

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464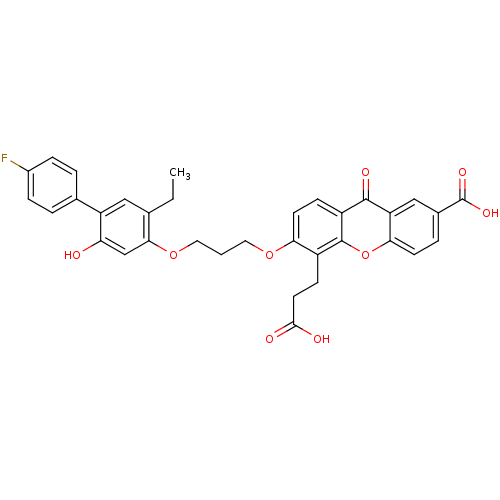 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029477 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-2-hydroxy-biphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889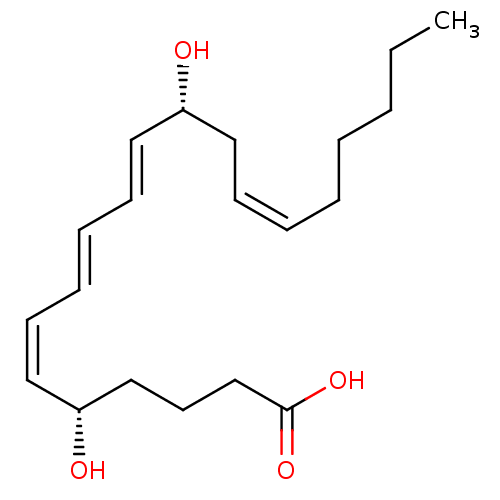 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029477 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-2-hydroxy-biphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029482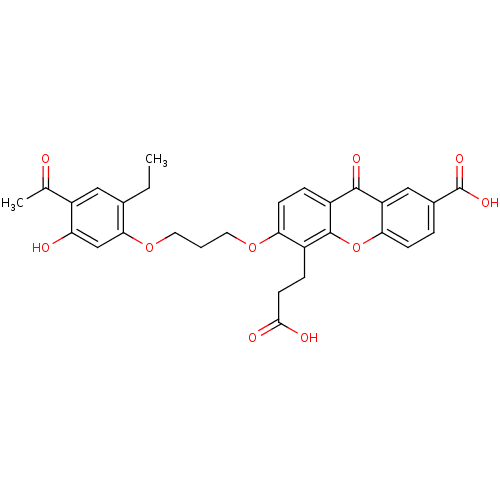 (6-[3-(4-Acetyl-2-ethyl-5-hydroxy-phenoxy)-propoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029482 (6-[3-(4-Acetyl-2-ethyl-5-hydroxy-phenoxy)-propoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029462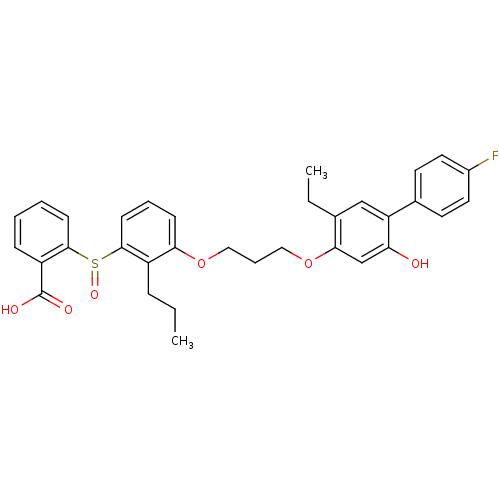 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50045092 (5-(2-Carboxy-ethyl)-6-[(E)-6-(4-methoxy-phenyl)-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition constant against binding of [3H]-LTB4 to human neutrophils | J Med Chem 36: 1726-34 (1993) BindingDB Entry DOI: 10.7270/Q2JQ1023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029448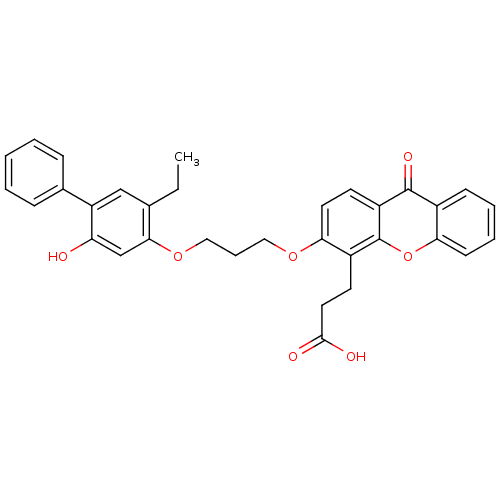 (3-{3-[3-(5-Ethyl-2-hydroxy-biphenyl-4-yloxy)-propo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029448 (3-{3-[3-(5-Ethyl-2-hydroxy-biphenyl-4-yloxy)-propo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029452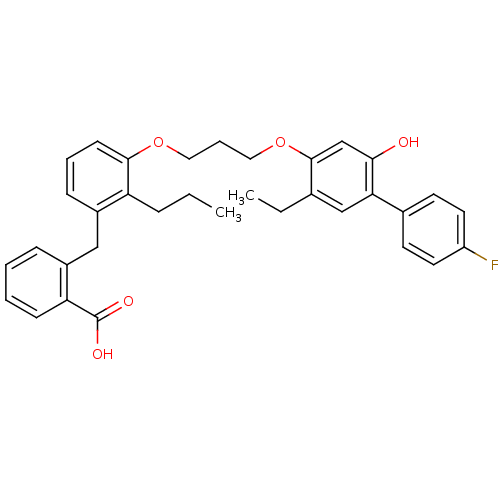 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029468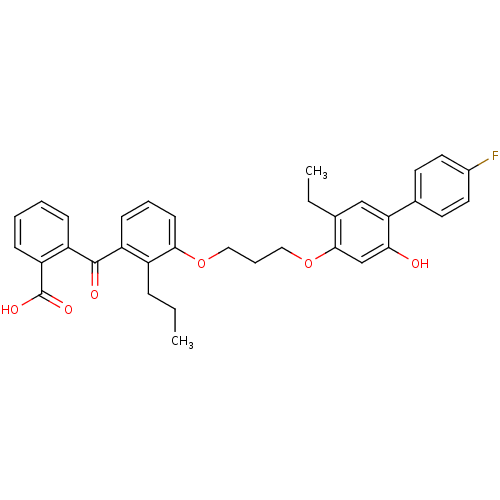 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029460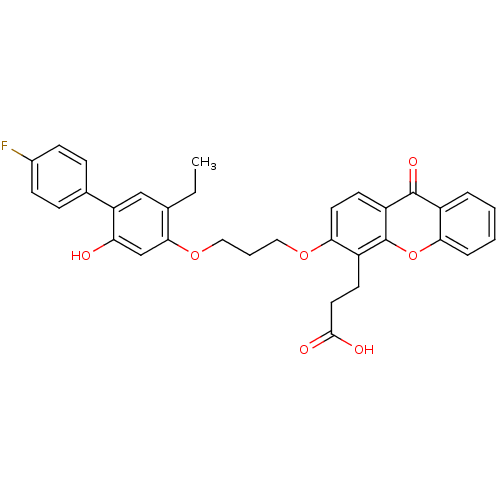 (3-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029467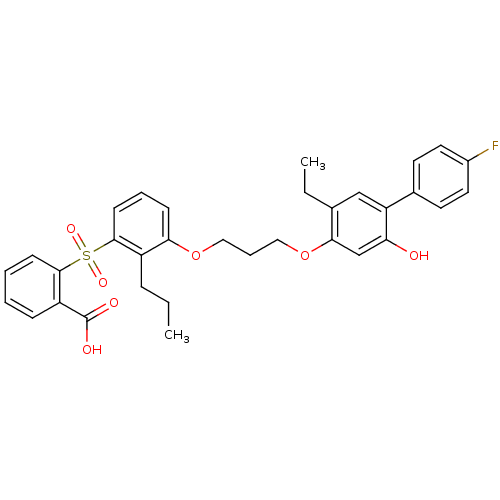 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029460 (3-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029484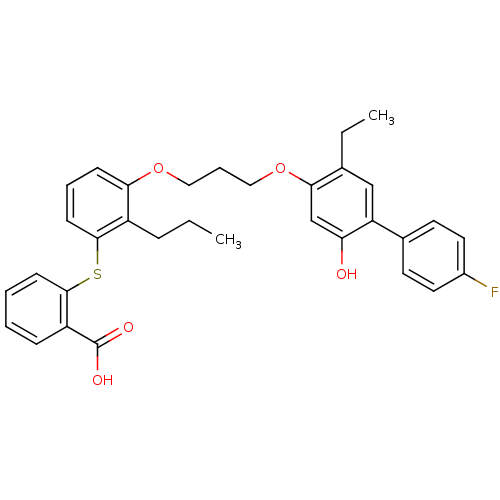 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132988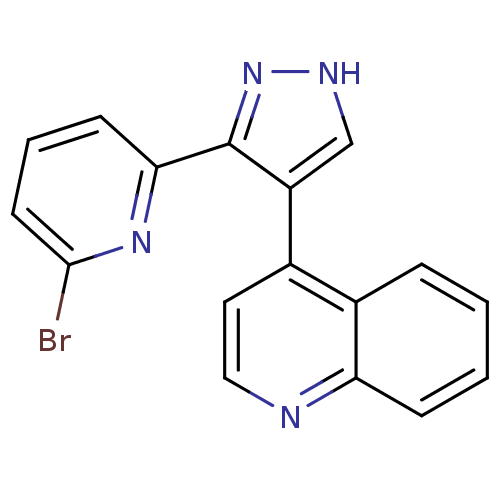 (4-[3-(6-Bromo-pyridin-2-yl)-1H-pyrazol-4-yl]-quino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50029464 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LTB4-induced up-regulation of human neutrophil CD11b/CD18 integrin | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM518497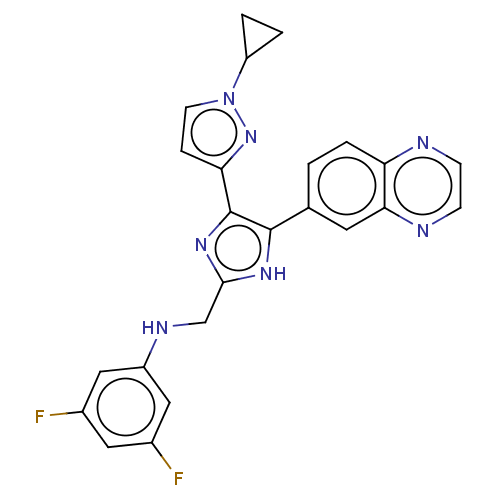 (US11124509, Compound 68) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplified compounds were screened in 1% DMSO (final concentration) by 3-fold serial dilutions from 10,000 nM to 0.316 nM to produce an IC50 or at s... | Citation and Details BindingDB Entry DOI: 10.7270/Q2474F1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50029460 (3-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611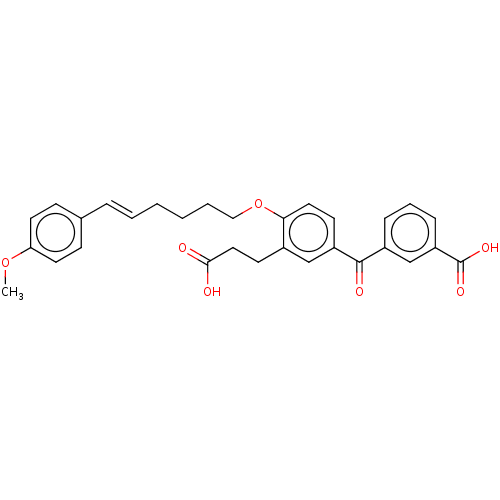 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-LTB4 to receptor on nonradioactive LTB4 | J Med Chem 36: 1726-34 (1993) BindingDB Entry DOI: 10.7270/Q2JQ1023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132988 (4-[3-(6-Bromo-pyridin-2-yl)-1H-pyrazol-4-yl]-quino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132989 (4-[3-(6-Methyl-pyridin-2-yl)-1H-pyrazol-4-yl]-quin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161301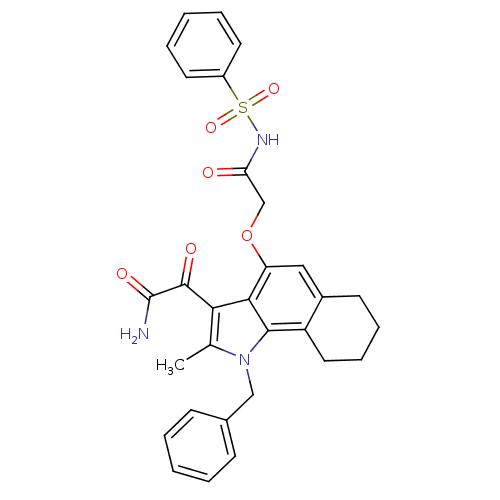 (2-[4-(2-Benzenesulfonylamino-2-oxo-ethoxy)-1-benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM518501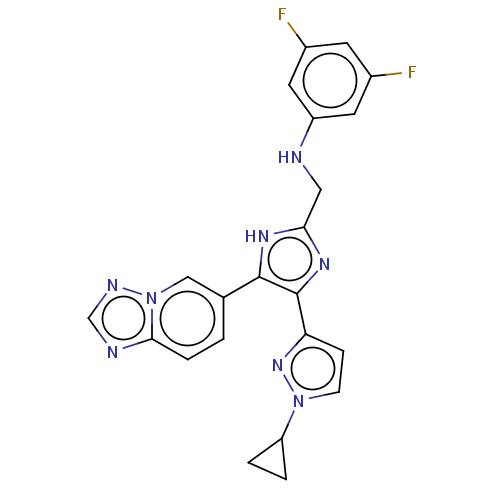 (US11124509, Compound 69) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplified compounds were screened in 1% DMSO (final concentration) by 