6-4-3-5-13(14)10-23/h3-9,12H,11H2,1-2H3,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50256040
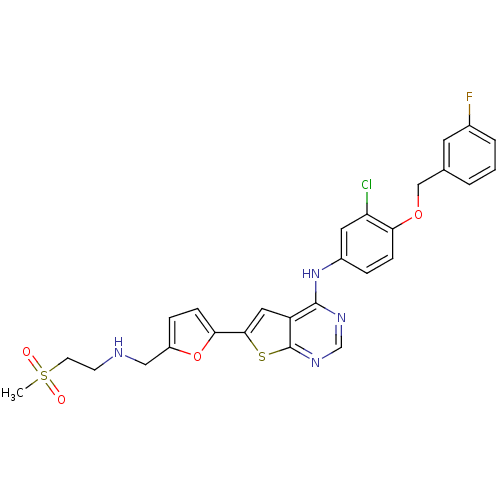
(CHEMBL480349 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1cc2c(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)ncnc2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)10-9-30-14-20-6-8-24(37-20)25-13-21-26(31-16-32-27(21)38-25)33-19-5-7-23(22(28)12-19)36-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30H,9-10,14-15H2,1H3,(H,31,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256020
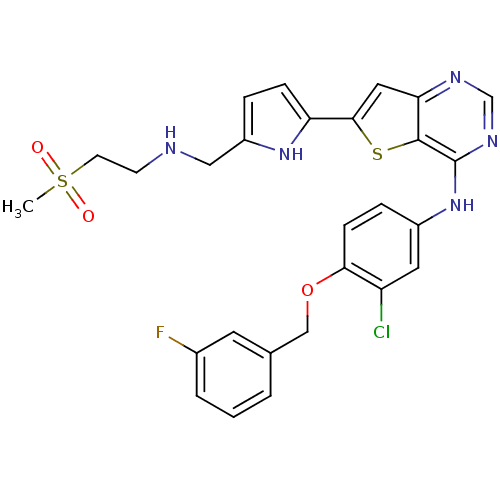
(CHEMBL475446 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H25ClFN5O3S2/c1-39(35,36)10-9-30-14-20-5-7-22(33-20)25-13-23-26(38-25)27(32-16-31-23)34-19-6-8-24(21(28)12-19)37-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30,33H,9-10,14-15H2,1H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256018

(CHEMBL473427 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)-c3cccs3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3OS2/c24-17-10-16(6-7-19(17)29-12-14-3-1-4-15(25)9-14)28-23-22-18(26-13-27-23)11-21(31-22)20-5-2-8-30-20/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28183
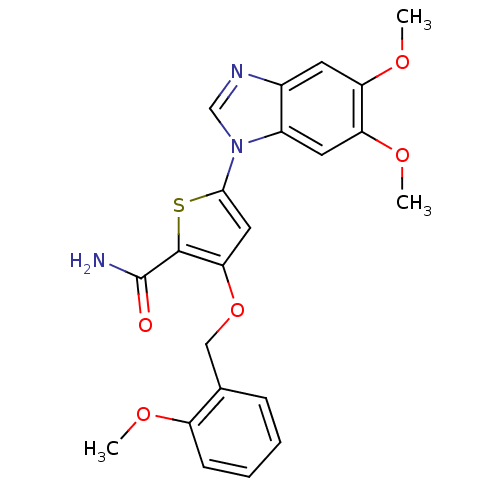
(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-[(2-me...)Show SMILES COc1ccccc1COc1cc(sc1C(N)=O)-n1cnc2cc(OC)c(OC)cc12 Show InChI InChI=1S/C22H21N3O5S/c1-27-16-7-5-4-6-13(16)11-30-19-10-20(31-21(19)22(23)26)25-12-24-14-8-17(28-2)18(29-3)9-15(14)25/h4-10,12H,11H2,1-3H3,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28174

