

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50568282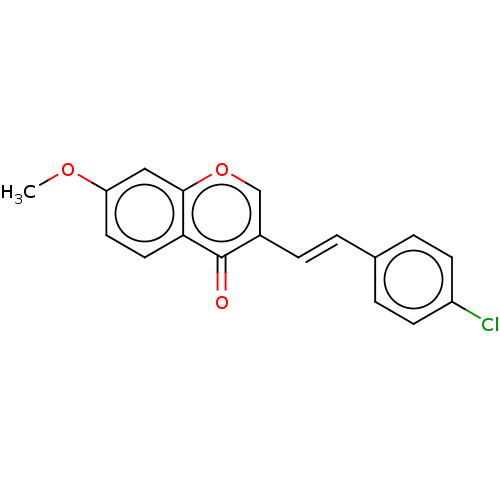 (CHEMBL4847545) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of human recombinant MAO-B by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2021.116255 BindingDB Entry DOI: 10.7270/Q2Z89H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171475 (CHEMBL190288 | {3-[2-(Diphenyl-ethanesulfonyl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171478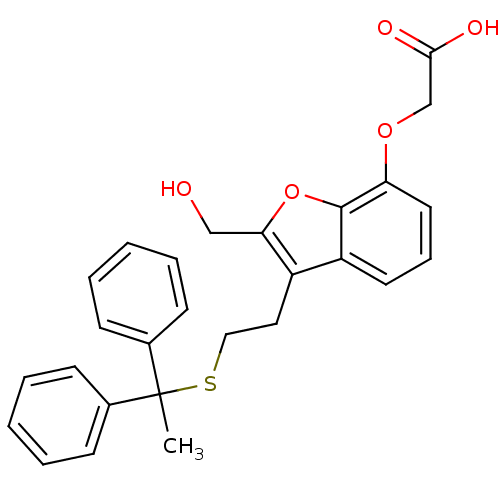 (CHEMBL190236 | {3-[2-(1,1-Diphenyl-ethylsulfanyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332808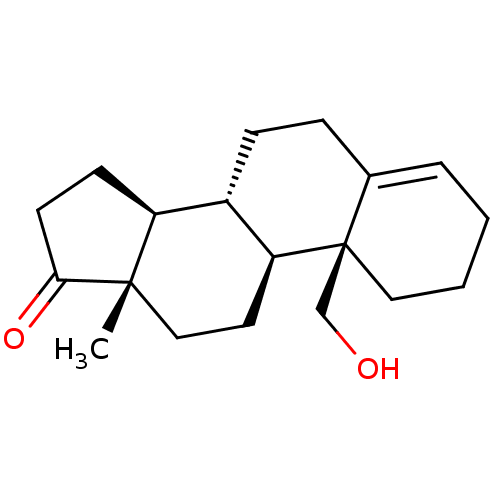 ((8R,9S,10S,13S,14S)-10-(hydroxymethyl)-13-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 1 uM [1-beta-3H]-androstenedione binding to human placental microsome Cytochrome P450 19A1 | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332807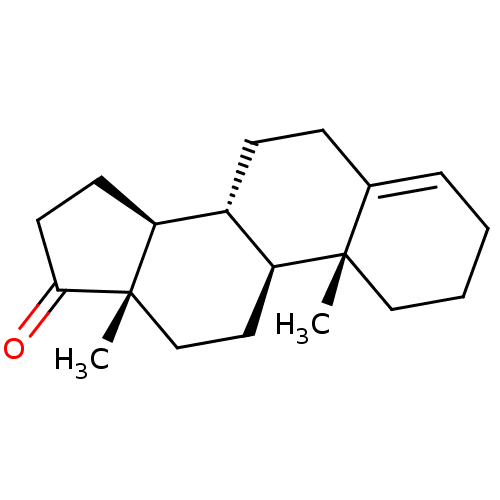 ((8R,9S,10R,13S,14S)-10,13-dimethyl-2,3,7,8,9,10,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50568281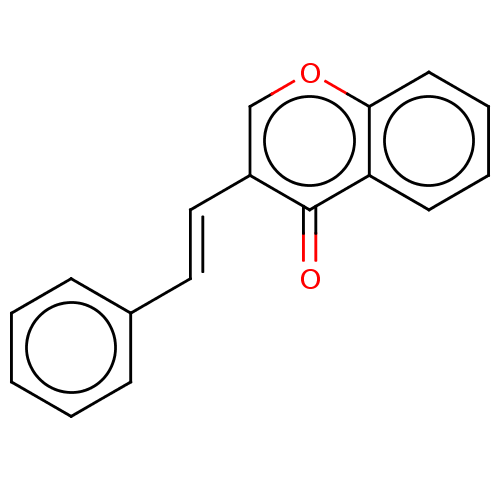 (CHEMBL1241163) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of human recombinant MAO-B by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2021.116255 BindingDB Entry DOI: 10.7270/Q2Z89H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025428 (10,13-Dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for aromatase cytochrome