3-fold serial dilutions from 10,000 nM to 0.316 nM to produce an IC50 or at s... | Citation and Details BindingDB Entry DOI: 10.7270/Q2474F1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50029477 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-2-hydroxy-biphen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161305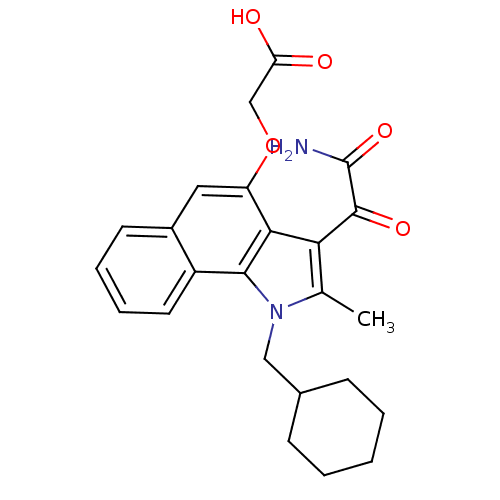 ((3-Aminooxalyl-1-cyclohexylmethyl-2-methyl-2,3-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM518482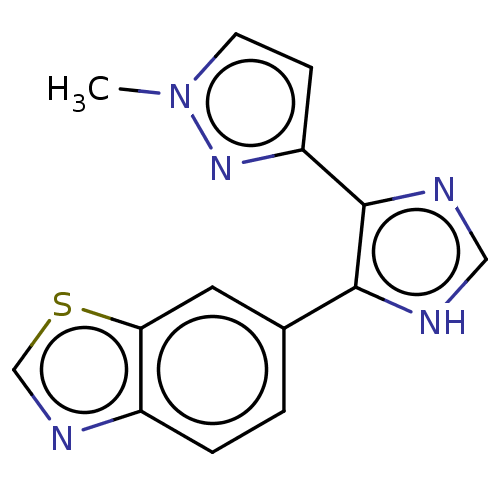 (US11124509, Compound 55) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplified compounds were screened in 1% DMSO (final concentration) by 3-fold serial dilutions from 10,000 nM to 0.316 nM to produce an IC50 or at s... | Citation and Details BindingDB Entry DOI: 10.7270/Q2474F1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50029448 (3-{3-[3-(5-Ethyl-2-hydroxy-biphenyl-4-yloxy)-propo...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM518474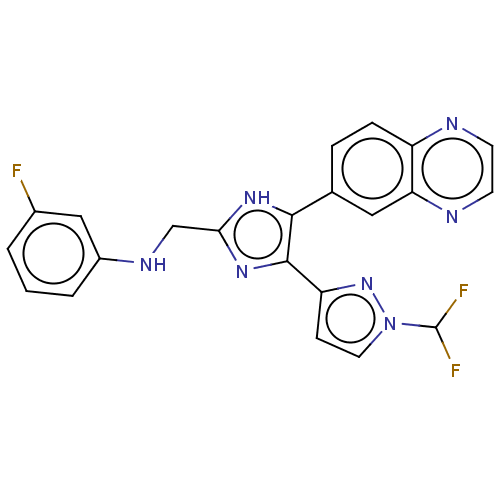 (US11124509, Compound 46) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplified compounds were screened in 1% DMSO (final concentration) by 3-fold serial