 Found 118 hits with Last Name = 'kassa' and Initial = 'j'
Found 118 hits with Last Name = 'kassa' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473
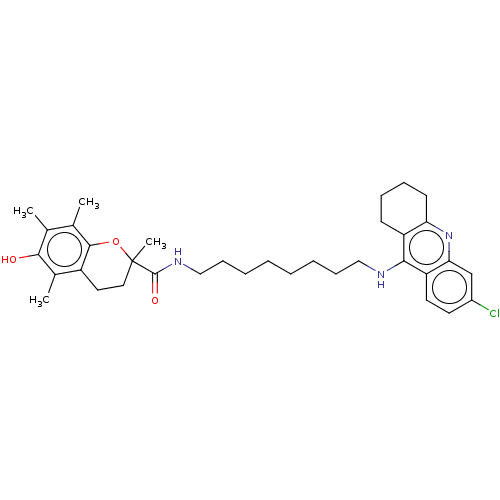
(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343062
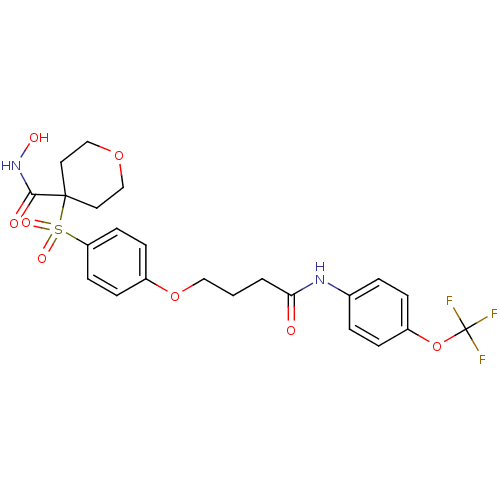
(CHEMBL1770709 | N-hydroxy-4-(4-(4-oxo-4-(4-(triflu...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H25F3N2O8S/c24-23(25,26)36-18-5-3-16(4-6-18)27-20(29)2-1-13-35-17-7-9-19(10-8-17)37(32,33)22(21(30)28-31)11-14-34-15-12-22/h3-10,31H,1-2,11-15H2,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343059

(4-(4-(4-(4-(dimethylamino)phenyl)-4-oxobutoxy)phen...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C24H30N2O7S/c1-26(2)19-7-5-18(6-8-19)22(27)4-3-15-33-20-9-11-21(12-10-20)34(30,31)24(23(28)25-29)13-16-32-17-14-24/h5-12,29H,3-4,13-17H2,1-2H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343061

(CHEMBL1770157 | N-hydroxy-4-(4-(4-(4-methoxyphenyl...)Show SMILES COc1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H28N2O8S/c1-31-18-6-4-17(5-7-18)24-21(26)3-2-14-33-19-8-10-20(11-9-19)34(29,30)23(22(27)25-28)12-15-32-16-13-23/h4-11,28H,2-3,12-16H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343051
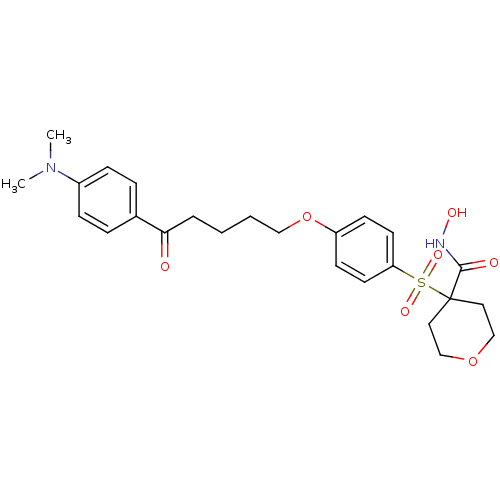
(4-(4-(5-(4-(dimethylamino)phenyl)-5-oxopentyloxy)p...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C25H32N2O7S/c1-27(2)20-8-6-19(7-9-20)23(28)5-3-4-16-34-21-10-12-22(13-11-21)35(31,32)25(24(29)26-30)14-17-33-18-15-25/h6-13,30H,3-5,14-18H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343054
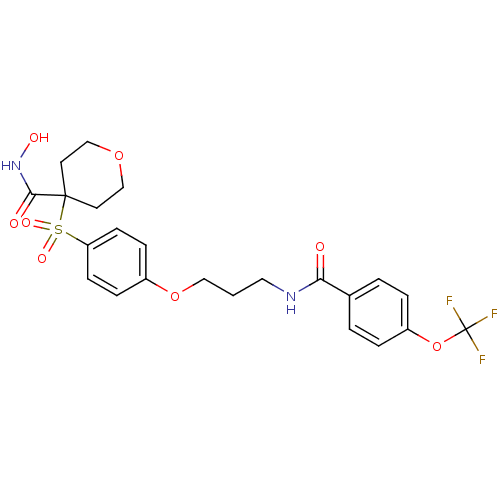
(CHEMBL1770702 | N-hydroxy-4-(4-(3-(4-(trifluoromet...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCNC(=O)c2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H25F3N2O8S/c24-23(25,26)36-18-4-2-16(3-5-18)20(29)27-12-1-13-35-17-6-8-19(9-7-17)37(32,33)22(21(30)28-31)10-14-34-15-11-22/h2-9,31H,1,10-15H2,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343066