P45019A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171477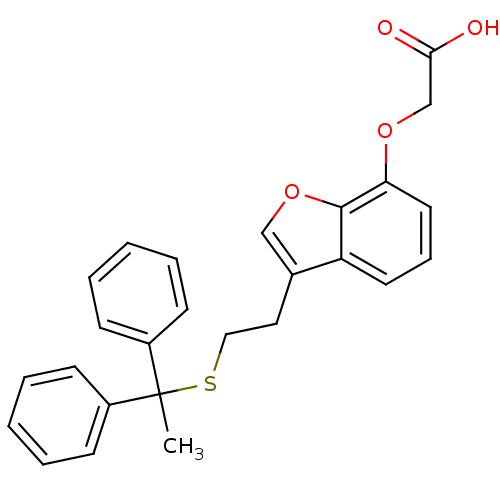 (CHEMBL191056 | {3-[2-(1,1-Diphenyl-ethylsulfanyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332807 ((8R,9S,10R,13S,14S)-10,13-dimethyl-2,3,7,8,9,10,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome cytochrome P450 19A1 with 1 uM [1-beta-3H]-androstenedione | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171483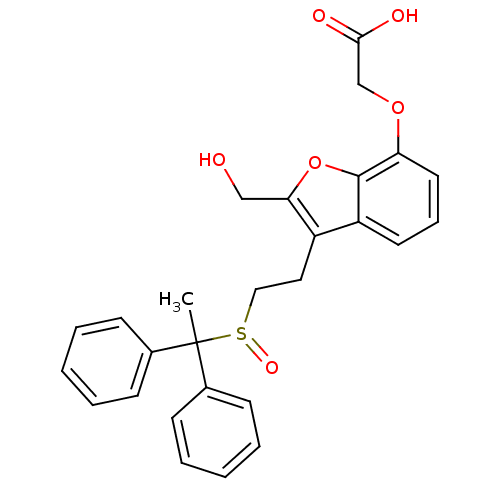 (CHEMBL365501 | {3-[2-(Diphenyl-ethanesulfinyl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039154 (10,13-Dimethyl-1,2,7,8,9,10,11,12,13,14,15,16-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171472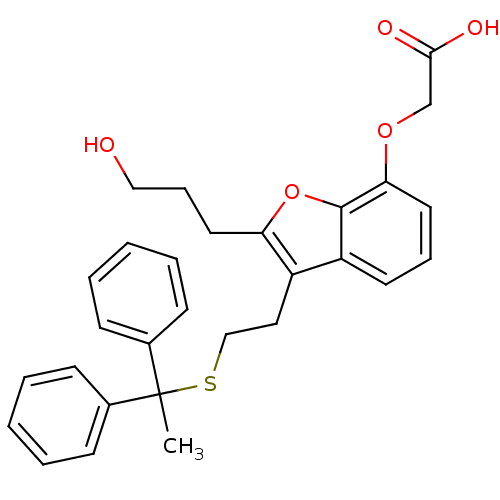 (CHEMBL190840 | [3-[2-(1,1-Diphenyl-ethylsulfanyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171474 (CHEMBL192662 | [3-[2-(1,1-Diphenyl-ethylsulfanyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171466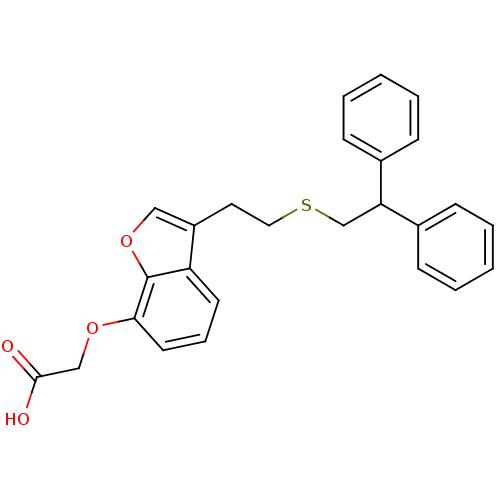 (CHEMBL361939 | {3-[2-(2,2-Diphenyl-ethylsulfanyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171466 (CHEMBL361939 | {3-[2-(2,2-Diphenyl-ethylsulfanyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171465 (CHEMBL190708 | [3-(2-Benzhydrylsulfanyl-ethyl)-2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50422015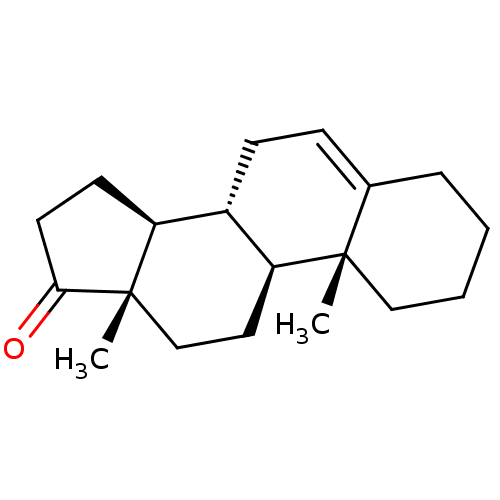 (CHEMBL2311169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039160 (13-Methyl-1,6,7,8,9,10,11,12,13,14,15,16-dodecahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for aromatase cytochrome P45019A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171480 (CHEMBL191118 | {2-Hydroxymethyl-3-[2-(2,2,2-triflu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039148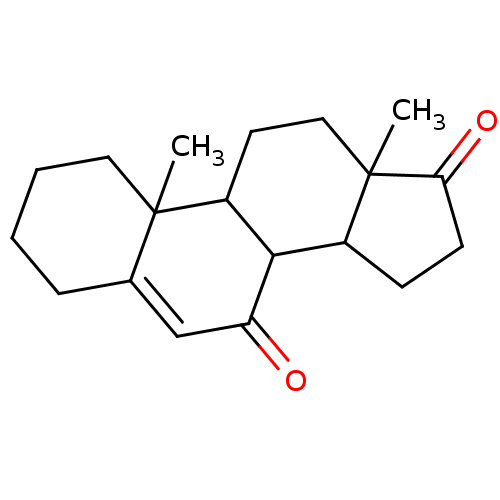 (10,13-Dimethyl-1,3,4,8,9,10,11,12,13,14,15,16-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Time dependent inactivation of Cytochrome P450 19A1 was obtained by kitz-wilson plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50009437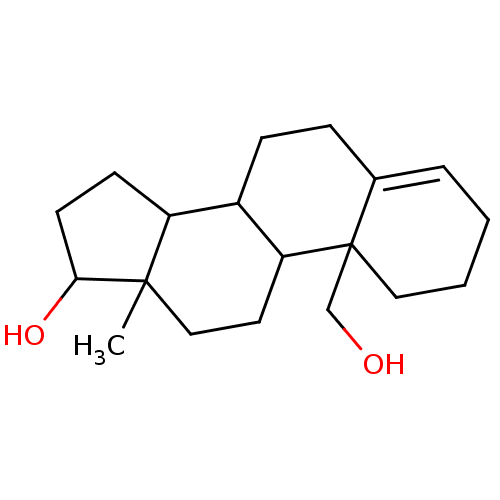 (10-Hydroxymethyl-13-methyl-2,3,6,7,8,9,10,11,12,13...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 1 uM [1-beta-3H]-androstenedione binding to human placental microsome Cytochrome P450 19A1 | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171479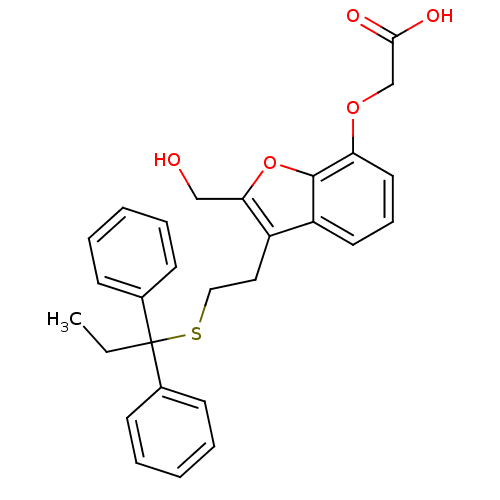 (CHEMBL189694 | {3-[2-(1,1-Diphenyl-propylsulfanyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039158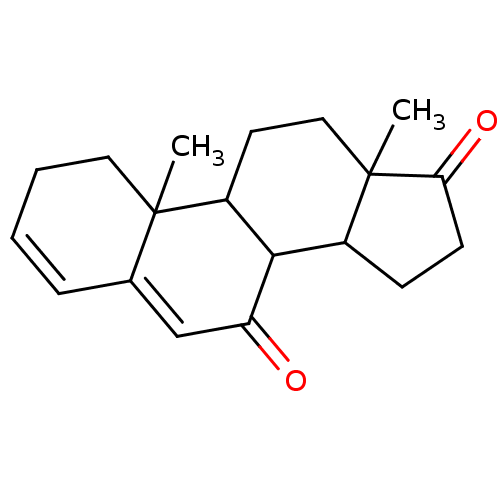 (10,13-Dimethyl-1,8,9,10,11,12,13,14,15,16-decahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Time dependent inactivation of Cytochrome P450 19A1 was obtained by kitz-wilson plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171474 (CHEMBL192662 | [3-[2-(1,1-Diphenyl-ethylsulfanyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039158 (10,13-Dimethyl-1,8,9,10,11,12,13,14,15,16-decahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50009430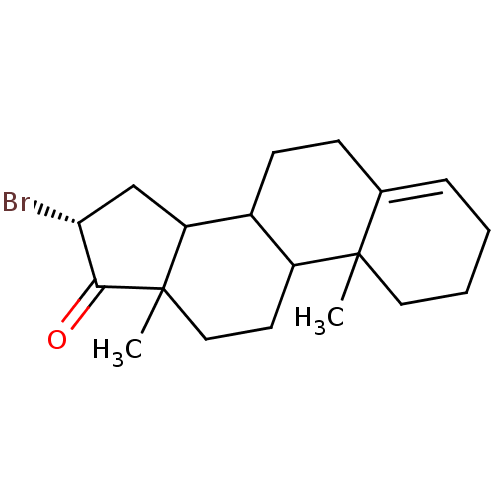 (16-Bromo-10,13-dimethyl-1,2,3,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome cytochrome P450 19A1 with 1 uM [1-beta-3H]-androstenedione | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039148 (10,13-Dimethyl-1,3,4,8,9,10,11,12,13,14,15,16-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171473 (CHEMBL187758 | [3-(3-Benzhydrylsulfanyl-propyl)-be...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50009436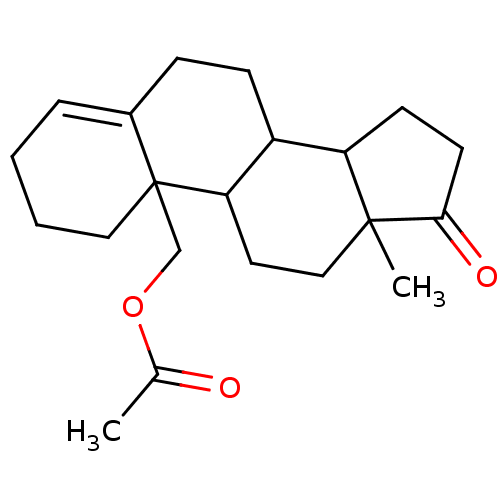 (Acetic acid 13-methyl-17-oxo-1,2,3,6,7,8,9,11,12,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome cytochrome P450 19A1 with 1 uM [1-beta-3H]-androstenedione | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171469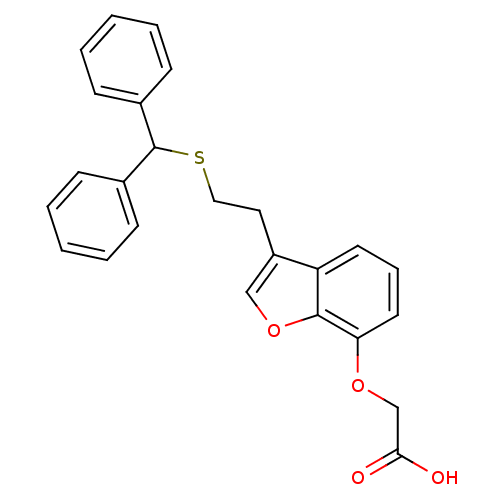 (CHEMBL188384 | [3-(2-Benzhydrylsulfanyl-ethyl)-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171484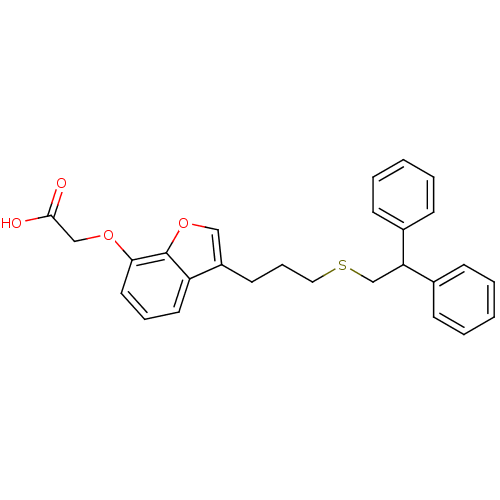 (CHEMBL188758 | {3-[3-(2,2-Diphenyl-ethylsulfanyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171469 (CHEMBL188384 | [3-(2-Benzhydrylsulfanyl-ethyl)-ben...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50009435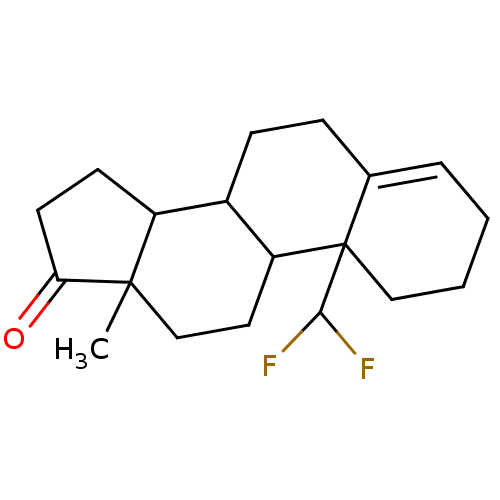 (10-Difluoromethyl-13-methyl-1,2,3,6,7,8,9,10,11,12...