(5-(5-methoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(trifl...)Show SMILES COc1ccc2n(cnc2c1)-c1cc(OCc2ccccc2C(F)(F)F)c(s1)C(N)=O Show InChI InChI=1S/C21H16F3N3O3S/c1-29-13-6-7-16-15(8-13)26-11-27(16)18-9-17(19(31-18)20(25)28)30-10-12-4-2-3-5-14(12)21(22,23)24/h2-9,11H,10H2,1H3,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256041
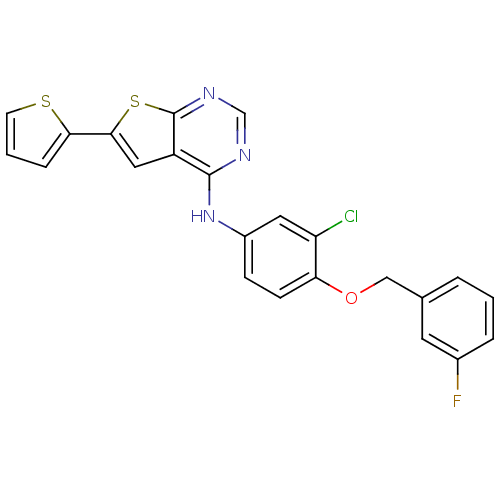
(CHEMBL506414 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4sc(cc34)-c3cccs3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3OS2/c24-18-10-16(6-7-19(18)29-12-14-3-1-4-15(25)9-14)28-22-17-11-21(20-5-2-8-30-20)31-23(17)27-13-26-22/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM25120

(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4C(F)(F)F)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C22H18F3N3O4S/c1-30-16-7-14-15(8-17(16)31-2)28(11-27-14)19-9-18(20(33-19)21(26)29)32-10-12-5-3-4-6-13(12)22(23,24)25/h3-9,11H,10H2,1-2H3,(H2,26,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28171

(3-[(2-bromophenyl)methoxy]-5-[6-(trifluoromethyl)-...)Show SMILES NC(=O)c1sc(cc1OCc1ccccc1Br)-n1cnc2ccc(cc12)C(F)(F)F Show InChI InChI=1S/C20H13BrF3N3O2S/c21-13-4-2-1-3-11(13)9-29-16-8-17(30-18(16)19(25)28)27-10-26-14-6-5-12(7-15(14)27)20(22,23)24/h1-8,10H,9H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256015

(CHEMBL479800 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)-c3ccco3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3O2S/c24-17-10-16(6-7-19(17)30-12-14-3-1-4-15(25)9-14)28-23-22-18(26-13-27-23)11-21(31-22)20-5-2-8-29-20/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28192
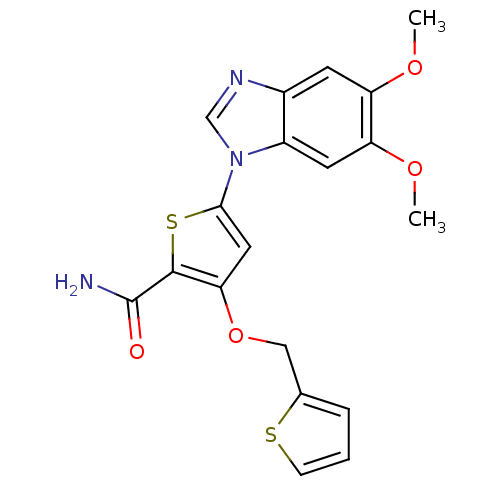
(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-(thiop...)Show SMILES COc1cc2ncn(-c3cc(OCc4cccs4)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C19H17N3O4S2/c1-24-14-6-12-13(7-15(14)25-2)22(10-21-12)17-8-16(18(28-17)19(20)23)26-9-11-4-3-5-27-11/h3-8,10H,9H2,1-2H3,(H2,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28166
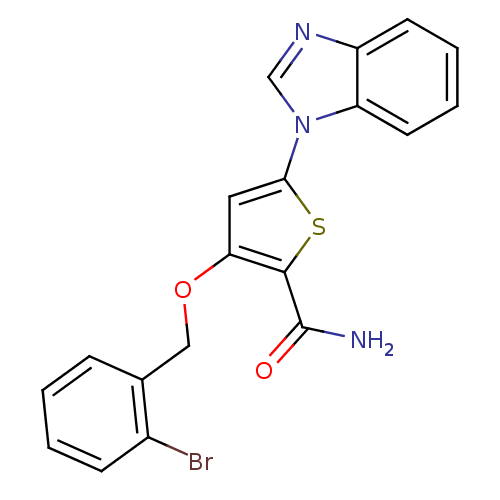
(5-(1H-1,3-benzodiazol-1-yl)-3-[(2-bromophenyl)meth...)Show InChI InChI=1S/C19H14BrN3O2S/c20-13-6-2-1-5-12(13)10-25-16-9-17(26-18(16)19(21)24)23-11-22-14-7-3-4-8-15(14)23/h1-9,11H,10H2,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28157

