 Found 468 hits with Last Name = 'ozaki' and Initial = 'k'
Found 468 hits with Last Name = 'ozaki' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dioxygenase
(Pseudomonas paucimobilis) | BDBM50111620
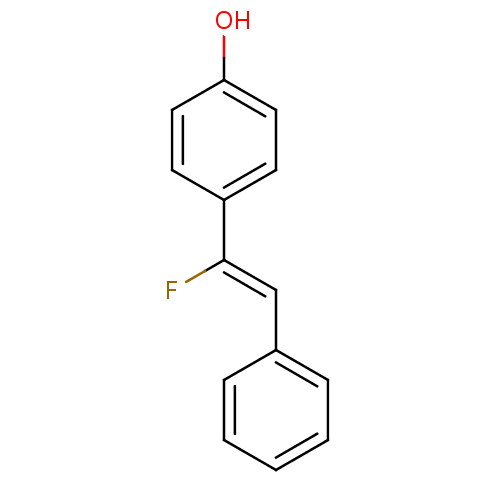
(4-((Z)-1-Fluoro-2-phenyl-vinyl)-phenol | CHEMBL147...)Show InChI InChI=1S/C14H11FO/c15-14(10-11-4-2-1-3-5-11)12-6-8-13(16)9-7-12/h1-10,16H/b14-10- | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN
Curated by ChEMBL
| Assay Description
Tested for the inhibition of Lignostilbene-alpha, beta-dioxygenase (LSD) |
Bioorg Med Chem Lett 12: 1139-42 (2002)
BindingDB Entry DOI: 10.7270/Q2KS6QV0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50103533
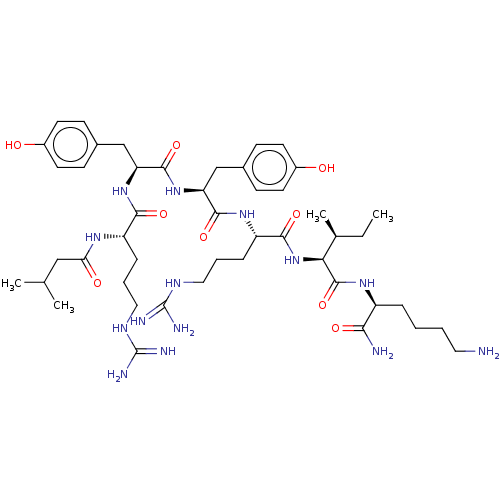
(CHEMBL3343947)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CC(C)C)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C47H76N14O9/c1-5-28(4)39(45(70)57-33(40(49)65)10-6-7-21-48)61-42(67)35(12-9-23-55-47(52)53)58-43(68)36(25-29-13-17-31(62)18-14-29)60-44(69)37(26-30-15-19-32(63)20-16-30)59-41(66)34(11-8-22-54-46(50)51)56-38(64)24-27(2)3/h13-20,27-28,33-37,39,62-63H,5-12,21-26,48H2,1-4H3,(H2,49,65)(H,56,64)(H,57,70)(H,58,68)(H,59,66)(H,60,69)(H,61,67)(H4,50,51,54)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor high affinity binding site expressed in African green monkey COS7 cells after 90 mins by top... |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50103534
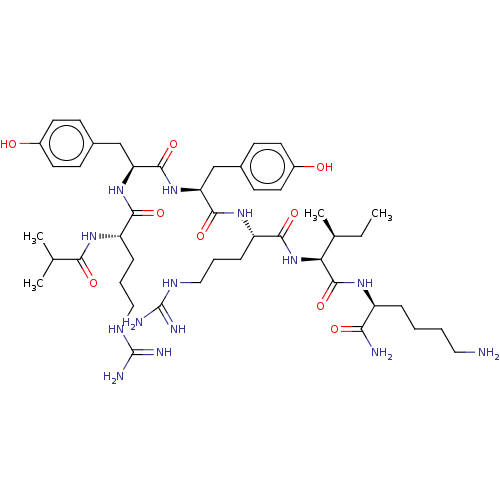
(CHEMBL3343949)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)C(C)C)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C46H74N14O9/c1-5-27(4)37(44(69)55-32(38(48)63)10-6-7-21-47)60-41(66)34(12-9-23-54-46(51)52)57-42(67)35(24-28-13-17-30(61)18-14-28)59-43(68)36(25-29-15-19-31(62)20-16-29)58-40(65)33(56-39(64)26(2)3)11-8-22-53-45(49)50/h13-20,26-27,32-37,61-62H,5-12,21-25,47H2,1-4H3,(H2,48,63)(H,55,69)(H,56,64)(H,57,67)(H,58,65)(H,59,68)(H,60,66)(H4,49,50,53)(H4,51,52,54)/t27-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor high affinity binding site expressed in African green monkey COS7 cells after 90 mins by top... |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Binding affinity to human ORL1 receptor expressed in African green monkey COS7 cells assessed per mg protein after 90 mins by Scatchard plot analysis |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50285779
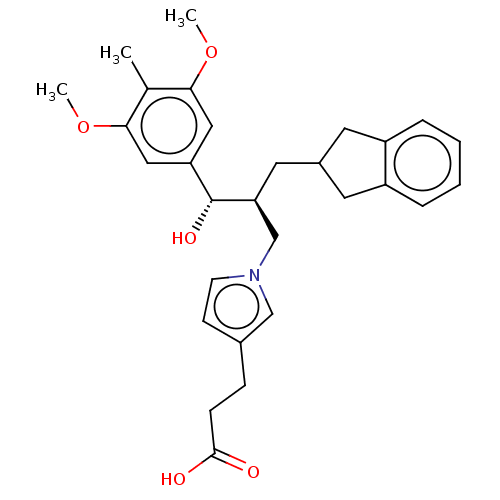
(CHEMBL4165205)Show SMILES COc1cc(cc(OC)c1C)[C@@H](O)[C@@H](CC1Cc2ccccc2C1)Cn1ccc(CCC(O)=O)c1 |r| Show InChI InChI=1S/C29H35NO5/c1-19-26(34-2)15-24(16-27(19)35-3)29(33)25(14-21-12-22-6-4-5-7-23(22)13-21)18-30-11-10-20(17-30)8-9-28(31)32/h4-7,10-11,15-17,21,25,29,33H,8-9,12-14,18H2,1-3H3,(H,31,32)/t25-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human LPA1 expressed in CHO cell membranes pretreated for 24 hrs prior to Fura-2-AM dye addition for 1 hr followed... |
ACS Med Chem Lett 8: 1281-1286 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00383
BindingDB Entry DOI: 10.7270/Q2PG1V8Z |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274454
(CHEMBL511107 | FGGFTGARKSARKKKNQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H137N29O22/c1-45(99-64(117)43-98-79(131)66(47(3)113)111-77(129)58(39-49-22-8-5-9-23-49)101-65(118)42-96-63(116)41-97-69(121)50(87)38-48-20-6-4-7-21-48)67(119)102-55(28-18-36-94-81(90)91)72(124)107-54(27-13-17-35-86)75(127)110-60(44-112)78(130)100-46(2)68(120)103-56(29-19-37-95-82(92)93)73(125)105-52(25-11-15-33-84)70(122)104-51(24-10-14-32-83)71(123)106-53(26-12-16-34-85)74(126)109-59(40-62(89)115)76(128)108-57(80(132)133)30-31-61(88)114/h4-9,20-23,45-47,50-60,66,112-113H,10-19,24-44,83-87H2,1-3H3,(H2,88,114)(H2,89,115)(H,96,116)(H,97,121)(H,98,131)(H,99,117)(H,100,130)(H,101,118)(H,102,119)(H,103,120)(H,104,122)(H,105,125)(H,106,123)(H,107,124)(H,108,128)(H,109,126)(H,110,127)(H,111,129)(H,132,133)(H4,90,91,94)(H4,92,93,95)/t45-,46-,47+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50285779

(CHEMBL4165205)Show SMILES COc1cc(cc(OC)c1C)[C@@H](O)[C@@H](CC1Cc2ccccc2C1)Cn1ccc(CCC(O)=O)c1 |r| Show InChI InChI=1S/C29H35NO5/c1-19-26(34-2)15-24(16-27(19)35-3)29(33)25(14-21-12-22-6-4-5-7-23(22)13-21)18-30-11-10-20(17-30)8-9-28(31)32/h4-7,10-11,15-17,21,25,29,33H,8-9,12-14,18H2,1-3H3,(H,31,32)/t25-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human LPA1 expressed in CHO cell membranes pretreated for 24 hrs prior to Fura-2-AM dye addition for 1 hr followed... |
ACS Med Chem Lett 8: 1281-1286 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00383
BindingDB Entry DOI: 10.7270/Q2PG1V8Z |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274452
(CHEMBL506825 | FGGFTGARKSARKKRNQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H137N31O22/c1-44(101-63(119)42-100-78(133)65(46(3)115)113-76(131)57(38-48-21-8-5-9-22-48)103-64(120)41-98-62(118)40-99-68(123)49(86)37-47-19-6-4-7-20-47)66(121)104-53(26-16-34-95-80(89)90)71(126)108-52(25-12-15-33-85)74(129)112-59(43-114)77(132)102-45(2)67(122)105-54(27-17-35-96-81(91)92)72(127)107-50(23-10-13-31-83)69(124)106-51(24-11-14-32-84)70(125)109-55(28-18-36-97-82(93)94)73(128)111-58(39-61(88)117)75(130)110-56(79(134)135)29-30-60(87)116/h4-9,19-22,44-46,49-59,65,114-115H,10-18,23-43,83-86H2,1-3H3,(H2,87,116)(H2,88,117)(H,98,118)(H,99,123)(H,100,133)(H,101,119)(H,102,132)(H,103,120)(H,104,121)(H,105,122)(H,106,124)(H,107,127)(H,108,126)(H,109,125)(H,110,130)(H,111,128)(H,112,129)(H,113,131)(H,134,135)(H4,89,90,95)(H4,91,92,96)(H4,93,94,97)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274449