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 443 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome cytochrome P450 19A1 with 1 uM [1-beta-3H]-androstenedione | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50009431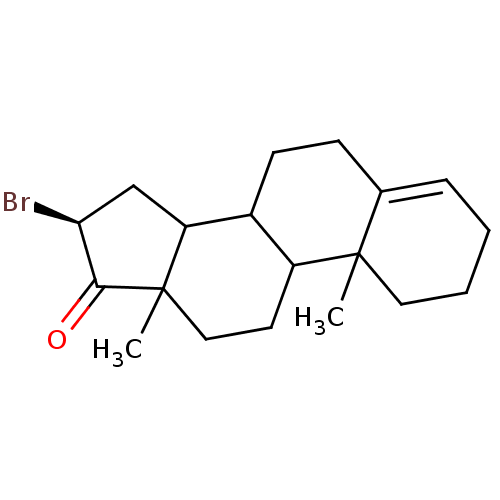 (16-Bromo-10,13-dimethyl-1,2,3,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome cytochrome P450 19A1 with 1 uM [1-beta-3H]-androstenedione | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171478 (CHEMBL190236 | {3-[2-(1,1-Diphenyl-ethylsulfanyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171482 (CHEMBL190735 | {3-[2-(3,3-Diphenyl-propylsulfanyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171477 (CHEMBL191056 | {3-[2-(1,1-Diphenyl-ethylsulfanyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171471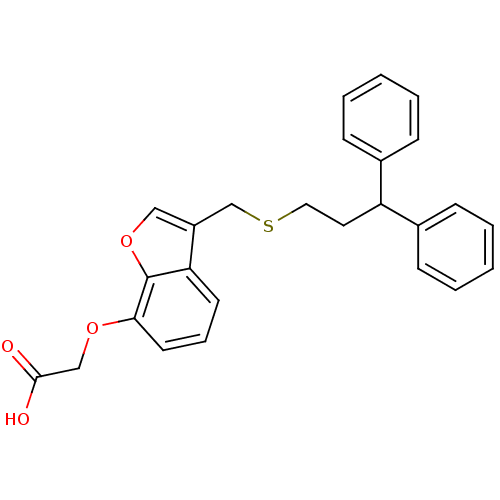 (CHEMBL189063 | [3-(3,3-Diphenyl-propylsulfanylmeth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171464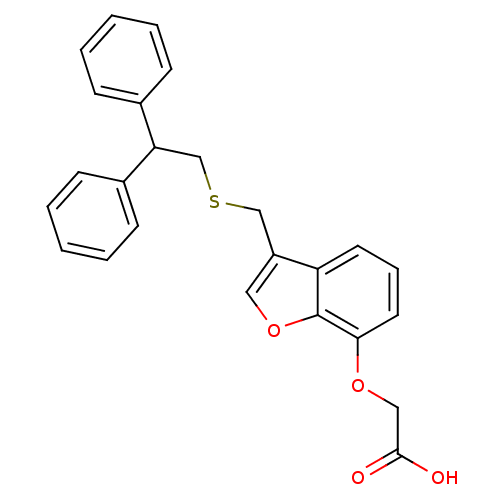 (CHEMBL187544 | [3-(2,2-Diphenyl-ethylsulfanylmethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171465 (CHEMBL190708 | [3-(2-Benzhydrylsulfanyl-ethyl)-2-h...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171480 (CHEMBL191118 | {2-Hydroxymethyl-3-[2-(2,2,2-triflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50009434 (10,13-Dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for human placental microsome cytochrome P450 19A1 with 1 uM [1-beta-3H]-androstenedione | J Med Chem 34: 2496-504 (1991) BindingDB Entry DOI: 