(5-(1H-1,3-benzodiazol-1-yl)-3-[(2-methylphenyl)met...)Show InChI InChI=1S/C20H17N3O2S/c1-13-6-2-3-7-14(13)11-25-17-10-18(26-19(17)20(21)24)23-12-22-15-8-4-5-9-16(15)23/h2-10,12H,11H2,1H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28158

(5-(1H-1,3-benzodiazol-1-yl)-3-[(3-methylphenyl)met...)Show InChI InChI=1S/C20H17N3O2S/c1-13-5-4-6-14(9-13)11-25-17-10-18(26-19(17)20(21)24)23-12-22-15-7-2-3-8-16(15)23/h2-10,12H,11H2,1H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28159

(5-(1H-1,3-benzodiazol-1-yl)-3-[(2-methoxyphenyl)me...)Show InChI InChI=1S/C20H17N3O3S/c1-25-16-9-5-2-6-13(16)11-26-17-10-18(27-19(17)20(21)24)23-12-22-14-7-3-4-8-15(14)23/h2-10,12H,11H2,1H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256040

(CHEMBL480349 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1cc2c(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)ncnc2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)10-9-30-14-20-6-8-24(37-20)25-13-21-26(31-16-32-27(21)38-25)33-19-5-7-23(22(28)12-19)36-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30H,9-10,14-15H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28173

(5-(6-methanesulfonyl-1H-1,3-benzodiazol-1-yl)-3-{[...)Show SMILES CS(=O)(=O)c1ccc2ncn(-c3cc(OCc4ccccc4C(F)(F)F)c(s3)C(N)=O)c2c1 Show InChI InChI=1S/C21H16F3N3O4S2/c1-33(29,30)13-6-7-15-16(8-13)27(11-26-15)18-9-17(19(32-18)20(25)28)31-10-12-4-2-3-5-14(12)21(22,23)24/h2-9,11H,10H2,1H3,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28176
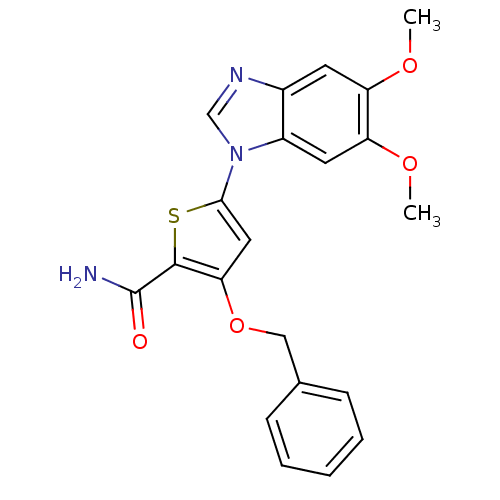
(3-(benzyloxy)-5-(5,6-dimethoxy-1H-1,3-benzodiazol-...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C21H19N3O4S/c1-26-16-8-14-15(9-17(16)27-2)24(12-23-14)19-10-18(20(29-19)21(22)25)28-11-13-6-4-3-5-7-13/h3-10,12H,11H2,1-2H3,(H2,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28162