(CHEMBL505874 | FGGFTGARKSARKRKNQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H137N31O22/c1-44(101-63(119)42-100-78(133)65(46(3)115)113-76(131)57(38-48-21-8-5-9-22-48)103-64(120)41-98-62(118)40-99-68(123)49(86)37-47-19-6-4-7-20-47)66(121)104-53(26-16-34-95-80(89)90)70(125)108-52(25-12-15-33-85)74(129)112-59(43-114)77(132)102-45(2)67(122)105-54(27-17-35-96-81(91)92)71(126)106-50(23-10-13-31-83)69(124)109-55(28-18-36-97-82(93)94)72(127)107-51(24-11-14-32-84)73(128)111-58(39-61(88)117)75(130)110-56(79(134)135)29-30-60(87)116/h4-9,19-22,44-46,49-59,65,114-115H,10-18,23-43,83-86H2,1-3H3,(H2,87,116)(H2,88,117)(H,98,118)(H,99,123)(H,100,133)(H,101,119)(H,102,132)(H,103,120)(H,104,121)(H,105,122)(H,106,126)(H,107,127)(H,108,125)(H,109,124)(H,110,130)(H,111,128)(H,112,129)(H,113,131)(H,134,135)(H4,89,90,95)(H4,91,92,96)(H4,93,94,97)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50333104
(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from recombinant ORL1 receptor expressed in COS7 cells by competitive receptor binding assay |
Bioorg Med Chem 17: 7904-8 (2009)
Article DOI: 10.1016/j.bmc.2009.10.026
BindingDB Entry DOI: 10.7270/Q28C9X65 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274449

(CHEMBL505874 | FGGFTGARKSARKRKNQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H137N31O22/c1-44(101-63(119)42-100-78(133)65(46(3)115)113-76(131)57(38-48-21-8-5-9-22-48)103-64(120)41-98-62(118)40-99-68(123)49(86)37-47-19-6-4-7-20-47)66(121)104-53(26-16-34-95-80(89)90)70(125)108-52(25-12-15-33-85)74(129)112-59(43-114)77(132)102-45(2)67(122)105-54(27-17-35-96-81(91)92)71(126)106-50(23-10-13-31-83)69(124)109-55(28-18-36-97-82(93)94)72(127)107-51(24-11-14-32-84)73(128)111-58(39-61(88)117)75(130)110-56(79(134)135)29-30-60(87)116/h4-9,19-22,44-46,49-59,65,114-115H,10-18,23-43,83-86H2,1-3H3,(H2,87,116)(H2,88,117)(H,98,118)(H,99,123)(H,100,133)(H,101,119)(H,102,132)(H,103,120)(H,104,121)(H,105,122)(H,106,126)(H,107,127)(H,108,125)(H,109,124)(H,110,130)(H,111,128)(H,112,129)(H,113,131)(H,134,135)(H4,89,90,95)(H4,91,92,96)(H4,93,94,97)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells by competitive binding assay |
Bioorg Med Chem 17: 5683-7 (2009)
Article DOI: 10.1016/j.bmc.2009.06.015
BindingDB Entry DOI: 10.7270/Q2GB2435 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50296841
(CHEMBL556388 | FGGFTGARKSARKWRNQ)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:1.1,73.75,89.90,42.44,112.115,26.27,131.134,58.59,3.3,wD:78.79,7.15,47.48,98.99,123.126,67.68,(7.64,-2.95,;8.97,-3.72,;10.31,-2.96,;8.96,-5.26,;7.62,-6.03,;6.29,-5.25,;6.3,-3.71,;4.95,-6.01,;4.94,-7.55,;6.27,-8.33,;6.25,-9.86,;7.58,-10.64,;8.92,-9.88,;8.93,-8.33,;7.61,-7.57,;3.62,-5.23,;2.29,-6,;2.28,-7.54,;.96,-5.22,;-.38,-5.98,;-1.71,-5.2,;-1.7,-3.66,;-3.05,-5.97,;-4.38,-5.19,;-5.72,-5.95,;-5.73,-7.49,;-7.04,-5.17,;-8.38,-5.94,;-7.03,-3.63,;-8.36,-2.86,;-8.36,-1.31,;-9.69,-.53,;-11.03,-1.3,;-11.03,-2.85,;-9.7,-3.61,;10.29,-6.04,;10.28,-7.58,;11.63,-5.28,;12.96,-6.06,;14.29,-5.29,;14.3,-3.75,;15.62,-6.07,;16.96,-5.31,;16.97,-3.77,;18.29,-6.09,;18.28,-7.63,;19.63,-5.32,;20.96,-6.1,;20.95,-7.64,;19.61,-8.4,;19.6,-9.94,;18.26,-10.71,;18.26,-12.25,;16.92,-13.01,;19.59,-13.02,;22.3,-5.34,;22.31,-3.8,;23.63,-6.12,;24.96,-5.35,;24.97,-3.81,;26.31,-3.05,;26.32,-1.51,;27.66,-.75,;27.67,.79,;26.29,-6.13,;26.28,-7.67,;27.63,-5.37,;28.96,-6.15,;28.95,-7.69,;27.61,-8.45,;30.3,-5.38,;30.31,-3.84,;31.63,-6.16,;32.97,-5.4,;32.98,-3.86,;34.29,-6.18,;34.28,-7.72,;35.63,-5.41,;36.96,-6.19,;36.95,-7.73,;35.61,-8.49,;35.61,-10.03,;34.27,-10.8,;34.26,-12.34,;32.93,-13.1,;35.59,-13.11,;38.3,-5.43,;38.31,-3.89,;39.63,-6.21,;40.97,-5.44,;40.98,-3.9,;42.32,-3.14,;42.33,-1.6,;43.67,-.84,;43.68,.7,;42.3,-6.22,;42.29,-7.76,;43.64,-5.46,;44.96,-6.24,;44.95,-7.78,;46.28,-8.55,;47.68,-7.93,;48.7,-9.08,;47.93,-10.4,;48.39,-11.85,;47.36,-12.97,;45.87,-12.64,;45.41,-11.19,;46.44,-10.07,;46.3,-5.47,;46.31,-3.93,;47.63,-6.25,;48.97,-5.49,;48.98,-3.95,;50.31,-3.19,;50.32,-1.66,;51.65,-.9,;51.66,.63,;52.99,1.39,;50.34,1.41,;50.3,-6.27,;50.29,-7.81,;51.64,-5.51,;52.97,-6.28,;52.96,-7.82,;54.29,-8.61,;55.62,-7.84,;54.28,-10.15,;54.31,-5.53,;54.32,-3.99,;55.63,-6.3,;56.97,-5.54,;56.98,-4,;58.32,-3.23,;58.33,-1.69,;57,-.92,;59.67,-.94,;58.3,-6.31,;59.64,-5.56,;58.29,-7.85,)| Show InChI InChI=1S/C87H135N31O22/c1-46(106-68(124)44-105-83(138)70(48(3)120)118-81(136)61(38-50-21-8-5-9-22-50)108-69(125)43-103-67(123)42-104-73(128)53(90)37-49-19-6-4-7-20-49)71(126)109-57(27-16-34-99-85(93)94)74(129)112-56(26-13-15-33-89)78(133)117-64(45-119)82(137)107-47(2)72(127)110-58(28-17-35-100-86(95)96)75(130)111-55(25-12-14-32-88)76(131)115-62(39-51-41-102-54-24-11-10-23-52(51)54)79(134)113-59(29-18-36-101-87(97)98)77(132)116-63(40-66(92)122)80(135)114-60(84(139)140)30-31-65(91)121/h4-11,19-24,41,46-48,53,55-64,70,102,119-120H,12-18,25-40,42-45,88-90H2,1-3H3,(H2,91,121)(H2,92,122)(H,103,123)(H,104,128)(H,105,138)(H,106,124)(H,107,137)(H,108,125)(H,109,126)(H,110,127)(H,111,130)(H,112,129)(H,113,134)(H,114,135)(H,115,131)(H,116,132)(H,117,133)(H,118,136)(H,139,140)(H4,93,94,99)(H4,95,96,100)(H4,97,98,101)/t46-,47-,48+,53-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,70-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells by competitive binding assay |