(CHEMBL1770712 | N-hydroxy-4-(4-(4-(4-methoxyphenox...)Show SMILES COc1ccc(OCCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H29NO8S/c1-29-18-4-6-19(7-5-18)31-14-2-3-15-32-20-8-10-21(11-9-20)33(27,28)23(22(25)24-26)12-16-30-17-13-23/h4-11,26H,2-3,12-17H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343058
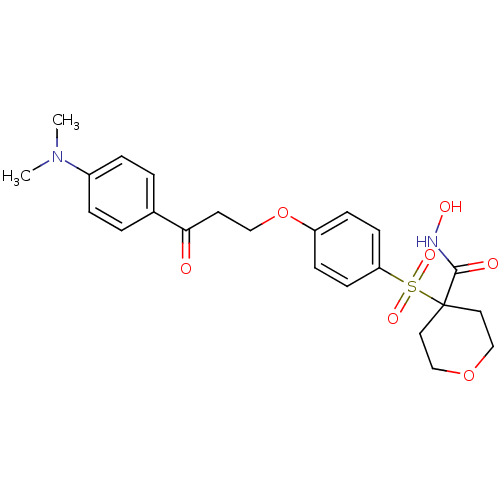
(4-(4-(3-(4-(dimethylamino)phenyl)-3-oxopropoxy)phe...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C23H28N2O7S/c1-25(2)18-5-3-17(4-6-18)21(26)11-14-32-19-7-9-20(10-8-19)33(29,30)23(22(27)24-28)12-15-31-16-13-23/h3-10,28H,11-16H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343064

(CHEMBL1770710 | N-hydroxy-4-(4-(4-oxo-4-(piperidin...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCC(=O)N2CCCCC2)cc1 Show InChI InChI=1S/C21H30N2O7S/c24-19(23-12-2-1-3-13-23)5-4-14-30-17-6-8-18(9-7-17)31(27,28)21(20(25)22-26)10-15-29-16-11-21/h6-9,26H,1-5,10-16H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343053
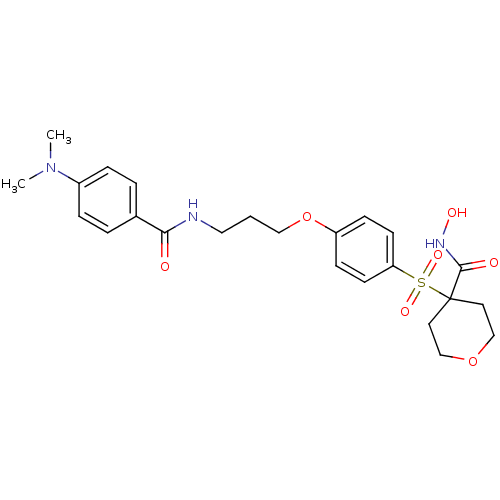
(4-(4-(3-(4-(dimethylamino)benzamido)propoxy)phenyl...)Show SMILES CN(C)c1ccc(cc1)C(=O)NCCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C24H31N3O7S/c1-27(2)19-6-4-18(5-7-19)22(28)25-14-3-15-34-20-8-10-21(11-9-20)35(31,32)24(23(29)26-30)12-16-33-17-13-24/h4-11,30H,3,12-17H2,1-2H3,(H,25,28)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343052
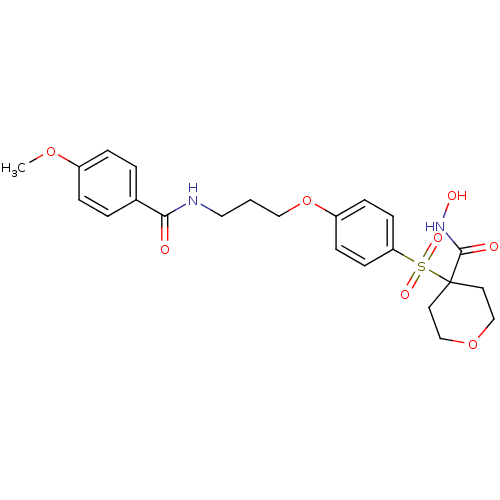
(CHEMBL1770700 | N-hydroxy-4-(4-(3-(4-methoxybenzam...)Show SMILES COc1ccc(cc1)C(=O)NCCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C23H28N2O8S/c1-31-18-5-3-17(4-6-18)21(26)24-13-2-14-33-19-7-9-20(10-8-19)34(29,30)23(22(27)25-28)11-15-32-16-12-23/h3-10,28H,2,11-16H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343060