(5-(1H-1,3-benzodiazol-1-yl)-3-[(2-chlorophenyl)met...)Show InChI InChI=1S/C19H14ClN3O2S/c20-13-6-2-1-5-12(13)10-25-16-9-17(26-18(16)19(21)24)23-11-22-14-7-3-4-8-15(14)23/h1-9,11H,10H2,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28187
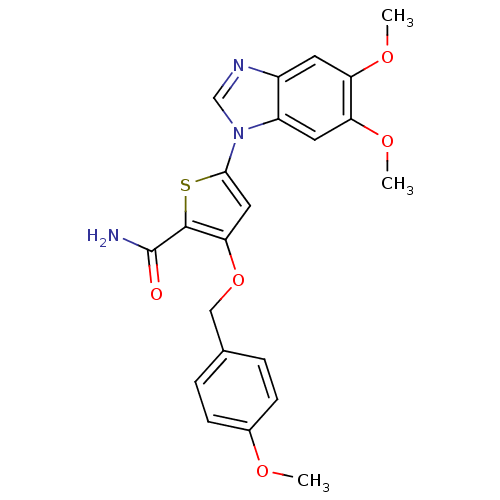
(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-[(4-me...)Show SMILES COc1ccc(COc2cc(sc2C(N)=O)-n2cnc3cc(OC)c(OC)cc23)cc1 Show InChI InChI=1S/C22H21N3O5S/c1-27-14-6-4-13(5-7-14)11-30-19-10-20(31-21(19)22(23)26)25-12-24-15-8-17(28-2)18(29-3)9-16(15)25/h4-10,12H,11H2,1-3H3,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50256021
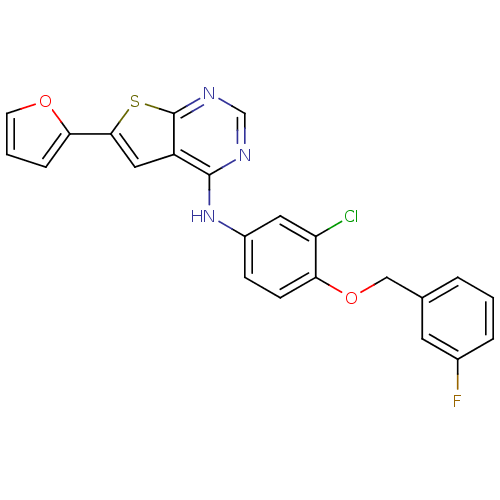
(CHEMBL514938 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4sc(cc34)-c3ccco3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3O2S/c24-18-10-16(6-7-19(18)30-12-14-3-1-4-15(25)9-14)28-22-17-11-21(20-5-2-8-29-20)31-23(17)27-13-26-22/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28198
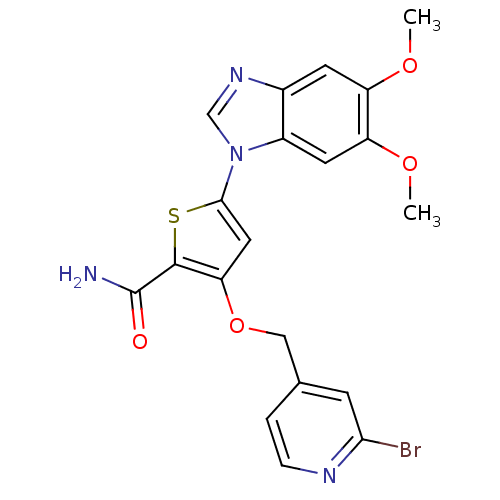
(3-[(2-bromopyridin-4-yl)methoxy]-5-(5,6-dimethoxy-...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccnc(Br)c4)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C20H17BrN4O4S/c1-27-14-6-12-13(7-15(14)28-2)25(10-24-12)18-8-16(19(30-18)20(22)26)29-9-11-3-4-23-17(21)5-11/h3-8,10H,9H2,1-2H3,(H2,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28167
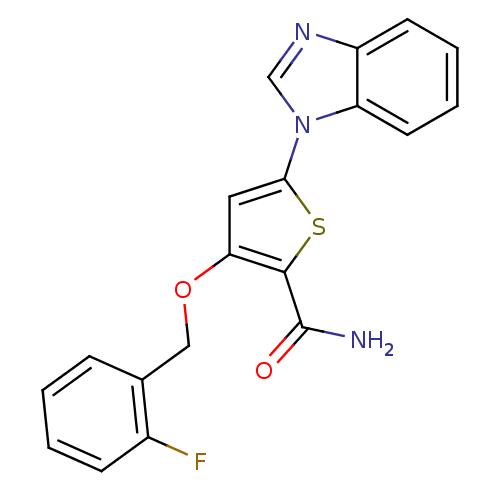
(5-(1H-1,3-benzodiazol-1-yl)-3-[(2-fluorophenyl)met...)Show InChI InChI=1S/C19H14FN3O2S/c20-13-6-2-1-5-12(13)10-25-16-9-17(26-18(16)19(21)24)23-11-22-14-7-3-4-8-15(14)23/h1-9,11H,10H2,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28165
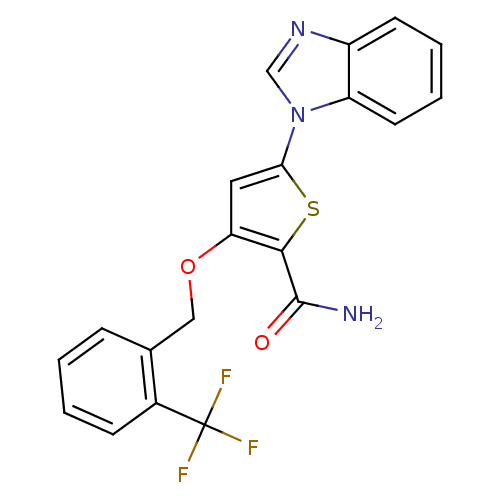
(5-(1H-1,3-benzodiazol-1-yl)-3-{[2-(trifluoromethyl...)Show SMILES NC(=O)c1sc(cc1OCc1ccccc1C(F)(F)F)-n1cnc2ccccc12 Show InChI InChI=1S/C20H14F3N3O2S/c21-20(22,23)13-6-2-1-5-12(13)10-28-16-9-17(29-18(16)19(24)27)26-11-25-14-7-3-4-8-15(14)26/h1-9,11H,10H2,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256021