Bioorg Med Chem 17: 5683-7 (2009)
Article DOI: 10.1016/j.bmc.2009.06.015
BindingDB Entry DOI: 10.7270/Q2GB2435 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50296842
(CHEMBL559130 | FGGFTGARKSARKWKNQ)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:1.1,73.75,89.90,42.44,26.27,112.115,129.132,58.59,3.3,wD:78.79,7.15,47.48,98.99,121.124,67.68,(8.85,-33.5,;10.18,-34.27,;11.52,-33.52,;10.17,-35.81,;8.84,-36.58,;7.5,-35.8,;7.51,-34.26,;6.17,-36.57,;6.16,-38.11,;7.49,-38.88,;7.47,-40.42,;8.8,-41.2,;10.14,-40.43,;10.14,-38.88,;8.83,-38.12,;4.84,-35.78,;3.5,-36.55,;3.5,-38.09,;2.17,-35.77,;.83,-36.54,;-.5,-35.75,;-.49,-34.21,;-1.84,-36.52,;-3.17,-35.74,;-4.51,-36.51,;-4.51,-38.05,;-5.83,-35.72,;-7.17,-36.49,;-5.82,-34.18,;-7.15,-33.41,;-7.14,-31.87,;-8.47,-31.09,;-9.81,-31.85,;-9.82,-33.4,;-8.48,-34.17,;11.5,-36.6,;11.5,-38.14,;12.84,-35.83,;14.17,-36.61,;15.51,-35.85,;15.51,-34.31,;16.84,-36.63,;18.18,-35.86,;18.18,-34.32,;19.51,-36.64,;19.5,-38.18,;20.84,-35.88,;22.18,-36.66,;22.17,-38.2,;20.83,-38.96,;20.82,-40.5,;19.48,-41.26,;19.47,-42.8,;18.13,-43.56,;20.8,-43.58,;23.51,-35.89,;23.52,-34.35,;24.84,-36.67,;26.18,-35.91,;26.19,-34.37,;27.52,-33.61,;27.53,-32.07,;28.87,-31.3,;28.88,-29.76,;27.51,-36.69,;27.5,-38.23,;28.85,-35.92,;30.18,-36.7,;30.17,-38.24,;28.83,-39,;31.52,-35.94,;31.52,-34.4,;32.85,-36.72,;34.18,-35.95,;34.19,-34.41,;35.51,-36.73,;35.5,-38.27,;36.85,-35.97,;38.18,-36.75,;38.17,-38.29,;36.83,-39.05,;36.82,-40.59,;35.48,-41.35,;35.47,-42.89,;34.14,-43.65,;36.8,-43.67,;39.52,-35.98,;39.52,-34.44,;40.85,-36.76,;42.19,-36,;42.19,-34.46,;43.53,-33.7,;43.54,-32.16,;44.88,-31.39,;44.89,-29.85,;43.52,-36.78,;43.51,-38.32,;44.86,-36.01,;46.18,-36.79,;46.17,-38.33,;47.5,-39.11,;48.91,-38.48,;49.93,-39.63,;49.16,-40.96,;49.63,-42.41,;48.61,-43.54,;47.1,-43.22,;46.63,-41.76,;47.66,-40.63,;47.52,-36.03,;47.53,-34.49,;48.85,-36.81,;50.19,-36.04,;50.2,-34.5,;51.53,-33.74,;51.54,-32.21,;52.87,-31.45,;52.88,-29.92,;51.52,-36.82,;51.51,-38.36,;52.86,-36.06,;54.19,-36.84,;54.18,-38.38,;55.51,-39.16,;56.84,-38.39,;55.5,-40.7,;55.53,-36.08,;55.53,-34.54,;56.85,-36.85,;58.19,-36.1,;58.2,-34.56,;59.54,-33.79,;59.54,-32.25,;58.21,-31.47,;60.88,-31.49,;59.52,-36.87,;60.86,-36.11,;59.51,-38.41,)| Show InChI InChI=1S/C87H135N29O22/c1-47(104-69(122)45-103-84(136)71(49(3)118)116-82(134)62(39-51-22-8-5-9-23-51)106-70(123)44-101-68(121)43-102-74(126)54(91)38-50-20-6-4-7-21-50)72(124)107-59(29-18-36-98-86(94)95)75(127)110-58(28-14-17-35-90)79(131)115-65(46-117)83(135)105-48(2)73(125)108-60(30-19-37-99-87(96)97)76(128)109-56(26-12-15-33-88)77(129)113-63(40-52-42-100-55-25-11-10-24-53(52)55)80(132)111-57(27-13-16-34-89)78(130)114-64(41-67(93)120)81(133)112-61(85(137)138)31-32-66(92)119/h4-11,20-25,42,47-49,54,56-65,71,100,117-118H,12-19,26-41,43-46,88-91H2,1-3H3,(H2,92,119)(H2,93,120)(H,101,121)(H,102,126)(H,103,136)(H,104,122)(H,105,135)(H,106,123)(H,107,124)(H,108,125)(H,109,128)(H,110,127)(H,111,132)(H,112,133)(H,113,129)(H,114,130)(H,115,131)(H,116,134)(H,137,138)(H4,94,95,98)(H4,96,97,99)/t47-,48-,49+,54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells by competitive binding assay |
Bioorg Med Chem 17: 5683-7 (2009)
Article DOI: 10.1016/j.bmc.2009.06.015
BindingDB Entry DOI: 10.7270/Q2GB2435 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274453
(CHEMBL507847 | FGGFTGARKSARKRRNQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H137N33O22/c1-43(103-62(121)41-102-77(135)64(45(3)117)115-75(133)56(37-47-20-8-5-9-21-47)105-63(122)40-100-61(120)39-101-67(125)48(85)36-46-18-6-4-7-19-46)65(123)106-51(24-14-32-96-79(88)89)69(127)109-50(23-11-13-31-84)73(131)114-58(42-116)76(134)104-44(2)66(124)107-52(25-15-33-97-80(90)91)70(128)108-49(22-10-12-30-83)68(126)110-53(26-16-34-98-81(92)93)71(129)111-54(27-17-35-99-82(94)95)72(130)113-57(38-60(87)119)74(132)112-55(78(136)137)28-29-59(86)118/h4-9,18-21,43-45,48-58,64,116-117H,10-17,22-42,83-85H2,1-3H3,(H2,86,118)(H2,87,119)(H,100,120)(H,101,125)(H,102,135)(H,103,121)(H,104,134)(H,105,122)(H,106,123)(H,107,124)(H,108,128)(H,109,127)(H,110,126)(H,111,129)(H,112,132)(H,113,130)(H,114,131)(H,115,133)(H,136,137)(H4,88,89,96)(H4,90,91,97)(H4,92,93,98)(H4,94,95,99)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50296843
