

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263570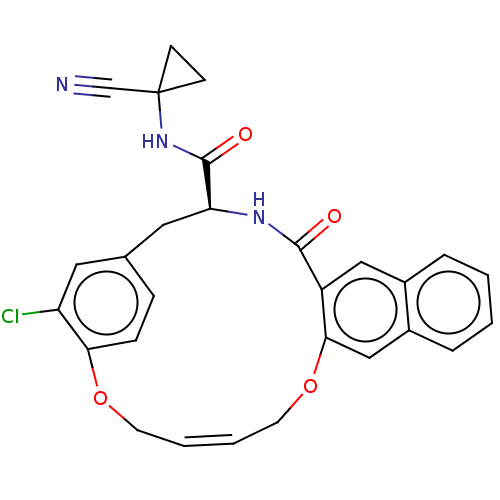 (CHEMBL4066422) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263570 (CHEMBL4066422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263572 (CHEMBL4092050) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263577 (CHEMBL4099651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210850 (US9290467, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM210854 (US9290467, 24 | US9290467, 25 | US9290467, 26) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263578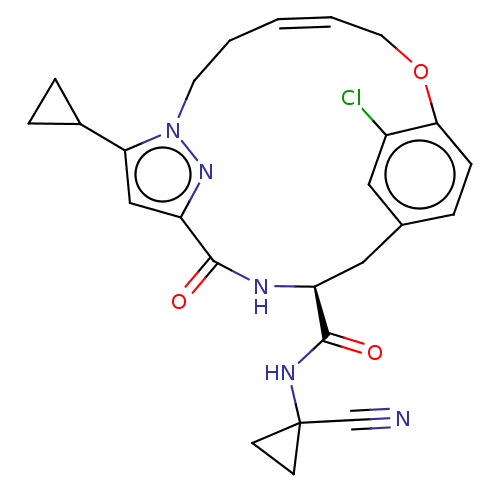 (CHEMBL4064172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210859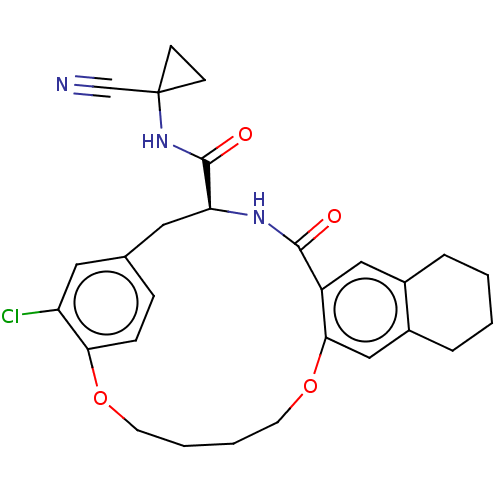 (US9290467, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50295588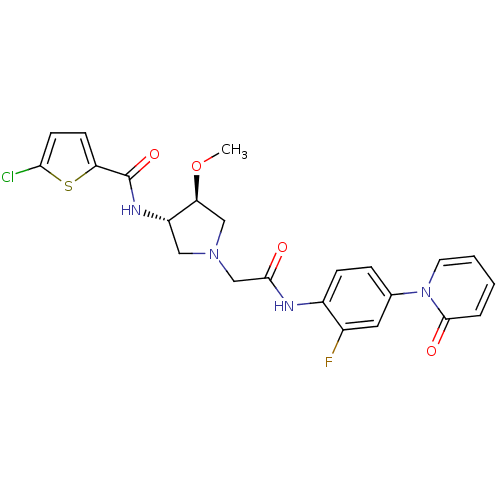 (5-chloro-N-((3S,4S)-1-(2-(2-fluoro-4-(2-oxopyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann - La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Eur J Med Chem 44: 2787-95 (2009) Article DOI: 10.1016/j.ejmech.2008.12.025 BindingDB Entry DOI: 10.7270/Q2CV4HSP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263571 (CHEMBL4062591) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50324743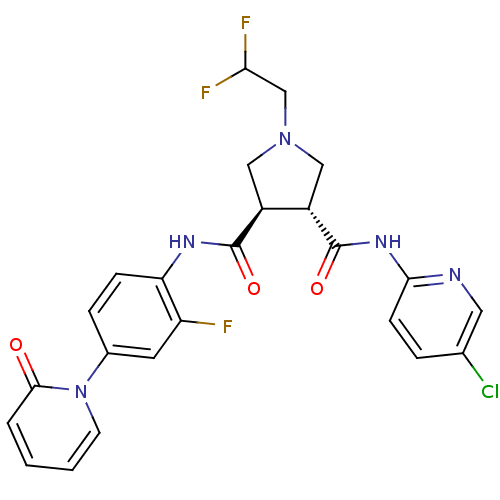 ((3R,4R)-1-(2,2-DIFLUORO-ETHYL)-PYRROLIDINE-3,4-DIC...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to rabbit factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263569 (CHEMBL4087751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263574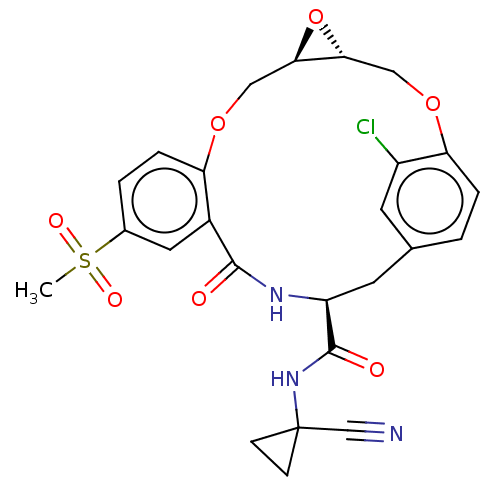 (CHEMBL4065847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263573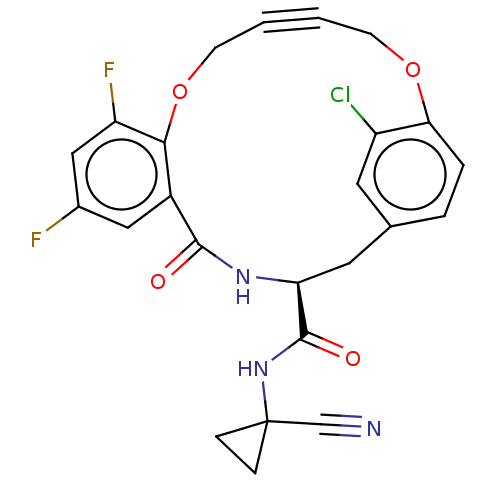 (CHEMBL4080708) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263573 (CHEMBL4080708) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263576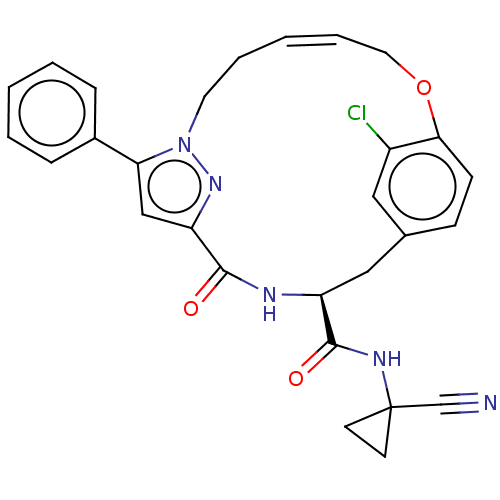 (CHEMBL4072767) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263584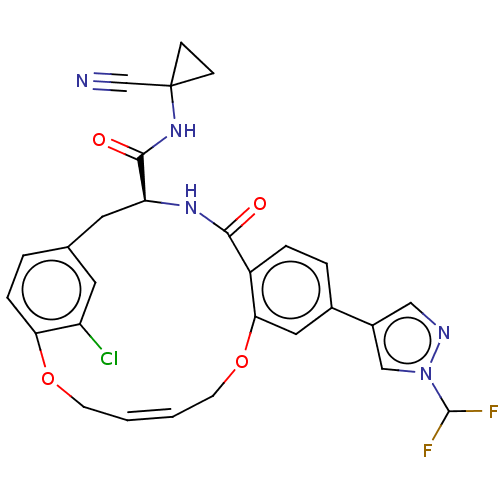 (CHEMBL4084294) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263568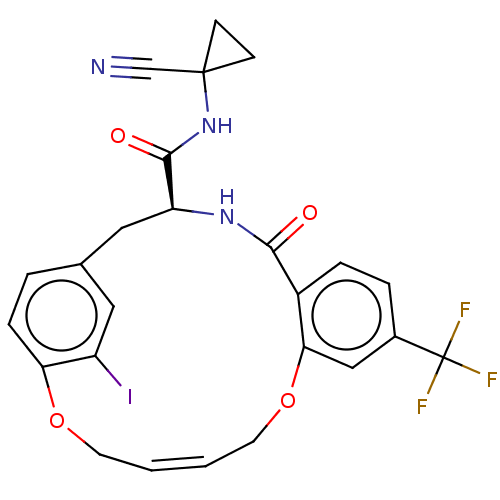 (CHEMBL4098931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263583 (CHEMBL4091192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50295583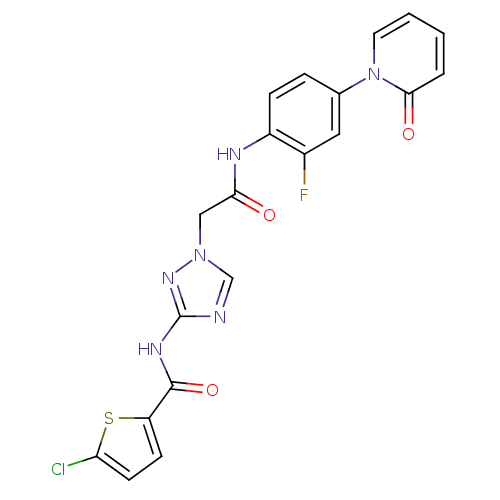 (4-chloro-N-(1-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann - La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Eur J Med Chem 44: 2787-95 (2009) Article DOI: 10.1016/j.ejmech.2008.12.025 BindingDB Entry DOI: 10.7270/Q2CV4HSP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50295581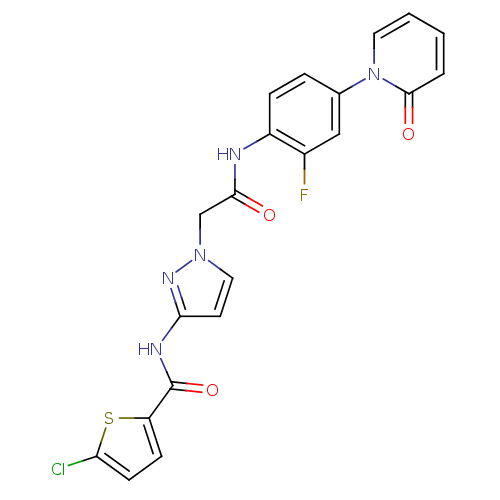 (4-chloro-N-(1-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann - La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Eur J Med Chem 44: 2787-95 (2009) Article DOI: 10.1016/j.ejmech.2008.12.025 BindingDB Entry DOI: 10.7270/Q2CV4HSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263569 (CHEMBL4087751) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263571 (CHEMBL4062591) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263584 (CHEMBL4084294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50295585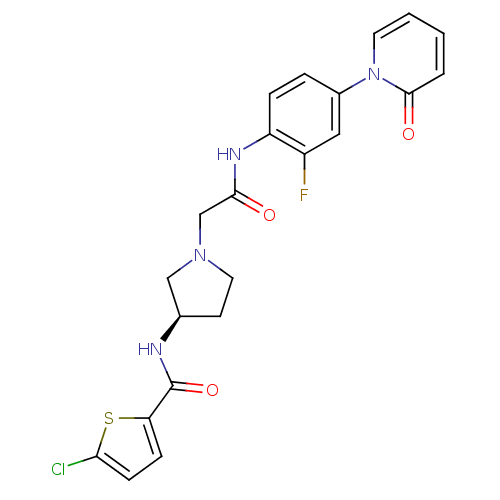 ((R)-4-chloro-N-(1-(2-(2-fluoro-4-(2-oxopyridin-1(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann - La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Eur J Med Chem 44: 2787-95 (2009) Article DOI: 10.1016/j.ejmech.2008.12.025 BindingDB Entry DOI: 