(4-(4-(4-(4-(dimethylamino)phenylamino)-4-oxobutoxy...)Show SMILES CN(C)c1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H31N3O7S/c1-27(2)19-7-5-18(6-8-19)25-22(28)4-3-15-34-20-9-11-21(12-10-20)35(31,32)24(23(29)26-30)13-16-33-17-14-24/h5-12,30H,3-4,13-17H2,1-2H3,(H,25,28)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343065
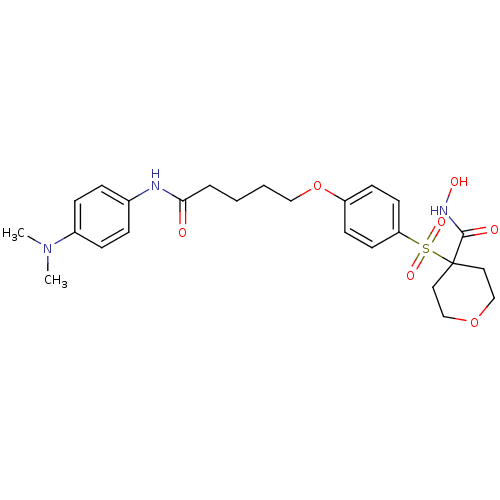
(4-(4-(5-(4-(dimethylamino)phenylamino)-5-oxopentyl...)Show SMILES CN(C)c1ccc(NC(=O)CCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C25H33N3O7S/c1-28(2)20-8-6-19(7-9-20)26-23(29)5-3-4-16-35-21-10-12-22(13-11-21)36(32,33)25(24(30)27-31)14-17-34-18-15-25/h6-13,31H,3-5,14-18H2,1-2H3,(H,26,29)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343065

(4-(4-(5-(4-(dimethylamino)phenylamino)-5-oxopentyl...)Show SMILES CN(C)c1ccc(NC(=O)CCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C25H33N3O7S/c1-28(2)20-8-6-19(7-9-20)26-23(29)5-3-4-16-35-21-10-12-22(13-11-21)36(32,33)25(24(30)27-31)14-17-34-18-15-25/h6-13,31H,3-5,14-18H2,1-2H3,(H,26,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343061

(CHEMBL1770157 | N-hydroxy-4-(4-(4-(4-methoxyphenyl...)Show SMILES COc1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H28N2O8S/c1-31-18-6-4-17(5-7-18)24-21(26)3-2-14-33-19-8-10-20(11-9-19)34(29,30)23(22(27)25-28)12-15-32-16-13-23/h4-11,28H,2-3,12-16H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50343065

(4-(4-(5-(4-(dimethylamino)phenylamino)-5-oxopentyl...)Show SMILES CN(C)c1ccc(NC(=O)CCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C25H33N3O7S/c1-28(2)20-8-6-19(7-9-20)26-23(29)5-3-4-16-35-21-10-12-22(13-11-21)36(32,33)25(24(30)27-31)14-17-34-18-15-25/h6-13,31H,3-5,14-18H2,1-2H3,(H,26,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343063