(CHEMBL514938 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4sc(cc34)-c3ccco3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3O2S/c24-18-10-16(6-7-19(18)30-12-14-3-1-4-15(25)9-14)28-22-17-11-21(20-5-2-8-29-20)31-23(17)27-13-26-22/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28181
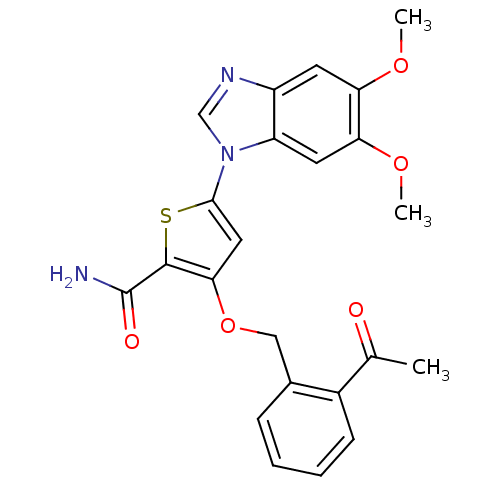
(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-[(2-ac...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccccc4C(C)=O)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C23H21N3O5S/c1-13(27)15-7-5-4-6-14(15)11-31-20-10-21(32-22(20)23(24)28)26-12-25-16-8-18(29-2)19(30-3)9-17(16)26/h4-10,12H,11H2,1-3H3,(H2,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28193