10.7270/Q2CV4HSP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324757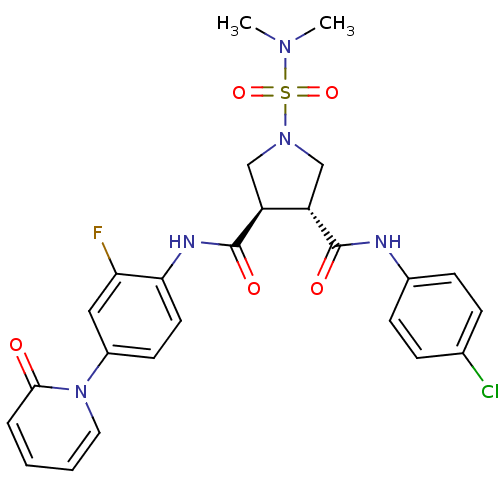 ((3R,4R)-N3-(4-chlorophenyl)-1-(N,N-dimethylsulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324771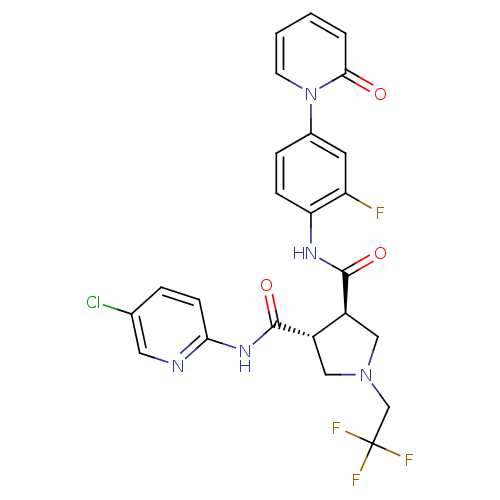 ((3R,4R)-N3-(5-chloropyridin-2-yl)-N4-(2-fluoro-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263568 (CHEMBL4098931) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324755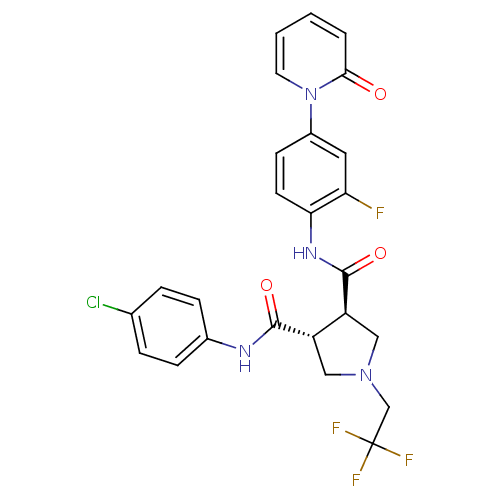 ((3R,4R)-N-(4-CHLOROPHENYL)-N'-[2-FLUORO-4-(2-OXOPY...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324754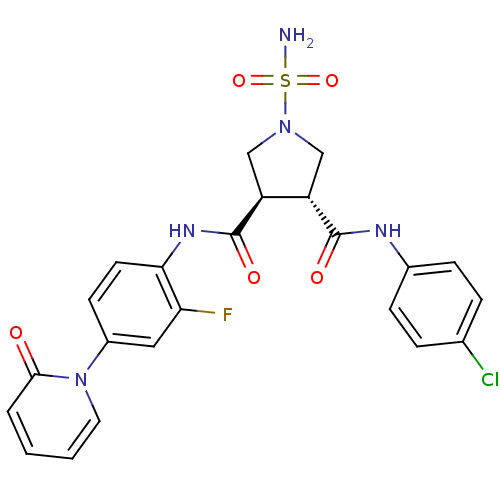 ((3R,4R)-1-SULFAMOYL-PYRROLIDINE-3,4-DICARBOXYLIC A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50295587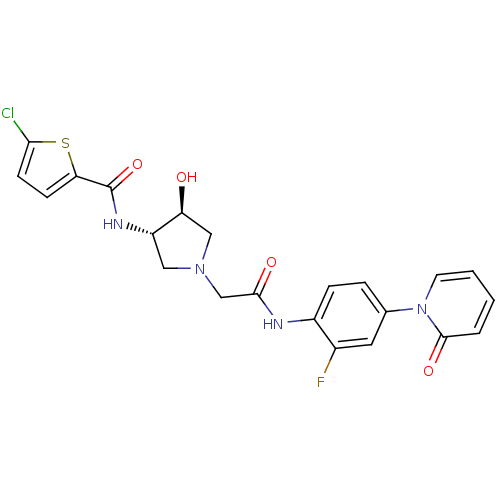 (5-Chloro-thiophene-2-carboxylic acid ((3S,4S)-1-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann - La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Eur J Med Chem 44: 2787-95 (2009) Article DOI: 10.1016/j.ejmech.2008.12.025 BindingDB Entry DOI: 10.7270/Q2CV4HSP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50295589 (5-chloro-N-((3S,4S)-4-ethoxy-1-(2-(2-fluoro-4-(2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann - La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Eur J Med Chem 44: 2787-95 (2009) Article DOI: 10.1016/j.ejmech.2008.12.025 BindingDB Entry DOI: 10.7270/Q2CV4HSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324758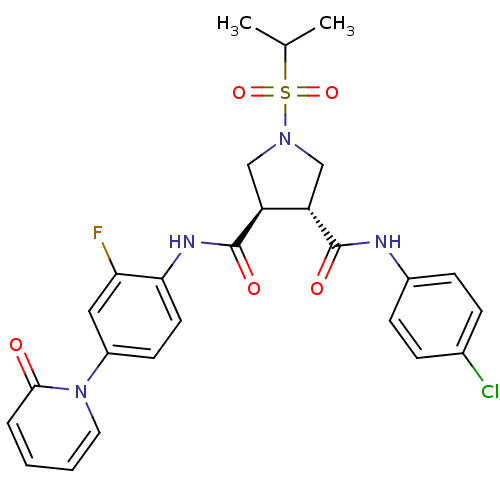 ((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210853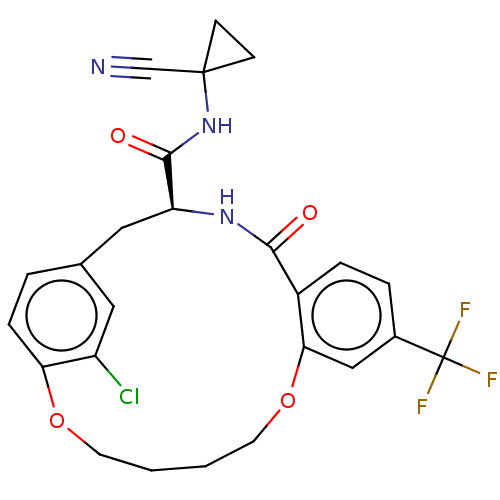 (US9290467, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324752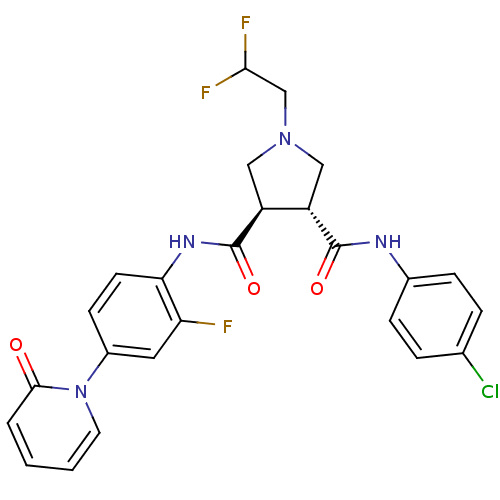 ((3R,4R)-N3-(4-chlorophenyl)-1-(2,2-difluoroethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324767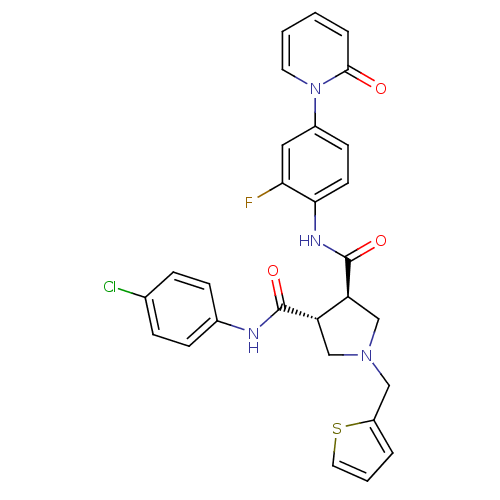 ((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324749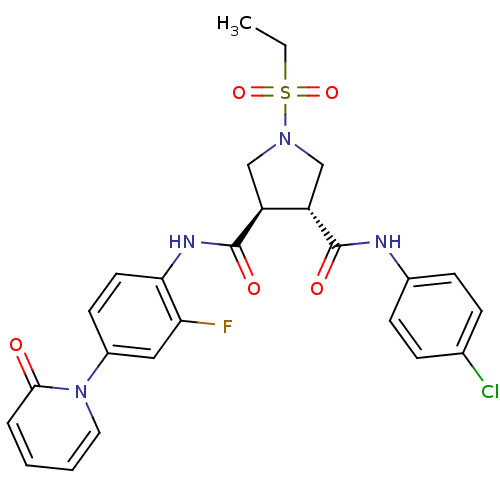 ((3R,4R)-N3-(4-chlorophenyl)-1-(ethylsulfonyl)-N4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM210853 (US9290467, 23) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50295584 (4-chloro-N-(2-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann - La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Eur J Med Chem 44: 2787-95 (2009) Article DOI: 