(CHEMBL555757 | FGGFTGARKSARKRWNQ)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:1.1,73.75,89.90,42.44,109.110,26.27,131.134,58.59,3.3,wD:78.79,7.15,47.48,98.99,123.126,67.68,(7.43,-25.86,;8.76,-26.64,;10.1,-25.88,;8.75,-28.18,;7.41,-28.94,;6.08,-28.16,;6.09,-26.62,;4.74,-28.93,;4.73,-30.47,;6.06,-31.24,;6.05,-32.78,;7.37,-33.56,;8.72,-32.79,;8.72,-31.24,;7.4,-30.48,;3.41,-28.15,;2.08,-28.91,;2.07,-30.45,;.75,-28.13,;-.59,-28.9,;-1.92,-28.12,;-1.91,-26.58,;-3.26,-28.88,;-4.59,-28.1,;-5.93,-28.87,;-5.94,-30.41,;-7.25,-28.09,;-8.59,-28.85,;-7.24,-26.55,;-8.57,-25.77,;-8.57,-24.23,;-9.89,-23.45,;-11.24,-24.21,;-11.25,-25.76,;-9.91,-26.53,;10.08,-28.96,;10.07,-30.5,;11.42,-28.19,;12.75,-28.97,;14.08,-28.21,;14.09,-26.67,;15.41,-28.99,;16.75,-28.22,;16.76,-26.68,;18.08,-29,;18.07,-30.54,;19.42,-28.24,;20.75,-29.02,;20.74,-30.56,;19.4,-31.32,;19.39,-32.86,;18.06,-33.62,;18.05,-35.16,;16.71,-35.92,;19.38,-35.94,;22.09,-28.25,;22.1,-26.71,;23.42,-29.03,;24.75,-28.27,;24.76,-26.73,;26.1,-25.97,;26.11,-24.43,;27.45,-23.66,;27.46,-22.12,;26.08,-29.05,;26.07,-30.59,;27.42,-28.28,;28.75,-29.06,;28.74,-30.6,;27.4,-31.36,;30.09,-28.3,;30.1,-26.76,;31.42,-29.08,;32.76,-28.31,;32.77,-26.77,;34.08,-29.09,;34.07,-30.63,;35.42,-28.33,;36.75,-29.11,;36.74,-30.65,;35.41,-31.41,;35.4,-32.95,;34.06,-33.71,;34.05,-35.25,;32.72,-36.01,;35.38,-36.03,;38.09,-28.34,;38.1,-26.8,;39.42,-29.12,;40.76,-28.36,;40.77,-26.82,;42.11,-26.06,;42.12,-24.52,;43.46,-23.75,;43.47,-22.21,;42.09,-29.14,;42.08,-30.68,;43.43,-28.37,;44.75,-29.15,;44.75,-30.69,;43.41,-31.45,;43.41,-32.99,;42.07,-33.74,;42.06,-35.28,;40.73,-36.04,;43.39,-36.05,;46.09,-28.39,;46.1,-26.85,;47.42,-29.17,;48.76,-28.4,;48.77,-26.86,;50.1,-26.1,;51.49,-26.73,;52.52,-25.6,;51.76,-24.27,;52.25,-22.82,;51.23,-21.68,;49.72,-21.99,;49.25,-23.44,;50.26,-24.58,;50.09,-29.18,;50.08,-30.72,;51.43,-28.43,;52.76,-29.2,;52.75,-30.74,;54.08,-31.52,;55.42,-30.76,;54.08,-33.06,;54.1,-28.44,;54.11,-26.9,;55.42,-29.22,;56.76,-28.46,;56.77,-26.92,;58.11,-26.15,;58.12,-24.61,;56.79,-23.84,;59.46,-23.85,;58.09,-29.23,;59.43,-28.47,;58.09,-30.77,)| Show InChI InChI=1S/C87H135N31O22/c1-46(106-68(124)44-105-83(138)70(48(3)120)118-81(136)61(38-50-21-8-5-9-22-50)108-69(125)43-103-67(123)42-104-73(128)53(90)37-49-19-6-4-7-20-49)71(126)109-57(27-16-34-99-85(93)94)75(130)112-56(26-13-15-33-89)78(133)117-64(45-119)82(137)107-47(2)72(127)110-58(28-17-35-100-86(95)96)76(131)111-55(25-12-14-32-88)74(129)113-59(29-18-36-101-87(97)98)77(132)115-62(39-51-41-102-54-24-11-10-23-52(51)54)79(134)116-63(40-66(92)122)80(135)114-60(84(139)140)30-31-65(91)121/h4-11,19-24,41,46-48,53,55-64,70,102,119-120H,12-18,25-40,42-45,88-90H2,1-3H3,(H2,91,121)(H2,92,122)(H,103,123)(H,104,128)(H,105,138)(H,106,124)(H,107,137)(H,108,125)(H,109,126)(H,110,127)(H,111,131)(H,112,130)(H,113,129)(H,114,135)(H,115,132)(H,116,134)(H,117,133)(H,118,136)(H,139,140)(H4,93,94,99)(H4,95,96,100)(H4,97,98,101)/t46-,47-,48+,53-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,70-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells by competitive binding assay |
Bioorg Med Chem 17: 5683-7 (2009)
Article DOI: 10.1016/j.bmc.2009.06.015
BindingDB Entry DOI: 10.7270/Q2GB2435 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50296844
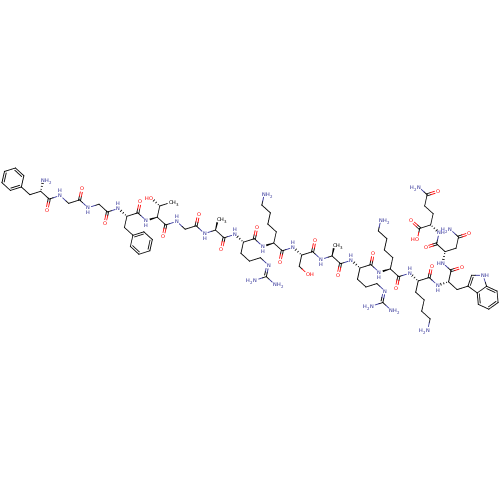
(CHEMBL558807 | FGGFTGARKSARKKWNQ)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:1.1,73.75,89.90,42.44,26.27,107.108,129.132,58.59,3.3,wD:78.79,7.15,47.48,98.99,121.124,67.68,(7.77,-3.8,;9.1,-4.57,;10.44,-3.81,;9.09,-6.11,;7.75,-6.88,;6.42,-6.1,;6.43,-4.56,;5.08,-6.86,;5.07,-8.4,;6.4,-9.18,;6.39,-10.71,;7.71,-11.49,;9.06,-10.73,;9.06,-9.18,;7.74,-8.42,;3.75,-6.08,;2.42,-6.85,;2.41,-8.39,;1.09,-6.07,;-.25,-6.83,;-1.58,-6.05,;-1.57,-4.51,;-2.92,-6.82,;-4.25,-6.04,;-5.59,-6.8,;-5.6,-8.34,;-6.91,-6.02,;-8.25,-6.79,;-6.9,-4.48,;-8.23,-3.71,;-8.22,-2.17,;-9.55,-1.38,;-10.89,-2.15,;-10.9,-3.7,;-9.57,-4.46,;10.42,-6.89,;10.41,-8.43,;11.76,-6.13,;13.09,-6.91,;14.42,-6.14,;14.43,-4.6,;15.75,-6.92,;17.09,-6.16,;17.1,-4.62,;18.42,-6.94,;18.41,-8.48,;19.76,-6.17,;21.09,-6.95,;21.08,-8.49,;19.74,-9.25,;19.73,-10.79,;18.39,-11.56,;18.38,-13.1,;17.05,-13.86,;19.72,-13.87,;22.43,-6.19,;22.44,-4.65,;23.76,-6.97,;25.09,-6.2,;25.1,-4.66,;26.44,-3.9,;26.45,-2.36,;27.79,-1.6,;27.79,-.06,;26.42,-6.98,;26.41,-8.52,;27.76,-6.22,;29.09,-7,;29.08,-8.54,;27.74,-9.3,;30.43,-6.23,;30.44,-4.69,;31.76,-7.01,;33.1,-6.25,;33.11,-4.71,;34.42,-7.03,;34.41,-8.57,;35.76,-6.26,;37.09,-7.04,;37.08,-8.58,;35.74,-9.34,;35.73,-10.88,;34.39,-11.65,;34.38,-13.19,;33.05,-13.95,;35.72,-13.96,;38.43,-6.28,;38.44,-4.74,;39.76,-7.06,;41.1,-6.29,;41.11,-4.75,;42.45,-3.99,;42.45,-2.45,;43.79,-1.69,;43.8,-.15,;42.43,-7.07,;42.42,-8.61,;43.77,-6.31,;45.09,-7.09,;45.08,-8.63,;46.41,-9.4,;46.4,-10.95,;47.72,-11.72,;47.72,-13.26,;46.43,-6.32,;46.44,-4.78,;47.76,-7.1,;49.1,-6.34,;49.11,-4.8,;50.44,-4.04,;51.83,-4.67,;52.86,-3.53,;52.1,-2.21,;52.58,-.76,;51.56,.39,;50.06,.07,;49.58,-1.38,;50.6,-2.51,;50.43,-7.12,;50.42,-8.66,;51.77,-6.36,;53.1,-7.13,;53.09,-8.67,;54.42,-9.46,;55.75,-8.69,;54.41,-11,;54.44,-6.38,;54.44,-4.84,;55.76,-7.15,;57.1,-6.39,;57.11,-4.85,;58.44,-4.09,;58.45,-2.55,;57.12,-1.77,;59.79,-1.79,;58.43,-7.17,;59.77,-6.41,;58.42,-8.71,)| Show InChI InChI=1S/C87H135N29O22/c1-47(104-69(122)45-103-84(136)71(49(3)118)116-82(134)62(39-51-22-8-5-9-23-51)106-70(123)44-101-68(121)43-102-74(126)54(91)38-50-20-6-4-7-21-50)72(124)107-59(29-18-36-98-86(94)95)76(128)111-58(28-14-17-35-90)79(131)115-65(46-117)83(135)105-48(2)73(125)108-60(30-19-37-99-87(96)97)77(129)109-56(26-12-15-33-88)75(127)110-57(27-13-16-34-89)78(130)113-63(40-52-42-100-55-25-11-10-24-53(52)55)80(132)114-64(41-67(93)120)81(133)112-61(85(137)138)31-32-66(92)119/h4-11,20-25,42,47-49,54,56-65,71,100,117-118H,12-19,26-41,43-46,88-91H2,1-3H3,(H2,92,119)(H2,93,120)(H,101,121)(H,102,126)(H,103,136)(H,104,122)(H,105,135)(H,106,123)(H,107,124)(H,108,125)(H,109,129)(H,110,127)(H,111,128)(H,112,133)(H,113,130)(H,114,132)(H,115,131)(H,116,134)(H,137,138)(H4,94,95,98)(H4,96,97,99)/t47-,48-,49+,54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells by competitive binding assay |