(CHEMBL1770697 | N-hydroxy-4-(4-(4-(methyl(4-(trifl...)Show SMILES CN(C(=O)CCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H27F3N2O8S/c1-29(17-4-6-19(7-5-17)37-24(25,26)27)21(30)3-2-14-36-18-8-10-20(11-9-18)38(33,34)23(22(31)28-32)12-15-35-16-13-23/h4-11,32H,2-3,12-16H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343066

(CHEMBL1770712 | N-hydroxy-4-(4-(4-(4-methoxyphenox...)Show SMILES COc1ccc(OCCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H29NO8S/c1-29-18-4-6-19(7-5-18)31-14-2-3-15-32-20-8-10-21(11-9-20)33(27,28)23(22(25)24-26)12-16-30-17-13-23/h4-11,26H,2-3,12-17H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50343062

(CHEMBL1770709 | N-hydroxy-4-(4-(4-oxo-4-(4-(triflu...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H25F3N2O8S/c24-23(25,26)36-18-5-3-16(4-6-18)27-20(29)2-1-13-35-17-7-9-19(10-8-17)37(32,33)22(21(30)28-31)11-14-34-15-12-22/h3-10,31H,1-2,11-15H2,(H,27,29)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343050
(4-(4-(4-(4-(dimethylamino)benzoyl)piperidin-1-yl)p...)Show SMILES CN(C)c1ccc(cc1)C(=O)C1CCN(CC1)c1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C26H33N3O6S/c1-28(2)21-5-3-19(4-6-21)24(30)20-11-15-29(16-12-20)22-7-9-23(10-8-22)36(33,34)26(25(31)27-32)13-17-35-18-14-26/h3-10,20,32H,11-18H2,1-2H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343055
(4-(4-(3-(2,4-dimethylbenzamido)propoxy)phenylsulfo...)Show SMILES Cc1ccc(C(=O)NCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)c(C)c1 Show InChI InChI=1S/C24H30N2O7S/c1-17-4-9-21(18(2)16-17)22(27)25-12-3-13-33-19-5-7-20(8-6-19)34(30,31)24(23(28)26-29)10-14-32-15-11-24/h4-9,16,29H,3,10-15H2,1-2H3,(H,25,27)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM11885

(CHEMBL1082381 | N-Hydroxy-4-{[4-(phenylthio)phenyl...)Show SMILES ONC(=O)C1(CCN(CC#C)CC1)S(=O)(=O)c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C21H22N2O4S2/c1-2-14-23-15-12-21(13-16-23,20(24)22-25)29(26,27)19-10-8-18(9-11-19)28-17-6-4-3-5-7-17/h1,3-11,25H,12-16H2,(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP14 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343056
(4-(4-(3-(cyclohexanecarboxamido)propoxy)phenylsulf...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCNC(=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H32N2O7S/c25-20(17-5-2-1-3-6-17)23-13-4-14-31-18-7-9-19(10-8-18)32(28,29)22(21(26)24-27)11-15-30-16-12-22/h7-10,17,27H,1-6,11-16H2,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50343057
(4-(4-(3-(2,2-dimethylpentanamido)propoxy)phenylsul...)Show SMILES CCCC(C)(C)C(=O)NCCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C22H34N2O7S/c1-4-10-21(2,3)19(25)23-13-5-14-31-17-6-8-18(9-7-17)32(28,29)22(20(26)24-27)11-15-30-16-12-22/h6-9,27H,4-5,10-16H2,1-3H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343060

(4-(4-(4-(4-(dimethylamino)phenylamino)-4-oxobutoxy...)Show SMILES CN(C)c1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H31N3O7S/c1-27(2)19-7-5-18(6-8-19)25-22(28)4-3-15-34-20-9-11-21(12-10-20)35(31,32)24(23(29)26-30)13-16-33-17-14-24/h5-12,30H,3-4,13-17H2,1-2H3,(H,25,28)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343062

(CHEMBL1770709 | N-hydroxy-4-(4-(4-oxo-4-(4-(triflu...)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(OCCCC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C23H25F3N2O8S/c24-23(25,26)36-18-5-3-16(4-6-18)27-20(29)2-1-13-35-17-7-9-19(10-8-17)37(32,33)22(21(30)28-31)11-14-34-15-12-22/h3-10,31H,1-2,11-15H2,(H,27,29)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343058

(4-(4-(3-(4-(dimethylamino)phenyl)-3-oxopropoxy)phe...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C23H28N2O7S/c1-25(2)18-5-3-17(4-6-18)21(26)11-14-32-19-7-9-20(10-8-19)33(29,30)23(22(27)24-28)12-15-31-16-13-23/h3-10,28H,11-16H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343059