10.1016/j.ejmech.2008.12.025 BindingDB Entry DOI: 10.7270/Q2CV4HSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50237034 (CHEMBL4061626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human Cathepsin L by fluorescence assay based Cheng-Prusoff equation analysis | J Med Chem 60: 2485-2497 (2017) Article DOI: 10.1021/acs.jmedchem.6b01881 BindingDB Entry DOI: 10.7270/Q2PV6NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50237035 (CHEMBL4099881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development (pRED) Curated by ChEMBL | Assay Description Inhibition of human Cathepsin L | J Med Chem 60: 2485-2497 (2017) Article DOI: 10.1021/acs.jmedchem.6b01881 BindingDB Entry DOI: 10.7270/Q2PV6NN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324787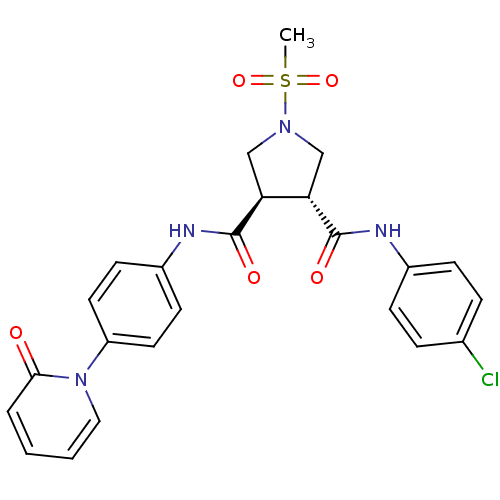 ((3R,4R)-N3-(4-chlorophenyl)-1-(methylsulfonyl)-N4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50295593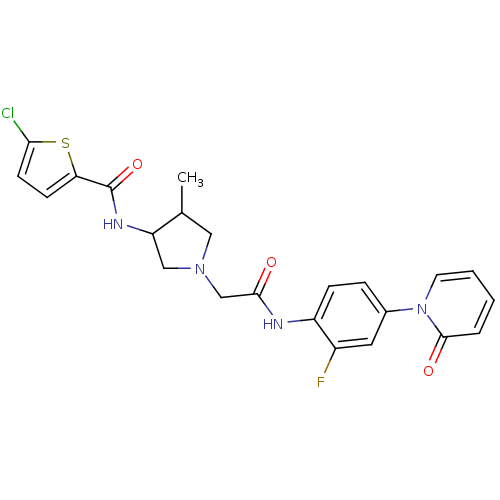 ((+/-)5-chloro-N-(1-(2-(2-fluoro-4-(2-oxopyridin-1(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann - La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Eur J Med Chem 44: 2787-95 (2009) Article