Bioorg Med Chem 17: 5683-7 (2009)
Article DOI: 10.1016/j.bmc.2009.06.015
BindingDB Entry DOI: 10.7270/Q2GB2435 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274462
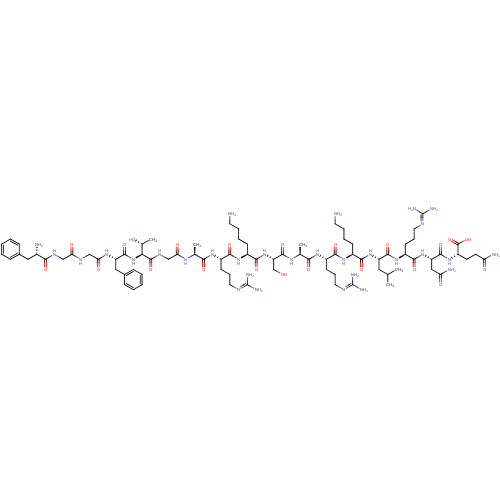
(CHEMBL526333 | FGGFTGARKSARKLRNQ)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H136N30O22/c1-43(2)35-56(74(128)107-54(27-18-34-96-82(92)93)72(126)110-58(38-61(87)116)75(129)108-55(79(133)134)28-29-60(86)115)109-71(125)50(23-12-14-30-83)105-70(124)53(26-17-33-95-81(90)91)104-67(121)45(4)101-77(131)59(42-113)111-73(127)51(24-13-15-31-84)106-69(123)52(25-16-32-94-80(88)89)103-66(120)44(3)100-63(118)41-99-78(132)65(46(5)114)112-76(130)57(37-48-21-10-7-11-22-48)102-64(119)40-97-62(117)39-98-68(122)49(85)36-47-19-8-6-9-20-47/h6-11,19-22,43-46,49-59,65,113-114H,12-18,23-42,83-85H2,1-5H3,(H2,86,115)(H2,87,116)(H,97,117)(H,98,122)(H,99,132)(H,100,118)(H,101,131)(H,102,119)(H,103,120)(H,104,121)(H,105,124)(H,106,123)(H,107,128)(H,108,129)(H,109,125)(H,110,126)(H,111,127)(H,112,130)(H,133,134)(H4,88,89,94)(H4,90,91,95)(H4,92,93,96)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274455
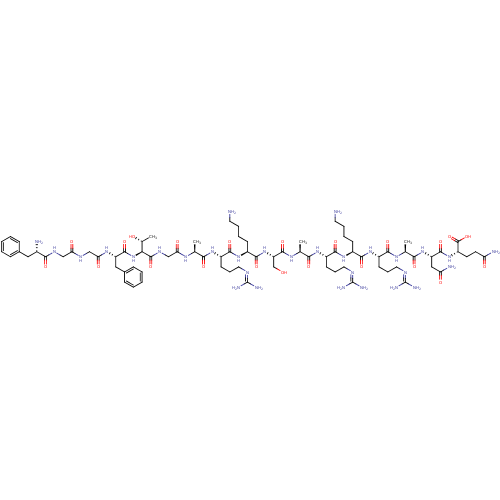
(CHEMBL500773 | FGGFTGARKSARKRANQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C79H130N30O22/c1-41(97-60(115)39-96-75(129)62(44(4)111)109-73(127)54(35-46-20-9-6-10-21-46)100-61(116)38-94-59(114)37-95-66(120)47(82)34-45-18-7-5-8-19-45)63(117)101-51(25-16-32-92-78(87)88)69(123)104-49(23-12-14-30-81)71(125)108-56(40-110)74(128)99-42(2)64(118)102-52(26-17-33-93-79(89)90)70(124)103-48(22-11-13-29-80)68(122)105-50(24-15-31-91-77(85)86)67(121)98-43(3)65(119)107-55(36-58(84)113)72(126)106-53(76(130)131)27-28-57(83)112/h5-10,18-21,41-44,47-56,62,110-111H,11-17,22-40,80-82H2,1-4H3,(H2,83,112)(H2,84,113)(H,94,114)(H,95,120)(H,96,129)(H,97,115)(H,98,121)(H,99,128)(H,100,116)(H,101,117)(H,102,118)(H,103,124)(H,104,123)(H,105,122)(H,106,126)(H,107,119)(H,108,125)(H,109,127)(H,130,131)(H4,85,86,91)(H4,87,88,92)(H4,89,90,93)/t41-,42-,43-,44+,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274461
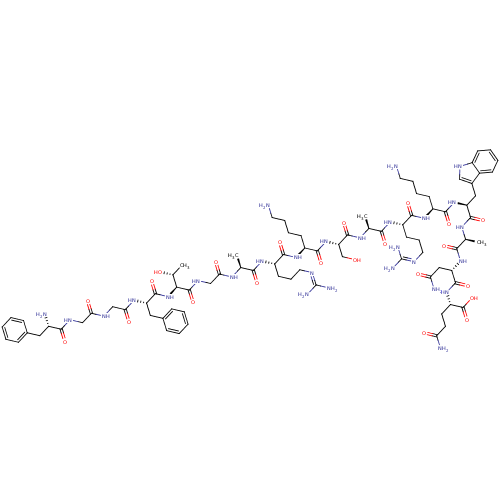
(CHEMBL504587 | FGGFTGARKSARKWANQ)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:26.27,3.3,1.1,42.44,58.59,73.75,89.90,112.116,125.128,wD:7.15,47.48,67.68,78.79,98.99,117.120,(9.16,.66,;10.48,-.11,;11.83,.66,;10.48,-1.65,;9.16,-2.42,;7.82,-1.64,;7.82,-.1,;6.49,-2.41,;6.49,-3.95,;7.82,-4.73,;9.15,-3.96,;10.49,-4.73,;10.48,-6.27,;9.15,-7.04,;7.81,-6.27,;5.15,-1.64,;3.82,-2.41,;3.82,-3.95,;2.48,-1.64,;1.15,-2.4,;-.19,-1.63,;-.19,-.09,;-1.52,-2.4,;-2.86,-1.63,;-4.19,-2.4,;-4.19,-3.94,;-5.52,-1.63,;-6.86,-2.4,;-5.52,-.09,;-4.2,.69,;-2.86,-.09,;-1.52,.68,;-1.52,2.22,;-2.85,2.99,;-4.19,2.22,;11.83,-2.42,;11.83,-3.96,;13.16,-1.65,;14.49,-2.42,;15.82,-1.66,;15.82,-.12,;17.16,-2.43,;18.5,-1.65,;18.5,-.12,;19.83,-2.43,;19.83,-3.97,;21.16,-1.66,;22.5,-2.43,;22.5,-3.98,;23.83,-4.74,;23.83,-6.29,;25.16,-7.06,;25.16,-8.61,;23.83,-9.38,;26.5,-9.37,;23.83,-1.66,;23.83,-.12,;25.17,-2.43,;26.5,-1.67,;26.5,-.13,;27.84,.64,;27.84,2.18,;29.18,2.95,;29.18,4.49,;27.84,-2.44,;27.84,-3.98,;29.17,-1.67,;30.51,-2.44,;30.51,-3.98,;31.84,-4.76,;31.84,-1.67,;31.84,-.13,;33.18,-2.45,;34.51,-1.68,;34.51,-.14,;35.85,-2.45,;35.85,-4,;37.18,-1.68,;38.52,-2.45,;38.52,-4,;39.85,-4.77,;39.85,-6.31,;41.18,-7.08,;41.18,-8.62,;39.84,-9.4,;42.52,-9.39,;39.85,-1.69,;39.85,-.15,;41.19,-2.46,;42.52,-1.69,;42.52,-.15,;43.85,.63,;43.85,2.16,;45.19,2.94,;45.19,4.47,;43.85,-2.46,;43.85,-4,;45.19,-1.69,;46.53,-2.47,;46.53,-4.01,;47.86,-4.77,;49.26,-4.15,;50.29,-5.3,;49.52,-6.63,;49.99,-8.1,;48.96,-9.24,;47.45,-8.92,;46.98,-7.45,;48.02,-6.31,;47.86,-1.69,;47.86,-.15,;49.19,-2.46,;50.53,-1.7,;50.53,-.16,;51.86,-2.47,;51.86,-4.02,;53.19,-1.7,;54.53,-2.47,;54.53,-4.01,;55.87,-4.79,;57.2,-4.01,;55.87,-6.33,;55.87,-1.7,;55.87,-.17,;57.21,-2.48,;58.53,-1.71,;58.53,-.17,;59.87,.6,;59.87,2.14,;58.54,2.91,;61.21,2.91,;59.87,-2.48,;61.2,-1.71,;59.87,-4.02,)| Show InChI InChI=1S/C84H128N28O22/c1-44(100-66(118)42-99-81(132)68(47(4)114)112-79(130)59(36-49-21-9-6-10-22-49)103-67(119)41-97-65(117)40-98-72(123)52(87)35-48-19-7-5-8-20-48)69(120)104-56(27-17-33-94-83(90)91)73(124)107-55(26-14-16-32-86)76(127)111-62(43-113)80(131)102-45(2)70(121)105-57(28-18-34-95-84(92)93)74(125)106-54(25-13-15-31-85)75(126)110-60(37-50-39-96-53-24-12-11-23-51(50)53)77(128)101-46(3)71(122)109-61(38-64(89)116)78(129)108-58(82(133)134)29-30-63(88)115/h5-12,19-24,39,44-47,52,54-62,68,96,113-114H,13-18,25-38,40-43,85-87H2,1-4H3,(H2,88,115)(H2,89,116)(H,97,117)(H,98,123)(H,99,132)(H,100,118)(H,101,128)(H,102,131)(H,103,119)(H,104,120)(H,105,121)(H,106,125)(H,107,124)(H,108,129)(H,109,122)(H,110,126)(H,111,127)(H,112,130)(H,133,134)(H4,90,91,94)(H4,92,93,95)/t44-,45-,46-,47+,52-,54-,55-,56-,57-,58-,59-,60-,61-,62-,68-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274463
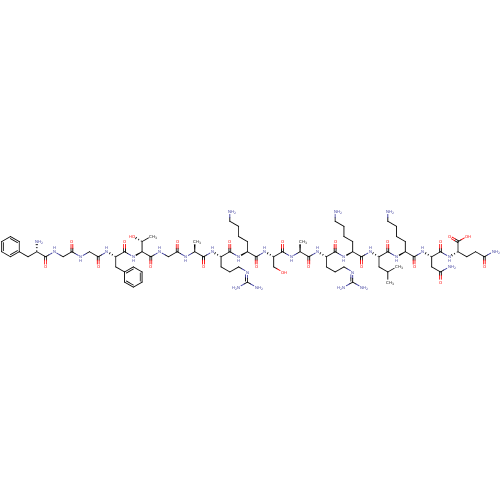