(4-(4-(4-(4-(dimethylamino)phenyl)-4-oxobutoxy)phen...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C24H30N2O7S/c1-26(2)19-7-5-18(6-8-19)22(27)4-3-15-33-20-9-11-21(12-10-20)34(30,31)24(23(28)25-29)13-16-32-17-14-24/h5-12,29H,3-4,13-17H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133449
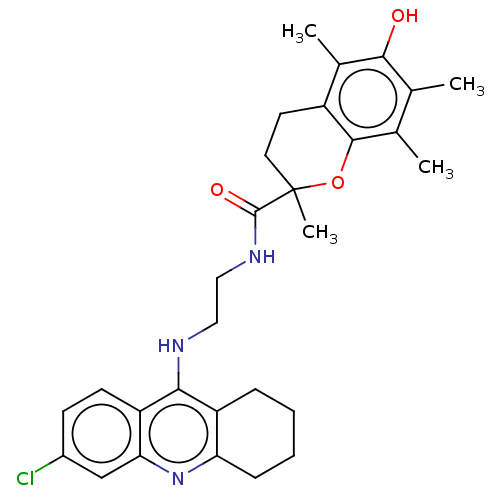
(CHEMBL3632988)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C29H34ClN3O3/c1-16-17(2)27-20(18(3)26(16)34)11-12-29(4,36-27)28(35)32-14-13-31-25-21-7-5-6-8-23(21)33-24-15-19(30)9-10-22(24)25/h9-10,15,34H,5-8,11-14H2,1-4H3,(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50343061

(CHEMBL1770157 | N-hydroxy-4-(4-(4-(4-methoxyphenyl...)Show SMILES COc1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H28N2O8S/c1-31-18-6-4-17(5-7-18)24-21(26)3-2-14-33-19-8-10-20(11-9-19)34(29,30)23(22(27)25-28)12-15-32-16-13-23/h4-11,28H,2-3,12-16H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50343061

(CHEMBL1770157 | N-hydroxy-4-(4-(4-(4-methoxyphenyl...)Show SMILES COc1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H28N2O8S/c1-31-18-6-4-17(5-7-18)24-21(26)3-2-14-33-19-8-10-20(11-9-19)34(29,30)23(22(27)25-28)12-15-32-16-13-23/h4-11,28H,2-3,12-16H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343051

(4-(4-(5-(4-(dimethylamino)phenyl)-5-oxopentyloxy)p...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C25H32N2O7S/c1-27(2)20-8-6-19(7-9-20)23(28)5-3-4-16-34-21-10-12-22(13-11-21)35(31,32)25(24(29)26-30)14-17-33-18-15-25/h6-13,30H,3-5,14-18H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50343063

(CHEMBL1770697 | N-hydroxy-4-(4-(4-(methyl(4-(trifl...)Show SMILES CN(C(=O)CCCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H27F3N2O8S/c1-29(17-4-6-19(7-5-17)37-24(25,26)27)21(30)3-2-14-36-18-8-10-20(11-9-18)38(33,34)23(22(31)28-32)12-15-35-16-13-23/h4-11,32H,2-3,12-16H2,1H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073117
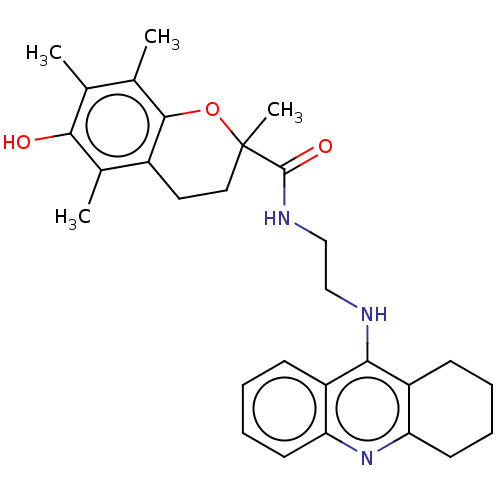
(CHEMBL3410951)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O3/c1-17-18(2)27-20(19(3)26(17)33)13-14-29(4,35-27)28(34)31-16-15-30-25-21-9-5-7-11-23(21)32-24-12-8-6-10-22(24)25/h5,7,9,11,33H,6,8,10,12-16H2,1-4H3,(H,30,32)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50343066