dilutions from 10,000 nM to 0.316 nM to produce an IC50 or at s... | Citation and Details BindingDB Entry DOI: 10.7270/Q2474F1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM518465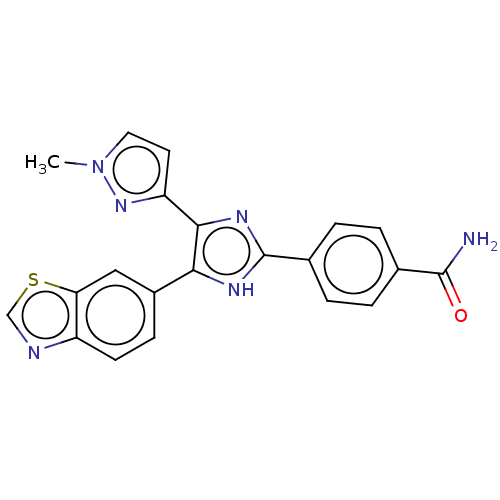 (US11124509, Compound 37) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplified compounds were screened in 1% DMSO (final concentration) by 3-fold serial dilutions from 10,000 nM to 0.316 nM to produce an IC50 or at s... | Citation and Details BindingDB Entry DOI: 10.7270/Q2474F1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161293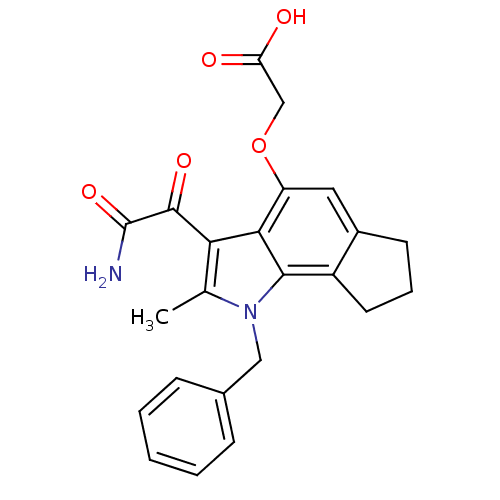 ((3-Aminooxalyl-1-benzyl-2-methyl-1,6,7,8-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50045092 (5-(2-Carboxy-ethyl)-6-[(E)-6-(4-methoxy-phenyl)-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of specific binding of LTB4 ( 0.1 nM) to receptors on intact human neutrophils | J Med Chem 36: 1726-34 (1993) BindingDB Entry DOI: 10.7270/Q2JQ1023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM518475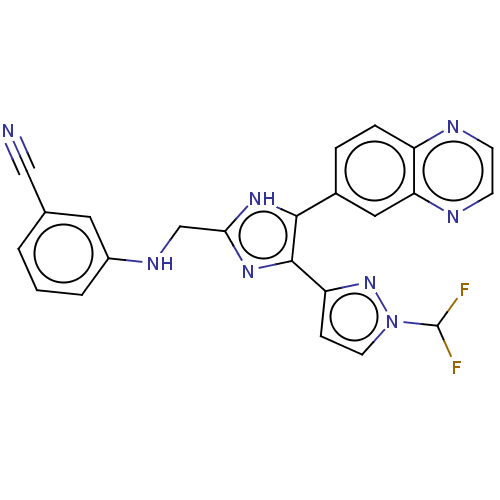 (US11124509, Compound 47) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplified compounds were screened in 1% DMSO (final