DOI: 10.1016/j.ejmech.2008.12.025 BindingDB Entry DOI: 10.7270/Q2CV4HSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324744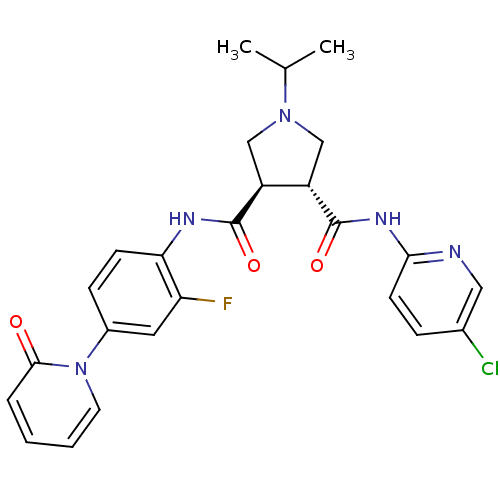 ((3R,4R)-N3-(5-chloropyridin-2-yl)-N4-(2-fluoro-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324746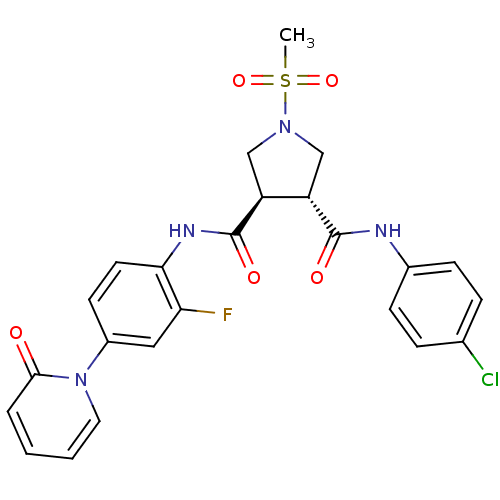 ((3R,4R)-1-METHANESULFONYL-PYRROLIDINE-3,4-DICARBOX...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324789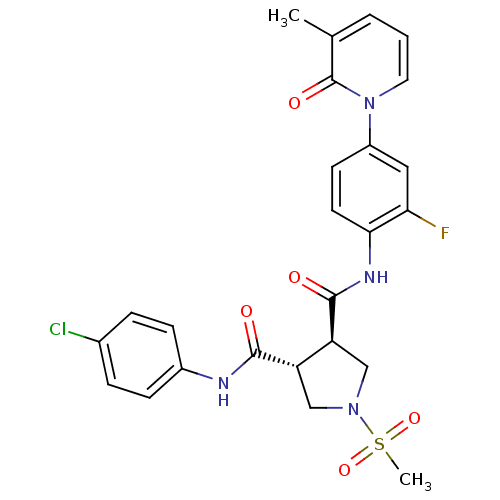 ((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263572 (CHEMBL4092050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263578 (CHEMBL4064172) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50324753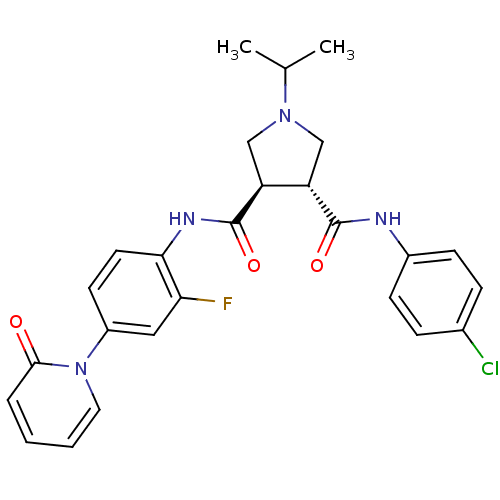 ((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50324745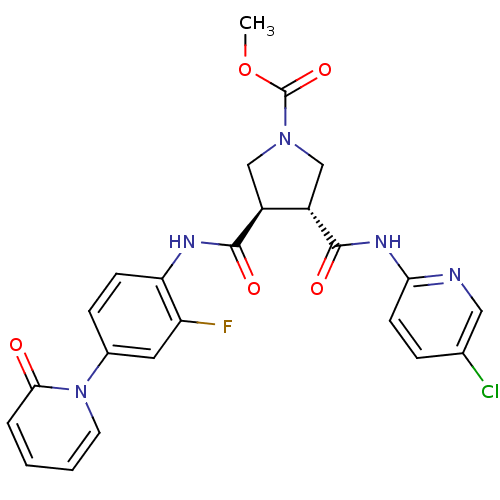 ((3R,4R)-methyl 3-(5-chloropyridin-2-ylcarbamoyl)-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to rabbit factor 10a | Bioorg Med Chem Lett 20: 5313-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.126 BindingDB Entry DOI: 10.7270/Q2542NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1598 total ) | Next | Last >> |