(CHEMBL1770712 | N-hydroxy-4-(4-(4-(4-methoxyphenox...)Show SMILES COc1ccc(OCCCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H29NO8S/c1-29-18-4-6-19(7-5-18)31-14-2-3-15-32-20-8-10-21(11-9-20)33(27,28)23(22(25)24-26)12-16-30-17-13-23/h4-11,26H,2-3,12-17H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073116
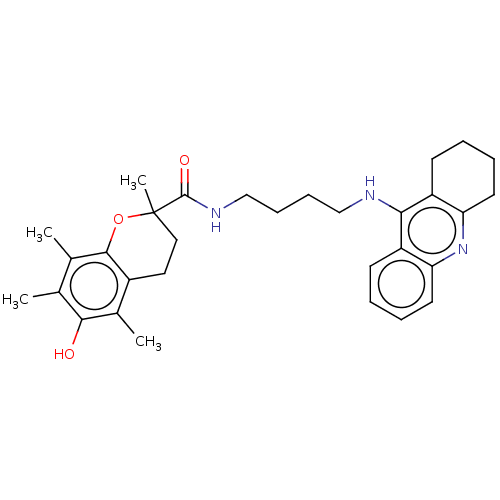
(CHEMBL3410952)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H39N3O3/c1-19-20(2)29-22(21(3)28(19)35)15-16-31(4,37-29)30(36)33-18-10-9-17-32-27-23-11-5-7-13-25(23)34-26-14-8-6-12-24(26)27/h5,7,11,13,35H,6,8-10,12,14-18H2,1-4H3,(H,32,34)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133472
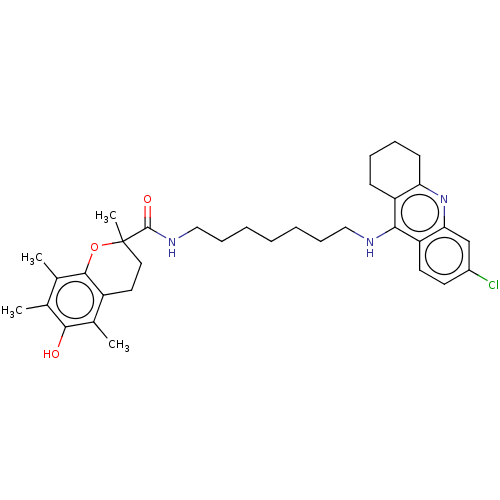
(CHEMBL3632993)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H44ClN3O3/c1-21-22(2)32-25(23(3)31(21)39)16-17-34(4,41-32)33(40)37-19-11-7-5-6-10-18-36-30-26-12-8-9-13-28(26)38-29-20-24(35)14-15-27(29)30/h14-15,20,39H,5-13,16-19H2,1-4H3,(H,36,38)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133450
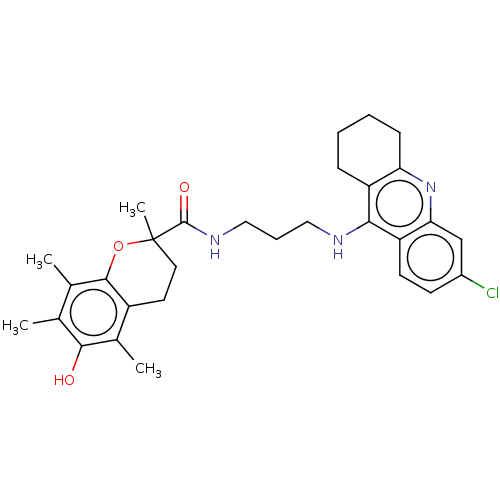
(CHEMBL3632989)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C30H36ClN3O3/c1-17-18(2)28-21(19(3)27(17)35)12-13-30(4,37-28)29(36)33-15-7-14-32-26-22-8-5-6-9-24(22)34-25-16-20(31)10-11-23(25)26/h10-11,16,35H,5-9,12-15H2,1-4H3,(H,32,34)(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133421
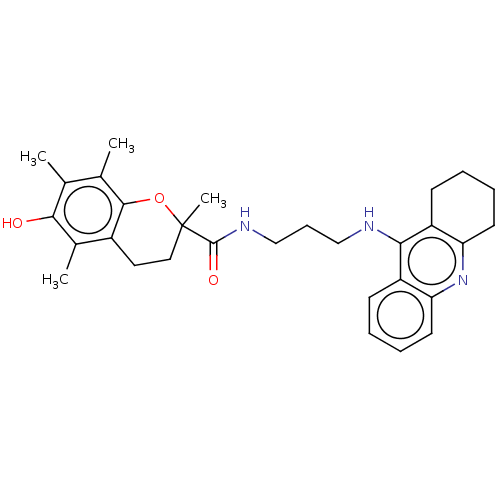
(CHEMBL3632987)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H37N3O3/c1-18-19(2)28-21(20(3)27(18)34)14-15-30(4,36-28)29(35)32-17-9-16-31-26-22-10-5-7-12-24(22)33-25-13-8-6-11-23(25)26/h5,7,10,12,34H,6,8-9,11,13-17H2,1-4H3,(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473