(CHEMBL503512 | FGGFTGARKSARKLKNQ)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H136N28O22/c1-44(2)36-57(75(126)105-52(25-13-16-32-84)73(124)108-59(39-62(88)114)76(127)106-56(80(131)132)29-30-61(87)113)107-72(123)51(24-12-15-31-83)103-71(122)55(28-19-35-94-82(91)92)102-68(119)46(4)99-78(129)60(43-111)109-74(125)53(26-14-17-33-85)104-70(121)54(27-18-34-93-81(89)90)101-67(118)45(3)98-64(116)42-97-79(130)66(47(5)112)110-77(128)58(38-49-22-10-7-11-23-49)100-65(117)41-95-63(115)40-96-69(120)50(86)37-48-20-8-6-9-21-48/h6-11,20-23,44-47,50-60,66,111-112H,12-19,24-43,83-86H2,1-5H3,(H2,87,113)(H2,88,114)(H,95,115)(H,96,120)(H,97,130)(H,98,116)(H,99,129)(H,100,117)(H,101,118)(H,102,119)(H,103,122)(H,104,121)(H,105,126)(H,106,127)(H,107,123)(H,108,124)(H,109,125)(H,110,128)(H,131,132)(H4,89,90,93)(H4,91,92,94)/t45-,46-,47+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274456
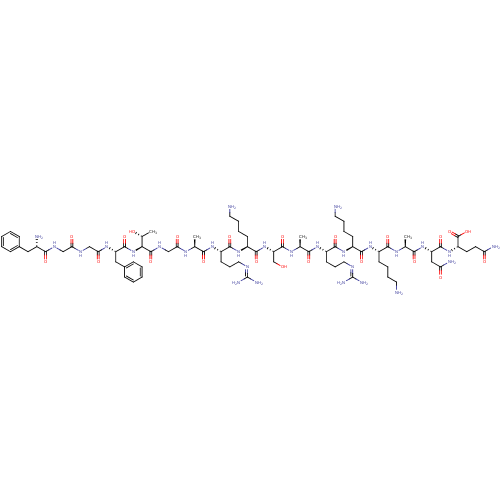
(CHEMBL505632 | FGGFTGARKSARKKANQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C79H130N28O22/c1-42(95-61(113)40-94-76(127)63(45(4)109)107-74(125)55(36-47-21-9-6-10-22-47)98-62(114)39-92-60(112)38-93-67(118)48(83)35-46-19-7-5-8-20-46)64(115)99-52(26-17-33-90-78(86)87)70(121)103-51(25-13-16-32-82)72(123)106-57(41-108)75(126)97-43(2)65(116)100-53(27-18-34-91-79(88)89)71(122)102-50(24-12-15-31-81)69(120)101-49(23-11-14-30-80)68(119)96-44(3)66(117)105-56(37-59(85)111)73(124)104-54(77(128)129)28-29-58(84)110/h5-10,19-22,42-45,48-57,63,108-109H,11-18,23-41,80-83H2,1-4H3,(H2,84,110)(H2,85,111)(H,92,112)(H,93,118)(H,94,127)(H,95,113)(H,96,119)(H,97,126)(H,98,114)(H,99,115)(H,100,116)(H,101,120)(H,102,122)(H,103,121)(H,104,124)(H,105,117)(H,106,123)(H,107,125)(H,128,129)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274461

(CHEMBL504587 | FGGFTGARKSARKWANQ)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:26.27,3.3,1.1,42.44,58.59,73.75,89.90,112.116,125.128,wD:7.15,47.48,67.68,78.79,98.99,117.120,(9.16,.66,;10.48,-.11,;11.83,.66,;10.48,-1.65,;9.16,-2.42,;7.82,-1.64,;7.82,-.1,;6.49,-2.41,;6.49,-3.95,;7.82,-4.73,;9.15,-3.96,;10.49,-4.73,;10.48,-6.27,;9.15,-7.04,;7.81,-6.27,;5.15,-1.64,;3.82,-2.41,;3.82,-3.95,;2.48,-1.64,;1.15,-2.4,;-.19,-1.63,;-.19,-.09,;-1.52,-2.4,;-2.86,-1.63,;-4.19,-2.4,;-4.19,-3.94,;-5.52,-1.63,;-6.86,-2.4,;-5.52,-.09,;-4.2,.69,;-2.86,-.09,;-1.52,.68,;-1.52,2.22,;-2.85,2.99,;-4.19,2.22,;11.83,-2.42,;11.83,-3.96,;13.16,-1.65,;14.49,-2.42,;15.82,-1.66,;15.82,-.12,;17.16,-2.43,;18.5,-1.65,;18.5,-.12,;19.83,-2.43,;19.83,-3.97,;21.16,-1.66,;22.5,-2.43,;22.5,-3.98,;23.83,-4.74,;23.83,-6.29,;25.16,-7.06,;25.16,-8.61,;23.83,-9.38,;26.5,-9.37,;23.83,-1.66,;23.83,-.12,;25.17,-2.43,;26.5,-1.67,;26.5,-.13,;27.84,.64,;27.84,2.18,;29.18,2.95,;29.18,4.49,;27.84,-2.44,;27.84,-3.98,;29.17,-1.67,;30.51,-2.44,;30.51,-3.98,;31.84,-4.76,;31.84,-1.67,;31.84,-.13,;33.18,-2.45,;34.51,-1.68,;34.51,-.14,;35.85,-2.45,;35.85,-4,;37.18,-1.68,;38.52,-2.45,;38.52,-4,;39.85,-4.77,;39.85,-6.31,;41.18,-7.08,;41.18,-8.62,;39.84,-9.4,;42.52,-9.39,;39.85,-1.69,;39.85,-.15,;41.19,-2.46,;42.52,-1.69,;42.52,-.15,;43.85,.63,;43.85,2.16,;45.19,2.94,;45.19,4.47,;43.85,-2.46,;43.85,-4,;45.19,-1.69,;46.53,-2.47,;46.53,-4.01,;47.86,-4.77,;49.26,-4.15,;50.29,-5.3,;49.52,-6.63,;49.99,-8.1,;48.96,-9.24,;47.45,-8.92,;46.98,-7.45,;48.02,-6.31,;47.86,-1.69,;47.86,-.15,;49.19,-2.46,;50.53,-1.7,;50.53,-.16,;51.86,-2.47,;51.86,-4.02,;53.19,-1.7,;54.53,-2.47,;54.53,-4.01,;55.87,-4.79,;57.2,-4.01,;55.87,-6.33,;55.87,-1.7,;55.87,-.17,;57.21,-2.48,;58.53,-1.71,;58.53,-.17,;59.87,.6,;59.87,2.14,;58.54,2.91,;61.21,2.91,;59.87,-2.48,;61.2,-1.71,;59.87,-4.02,)| Show InChI InChI=1S/C84H128N28O22/c1-44(100-66(118)42-99-81(132)68(47(4)114)112-79(130)59(36-49-21-9-6-10-22-49)103-67(119)41-97-65(117)40-98-72(123)52(87)35-48-19-7-5-8-20-48)69(120)104-56(27-17-33-94-83(90)91)73(124)107-55(26-14-16-32-86)76(127)111-62(43-113)80(131)102-45(2)70(121)105-57(28-18-34-95-84(92)93)74(125)106-54(25-13-15-31-85)75(126)110-60(37-50-39-96-53-24-12-11-23-51(50)53)77(128)101-46(3)71(122)109-61(38-64(89)116)78(129)108-58(82(133)134)29-30-63(88)115/h5-12,19-24,39,44-47,52,54-62,68,96,113-114H,13-18,25-38,40-43,85-87H2,1-4H3,(H2,88,115)(H2,89,116)(H,97,117)(H,98,123)(H,99,132)(H,100,118)(H,101,128)(H,102,131)(H,103,119)(H,104,120)(H,105,121)(H,106,125)(H,107,124)(H,108,129)(H,109,122)(H,110,126)(H,111,127)(H,112,130)(H,133,134)(H4,90,91,94)(H4,92,93,95)/t44-,45-,46-,47+,52-,54-,55-,56-,57-,58-,59-,60-,61-,62-,68-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells by competitive binding assay |
Bioorg Med Chem 17: 5683-7 (2009)
Article DOI: 10.1016/j.bmc.2009.06.015
BindingDB Entry DOI: 10.7270/Q2GB2435 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274451
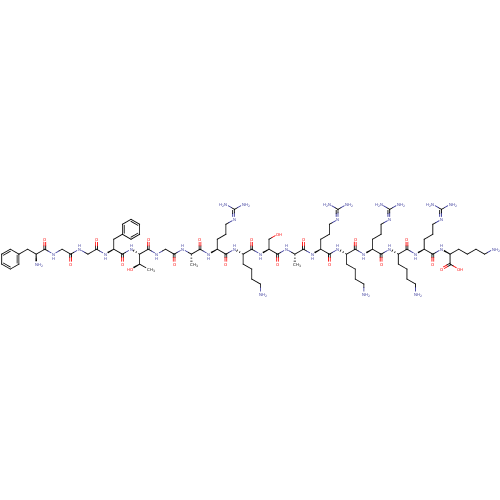
(CHEMBL505824 | FGGFTGARKSARKRKRK)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C85H147N33O20/c1-48(106-65(122)46-105-80(136)67(50(3)120)118-78(134)62(43-52-24-8-5-9-25-52)108-66(123)45-103-64(121)44-104-70(126)53(90)42-51-22-6-4-7-23-51)68(124)109-57(30-18-38-99-82(91)92)73(129)113-56(28-12-16-36-88)77(133)117-63(47-119)79(135)107-49(2)69(125)110-58(31-19-39-100-83(93)94)74(130)111-54(26-10-14-34-86)71(127)114-59(32-20-40-101-84(95)96)75(131)112-55(27-11-15-35-87)72(128)115-60(33-21-41-102-85(97)98)76(132)116-61(81(137)138)29-13-17-37-89/h4-9,22-25,48-50,53-63,67,119-120H,10-21,26-47,86-90H2,1-3H3,(H,103,121)(H,104,126)(H,105,136)(H,106,122)(H,107,135)(H,108,123)(H,109,124)(H,110,125)(H,111,130)(H,112,131)(H,113,129)(H,114,127)(H,115,128)(H,116,132)(H,117,133)(H,118,134)(H,137,138)(H4,91,92,99)(H4,93,94,100)(H4,95,96,101)(H4,97,98,102)/t48-,49-,50+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,67-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274464