10.7270/Q2MG7Q45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332833 ((8R,9S,10S,13S,14S)-10-(hydroxymethyl)-13-methyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039151 (13-Methyl-17-oxo-1,2,3,4,7,8,9,11,12,13,14,15,16,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for aromatase cytochrome P45019A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039155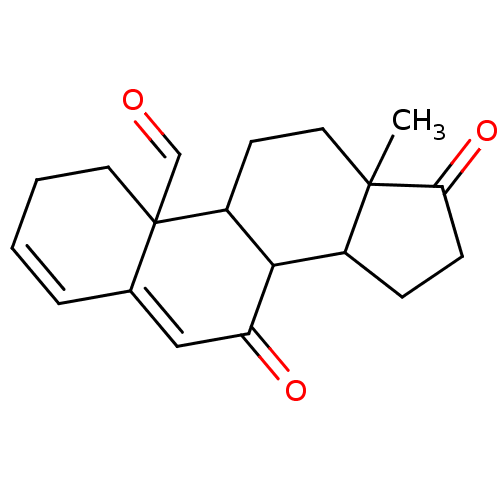 (13-Methyl-7,17-dioxo-1,2,7,8,9,11,12,13,14,15,16,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171475 (CHEMBL190288 | {3-[2-(Diphenyl-ethanesulfonyl)-eth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039161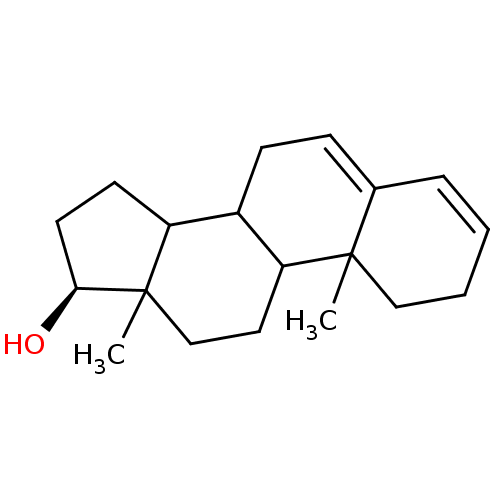 ((S)-10,13-Dimethyl-2,7,8,9,10,11,12,13,14,15,16,17...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50171470 (CHEMBL191162 | {3-[2-(1,1-Diphenyl-butylsulfanyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-APS-314d binding to prostacyclin receptors (IP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50039150 (13-Methyl-1,3,4,8,9,10,11,12,13,14,15,16-dodecahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50171464 (CHEMBL187544 | [3-(2,2-Diphenyl-ethylsulfanylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]-SQ-29,548 binding to thromboxane A2 receptors (TP) of human platelet membrane | J Med Chem 48: 5279-94 (2005) Article DOI: 10.1021/jm050194z BindingDB Entry DOI: 10.7270/Q2J67GGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 221 total ) | Next | Last >> |