(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133469
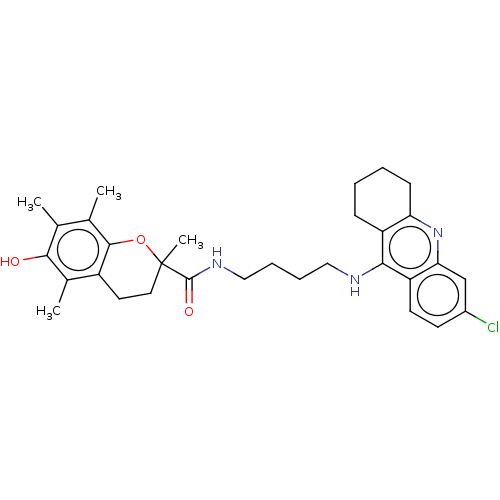
(CHEMBL3632990)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C31H38ClN3O3/c1-18-19(2)29-22(20(3)28(18)36)13-14-31(4,38-29)30(37)34-16-8-7-15-33-27-23-9-5-6-10-25(23)35-26-17-21(32)11-12-24(26)27/h11-12,17,36H,5-10,13-16H2,1-4H3,(H,33,35)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133471
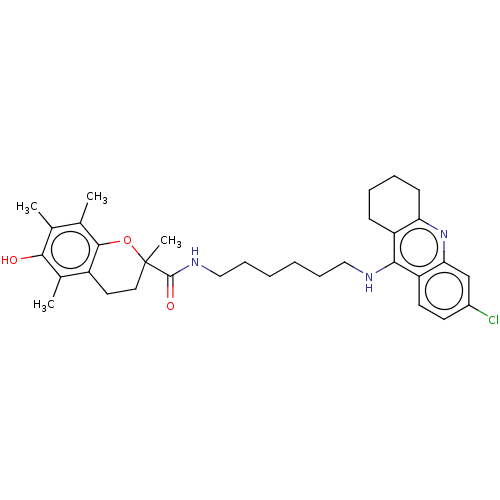
(CHEMBL3632992)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C33H42ClN3O3/c1-20-21(2)31-24(22(3)30(20)38)15-16-33(4,40-31)32(39)36-18-10-6-5-9-17-35-29-25-11-7-8-12-27(25)37-28-19-23(34)13-14-26(28)29/h13-14,19,38H,5-12,15-18H2,1-4H3,(H,35,37)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50343060

(4-(4-(4-(4-(dimethylamino)phenylamino)-4-oxobutoxy...)Show SMILES CN(C)c1ccc(NC(=O)CCCOc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H31N3O7S/c1-27(2)19-7-5-18(6-8-19)25-22(28)4-3-15-34-20-9-11-21(12-10-20)35(31,32)24(23(29)26-30)13-16-33-17-14-24/h5-12,30H,3-4,13-17H2,1-2H3,(H,25,28)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50343058

(4-(4-(3-(4-(dimethylamino)phenyl)-3-oxopropoxy)phe...)Show SMILES CN(C)c1ccc(cc1)C(=O)CCOc1ccc(cc1)S(=O)(=O)C1(CCOCC1)C(=O)NO Show InChI InChI=1S/C23H28N2O7S/c1-25(2)18-5-3-17(4-6-18)21(26)11-14-32-19-7-9-20(10-8-19)33(29,30)23(22(27)24-28)12-15-31-16-13-23/h3-10,28H,11-16H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
Bioorg Med Chem Lett 21: 2823-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.095
BindingDB Entry DOI: 10.7270/Q2CF9QD0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data