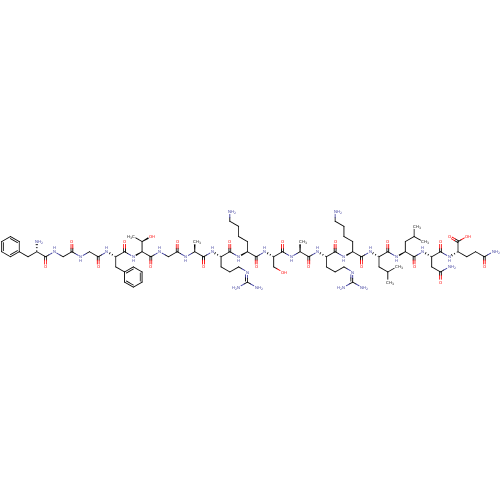
(CHEMBL504872 | FGGFTGARKSARKLLNQ)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H135N27O22/c1-43(2)34-56(74(124)106-57(35-44(3)4)75(125)107-59(38-62(87)113)76(126)104-55(80(130)131)28-29-61(86)112)105-72(122)51(24-14-16-30-83)102-71(121)54(27-19-33-93-82(90)91)101-68(118)46(6)98-78(128)60(42-110)108-73(123)52(25-15-17-31-84)103-70(120)53(26-18-32-92-81(88)89)100-67(117)45(5)97-64(115)41-96-79(129)66(47(7)111)109-77(127)58(37-49-22-12-9-13-23-49)99-65(116)40-94-63(114)39-95-69(119)50(85)36-48-20-10-8-11-21-48/h8-13,20-23,43-47,50-60,66,110-111H,14-19,24-42,83-85H2,1-7H3,(H2,86,112)(H2,87,113)(H,94,114)(H,95,119)(H,96,129)(H,97,115)(H,98,128)(H,99,116)(H,100,117)(H,101,118)(H,102,121)(H,103,120)(H,104,126)(H,105,122)(H,106,124)(H,107,125)(H,108,123)(H,109,127)(H,130,131)(H4,88,89,92)(H4,90,91,93)/t45-,46-,47+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274450
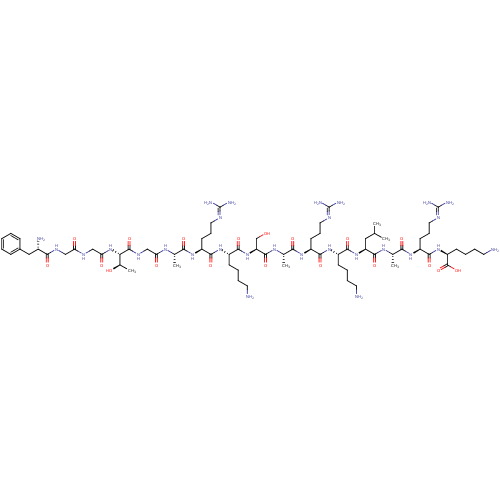
(CHEMBL499157 | FGGFTGARKSARKLARK)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C73H130N28O19/c1-39(2)33-52(67(116)91-41(4)59(108)95-50(26-18-32-86-73(82)83)64(113)98-51(70(119)120)23-12-15-29-76)99-65(114)46(21-10-13-27-74)96-63(112)49(25-17-31-85-72(80)81)94-60(109)42(5)92-68(117)53(38-102)100-66(115)47(22-11-14-28-75)97-62(111)48(24-16-30-84-71(78)79)93-58(107)40(3)90-55(105)37-89-69(118)57(43(6)103)101-56(106)36-87-54(104)35-88-61(110)45(77)34-44-19-8-7-9-20-44/h7-9,19-20,39-43,45-53,57,102-103H,10-18,21-38,74-77H2,1-6H3,(H,87,104)(H,88,110)(H,89,118)(H,90,105)(H,91,116)(H,92,117)(H,93,107)(H,94,109)(H,95,108)(H,96,112)(H,97,111)(H,98,113)(H,99,114)(H,100,115)(H,101,106)(H,119,120)(H4,78,79,84)(H4,80,81,85)(H4,82,83,86)/t40-,41-,42-,43+,45-,46-,47-,48-,49-,50-,51-,52-,53-,57-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50333104

(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human ORL1-Galpha fusion receptor in COS7 cells |
Bioorg Med Chem 16: 2635-44 (2008)
Article DOI: 10.1016/j.bmc.2007.11.043
BindingDB Entry DOI: 10.7270/Q2416XW9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor expressed in African green monkey COS7 cells after 90 mins by topcount analysis |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50372279
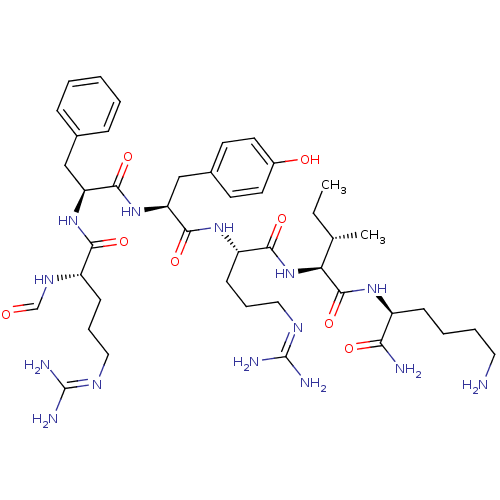
(CHEMBL271478)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6]=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C43H68N14O8/c1-3-26(2)35(41(65)53-30(36(45)60)13-7-8-20-44)57-38(62)32(15-10-22-51-43(48)49)54-39(63)34(24-28-16-18-29(59)19-17-28)56-40(64)33(23-27-11-5-4-6-12-27)55-37(61)31(52-25-58)14-9-21-50-42(46)47/h4-6,11-12,16-19,25-26,30-35,59H,3,7-10,13-15,20-24,44H2,1-2H3,(H2,45,60)(H,52,58)(H,53,65)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,46,47,50)(H4,48,49,51)/t26-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human ORL1-Galpha fusion receptor in COS7 cells |
Bioorg Med Chem 16: 2635-44 (2008)
Article DOI: 10.1016/j.bmc.2007.11.043
BindingDB Entry DOI: 10.7270/Q2416XW9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50333104

(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from recombinant ORL1 receptor expressed in COS7 cells by competitive receptor binding assay |
Bioorg Med Chem 17: 7904-8 (2009)
Article DOI: 10.1016/j.bmc.2009.10.026
BindingDB Entry DOI: 10.7270/Q28C9X65 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50333104

(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50372278
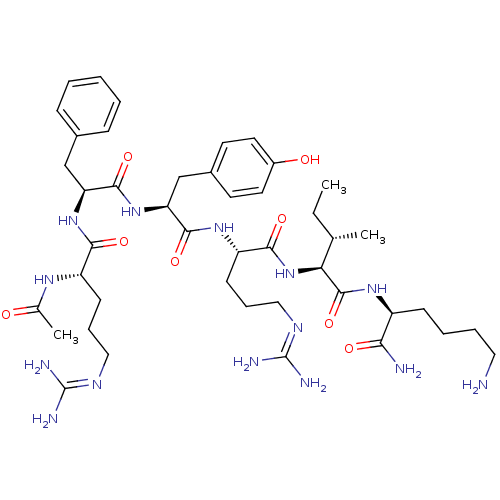
(CHEMBL256055)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C44H70N14O8/c1-4-26(2)36(42(66)54-31(37(46)61)14-8-9-21-45)58-39(63)33(16-11-23-52-44(49)50)55-40(64)35(25-29-17-19-30(60)20-18-29)57-41(65)34(24-28-12-6-5-7-13-28)56-38(62)32(53-27(3)59)15-10-22-51-43(47)48/h5-7,12-13,17-20,26,31-36,60H,4,8-11,14-16,21-25,45H2,1-3H3,(H2,46,61)(H,53,59)(H,54,66)(H,55,64)(H,56,62)(H,57,65)(H,58,63)(H4,47,48,51)(H4,49,50,52)/t26-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human ORL1-Galpha fusion receptor in COS7 cells |
Bioorg Med Chem 16: 2635-44 (2008)
Article DOI: 10.1016/j.bmc.2007.11.043
BindingDB Entry DOI: 10.7270/Q2416XW9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274448
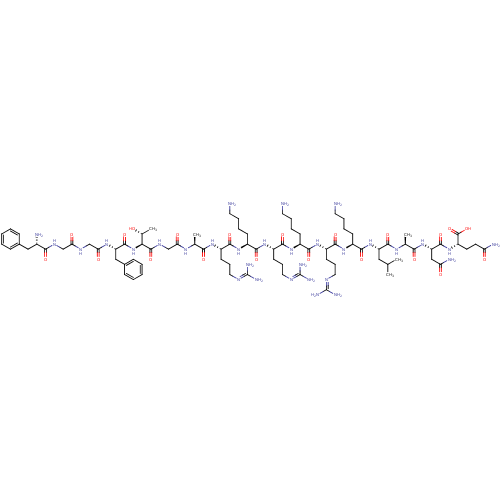
(CHEMBL504461 | FGGFTGARKRKRKLANQ)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C85H143N31O21/c1-46(2)39-60(78(132)105-48(4)70(124)114-62(42-64(91)119)79(133)113-59(82(136)137)31-32-63(90)118)115-77(131)55(27-14-17-35-88)110-76(130)58(30-20-38-100-85(96)97)112-73(127)54(26-13-16-34-87)109-75(129)57(29-19-37-99-84(94)95)111-72(126)53(25-12-15-33-86)108-74(128)56(28-18-36-98-83(92)93)107-69(123)47(3)104-66(121)45-103-81(135)68(49(5)117)116-80(134)61(41-51-23-10-7-11-24-51)106-67(122)44-101-65(120)43-102-71(125)52(89)40-50-21-8-6-9-22-50/h6-11,21-24,46-49,52-62,68,117H,12-20,25-45,86-89H2,1-5H3,(H2,90,118)(H2,91,119)(H,101,120)(H,102,125)(H,103,135)(H,104,121)(H,105,132)(H,106,122)(H,107,123)(H,108,128)(H,109,129)(H,110,130)(H,111,126)(H,112,127)(H,113,133)(H,114,124)(H,115,131)(H,116,134)(H,136,137)(H4,92,93,98)(H4,94,95,99)(H4,96,97,100)/t47-,48-,49+,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,68-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50190305