(5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-(thiop...)Show SMILES COc1cc2ncn(-c3cc(OCc4ccsc4)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C19H17N3O4S2/c1-24-14-5-12-13(6-15(14)25-2)22(10-21-12)17-7-16(18(28-17)19(20)23)26-8-11-3-4-27-9-11/h3-7,9-10H,8H2,1-2H3,(H2,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28191

(3-(cyclohexylmethoxy)-5-(5,6-dimethoxy-1H-1,3-benz...)Show SMILES COc1cc2ncn(-c3cc(OCC4CCCCC4)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C21H25N3O4S/c1-26-16-8-14-15(9-17(16)27-2)24(12-23-14)19-10-18(20(29-19)21(22)25)28-11-13-6-4-3-5-7-13/h8-10,12-13H,3-7,11H2,1-2H3,(H2,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28185
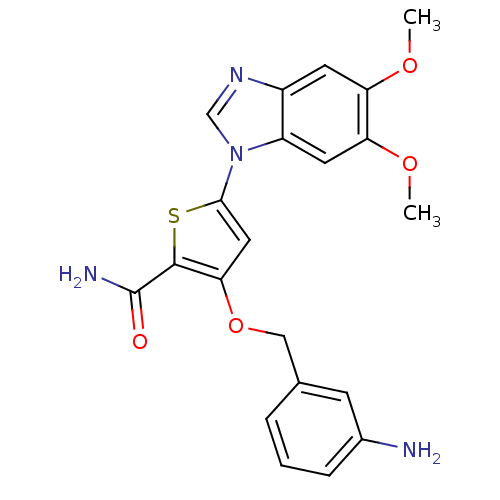
(3-[(3-aminophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,3...)Show SMILES COc1cc2ncn(-c3cc(OCc4cccc(N)c4)c(s3)C(N)=O)c2cc1OC Show InChI InChI=1S/C21H20N4O4S/c1-27-16-7-14-15(8-17(16)28-2)25(11-24-14)19-9-18(20(30-19)21(23)26)29-10-12-4-3-5-13(22)6-12/h3-9,11H,10,22H2,1-2H3,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM28168
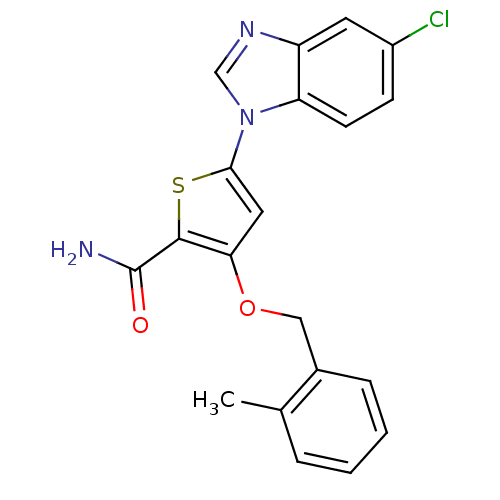
(5-(5-chloro-1H-1,3-benzodiazol-1-yl)-3-[(2-methylp...)Show SMILES Cc1ccccc1COc1cc(sc1C(N)=O)-n1cnc2cc(Cl)ccc12 Show InChI InChI=1S/C20H16ClN3O2S/c1-12-4-2-3-5-13(12)10-26-17-9-18(27-19(17)20(22)25)24-11-23-15-8-14(21)6-7-16(15)24/h2-9,11H,10H2,1H3,(H2,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK
| Assay Description
The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... |
Bioorg Med Chem Lett 19: 1018-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.041
BindingDB Entry DOI: 10.7270/Q26T0JZX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data