concentration) by 3-fold serial dilutions from 10,000 nM to 0.316 nM to produce an IC50 or at s... | Citation and Details BindingDB Entry DOI: 10.7270/Q2474F1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM518472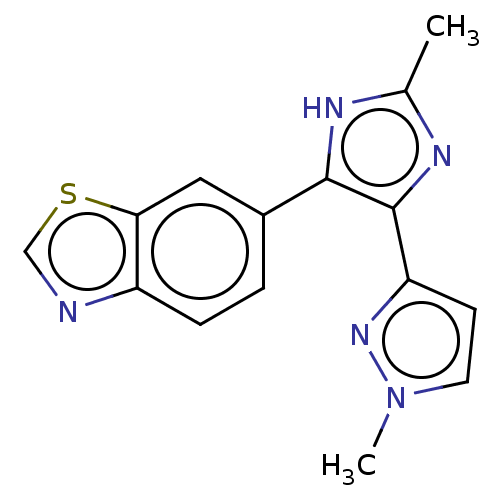 (US11124509, Compound 44) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplified compounds were screened in 1% DMSO (final concentration) by 3-fold serial dilutions from 10,000 nM to 0.316 nM to produce an IC50 or at s... | Citation and Details BindingDB Entry DOI: 10.7270/Q2474F1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132986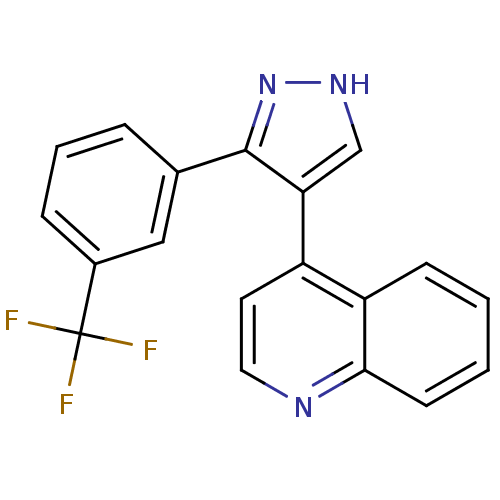 (4-(3-(3-(trifluoromethyl)phenyl)-1H-pyrazol-4-yl)q...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Mitogen-activated protein kinase p38 | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161299 ((3-Aminooxalyl-1-benzyl-2-ethyl-1,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161301 (2-[4-(2-Benzenesulfonylamino-2-oxo-ethoxy)-1-benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132989 (4-[3-(6-Methyl-pyridin-2-yl)-1H-pyrazol-4-yl]-quin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM518488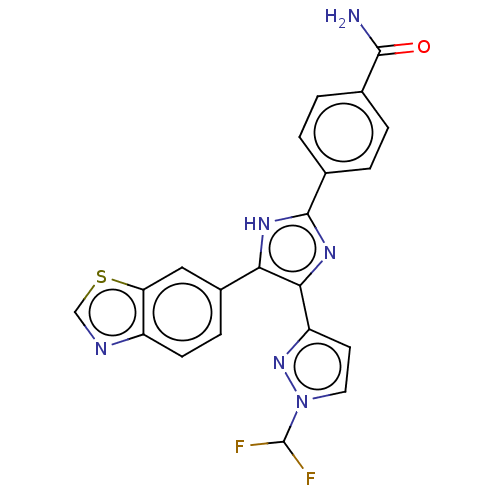 (US11124509, Compound 61) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exemplified compounds were screened in 1% DMSO (final concentration) by 3-fold serial dilutions from 10,000 nM to 0.316 nM to produce an IC50 or at s... | Citation and Details BindingDB Entry DOI: 10.7270/Q2474F1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161308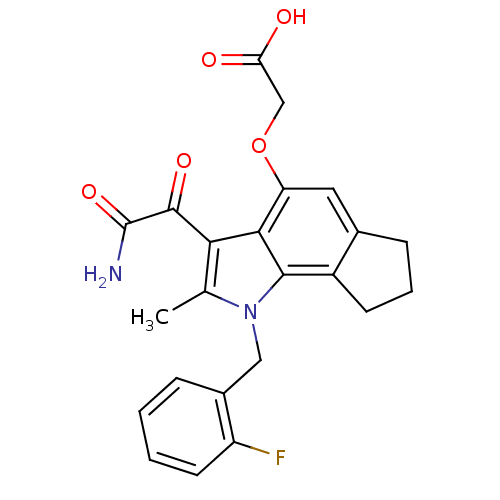 (CHEMBL179118 | [3-Aminooxalyl-1-(2-fluoro-benzyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161298 ((3-Aminooxalyl-1-benzyl-2-methyl-2,3,6,7,8,9-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161293 ((3-Aminooxalyl-1-benzyl-2-methyl-1,6,7,8-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161294 (CHEMBL179966 | [3-Aminooxalyl-1-(3-fluoro-benzyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161296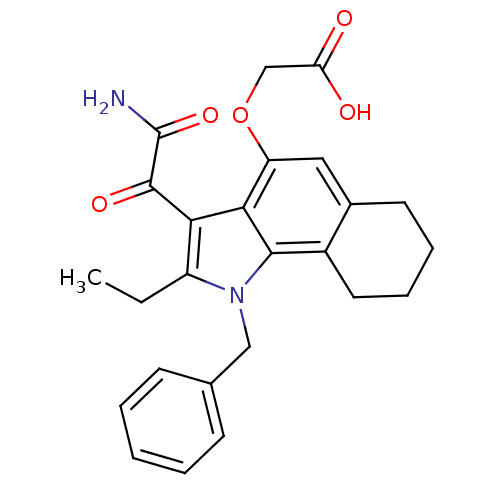 ((3-Aminooxalyl-1-benzyl-2-ethyl-2,3,6,7,8,9-hexahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161305 ((3-Aminooxalyl-1-cyclohexylmethyl-2-methyl-2,3-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161299 ((3-Aminooxalyl-1-benzyl-2-ethyl-1,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 215 total ) | Next | Last >> |