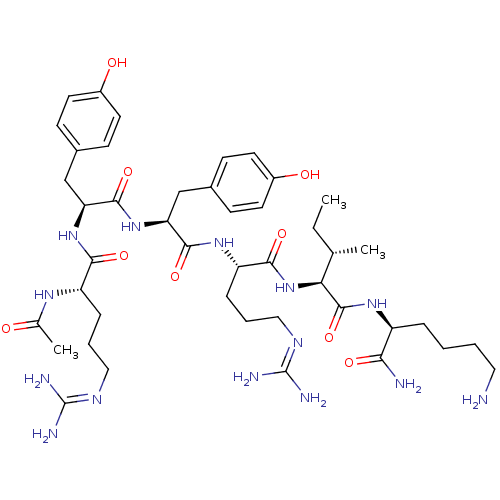
(Ac-RYYRIK-NH2 | CHEMBL437723)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C44H70N14O9/c1-4-25(2)36(42(67)54-31(37(46)62)9-5-6-20-45)58-39(64)33(11-8-22-52-44(49)50)55-40(65)34(23-27-12-16-29(60)17-13-27)57-41(66)35(24-28-14-18-30(61)19-15-28)56-38(63)32(53-26(3)59)10-7-21-51-43(47)48/h12-19,25,31-36,60-61H,4-11,20-24,45H2,1-3H3,(H2,46,62)(H,53,59)(H,54,67)(H,55,65)(H,56,63)(H,57,66)(H,58,64)(H4,47,48,51)(H4,49,50,52)/t25-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor expressed in African green monkey COS7 cells after 90 mins by topcount analysis |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Rattus norvegicus (Rat)) | BDBM149710

(US8975409, Example 38)Show SMILES CC(C)CC1(O)CCN(CC1)C(=O)Nc1cc(Oc2ccc(F)cc2)cc(c1)C(=O)Oc1ccc(cc1)C(C)(C)C(O)=O Show InChI InChI=1S/C33H37FN2O7/c1-21(2)20-33(41)13-15-36(16-14-33)31(40)35-25-17-22(18-28(19-25)42-26-11-7-24(34)8-12-26)29(37)43-27-9-5-23(6-10-27)32(3,4)30(38)39/h5-12,17-19,21,41H,13-16,20H2,1-4H3,(H,35,40)(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
ONO Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Chinese hamster ovary (CHO) cells overexpressing the human S1P2 gene were cultured in a Ham's F12 medium containing 10% fetal bovine serum (FBS), an ... |
US Patent US8975409 (2015)
BindingDB Entry DOI: 10.7270/Q2X34W60 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274467
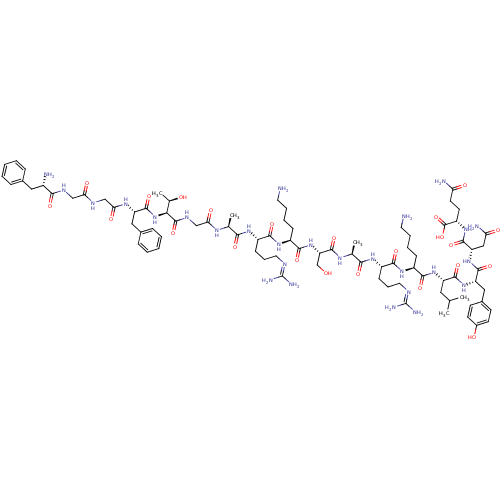
(CHEMBL505873 | FGGFTGARKSARKLYNQ)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C85H133N27O23/c1-45(2)36-59(77(128)109-61(39-51-26-28-52(115)29-27-51)78(129)110-62(40-65(90)117)79(130)107-58(83(134)135)30-31-64(89)116)108-75(126)54(22-12-14-32-86)105-74(125)57(25-17-35-96-85(93)94)104-71(122)47(4)101-81(132)63(44-113)111-76(127)55(23-13-15-33-87)106-73(124)56(24-16-34-95-84(91)92)103-70(121)46(3)100-67(119)43-99-82(133)69(48(5)114)112-80(131)60(38-50-20-10-7-11-21-50)102-68(120)42-97-66(118)41-98-72(123)53(88)37-49-18-8-6-9-19-49/h6-11,18-21,26-29,45-48,53-63,69,113-115H,12-17,22-25,30-44,86-88H2,1-5H3,(H2,89,116)(H2,90,117)(H,97,118)(H,98,123)(H,99,133)(H,100,119)(H,101,132)(H,102,120)(H,103,121)(H,104,122)(H,105,125)(H,106,124)(H,107,130)(H,108,126)(H,109,128)(H,110,129)(H,111,127)(H,112,131)(H,134,135)(H4,91,92,95)(H4,93,94,96)/t46-,47-,48+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,69-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274466

(CHEMBL525604 | FGGFTGARKSARKLFNQ)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C85H133N27O22/c1-46(2)37-59(77(127)109-61(40-52-25-13-8-14-26-52)78(128)110-62(41-65(90)116)79(129)107-58(83(133)134)31-32-64(89)115)108-75(125)54(27-15-17-33-86)105-74(124)57(30-20-36-96-85(93)94)104-71(121)48(4)101-81(131)63(45-113)111-76(126)55(28-16-18-34-87)106-73(123)56(29-19-35-95-84(91)92)103-70(120)47(3)100-67(118)44-99-82(132)69(49(5)114)112-80(130)60(39-51-23-11-7-12-24-51)102-68(119)43-97-66(117)42-98-72(122)53(88)38-50-21-9-6-10-22-50/h6-14,21-26,46-49,53-63,69,113-114H,15-20,27-45,86-88H2,1-5H3,(H2,89,115)(H2,90,116)(H,97,117)(H,98,122)(H,99,132)(H,100,118)(H,101,131)(H,102,119)(H,103,120)(H,104,121)(H,105,124)(H,106,123)(H,107,129)(H,108,125)(H,109,127)(H,110,128)(H,111,126)(H,112,130)(H,133,134)(H4,91,92,95)(H4,93,94,96)/t47-,48-,49+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,69-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Oryctolagus cuniculus) | BDBM50268430
((S)-N-((S)-1-amino-3-(3-tert-butyl-4-hydroxyphenyl...)Show SMILES CC(C)[C@H](N(C)C(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)c(c1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C28H40N4O4/c1-17(2)24(32(6)27(36)21(29)15-18-10-8-7-9-11-18)26(35)31-22(25(30)34)16-19-12-13-23(33)20(14-19)28(3,4)5/h7-14,17,21-22,24,33H,15-16,29H2,1-6H3,(H2,30,34)(H,31,35)/t21-,22-,24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]motilin from MTL receptor in rabbit duodenum homogenate |
Bioorg Med Chem Lett 19: 3426-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.059
BindingDB Entry DOI: 10.7270/Q2445MCH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274460
(CHEMBL504540 | FGGFTGARKSARKYANQ | OFQ/N,[Tyr14])Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H127N27O23/c1-43(97-64(116)41-96-79(130)66(46(4)111)109-77(128)57(36-48-19-9-6-10-20-48)100-65(117)40-94-63(115)39-95-70(121)51(85)35-47-17-7-5-8-18-47)67(118)101-54(23-15-33-92-81(88)89)71(122)104-53(22-12-14-32-84)74(125)108-60(42-110)78(129)99-44(2)68(119)102-55(24-16-34-93-82(90)91)72(123)103-52(21-11-13-31-83)73(124)107-58(37-49-25-27-50(112)28-26-49)75(126)98-45(3)69(120)106-59(38-62(87)114)76(127)105-56(80(131)132)29-30-61(86)113/h5-10,17-20,25-28,43-46,51-60,66,110-112H,11-16,21-24,29-42,83-85H2,1-4H3,(H2,86,113)(H2,87,114)(H,94,115)(H,95,121)(H,96,130)(H,97,116)(H,98,126)(H,99,129)(H,100,117)(H,101,118)(H,102,119)(H,103,123)(H,104,122)(H,105,127)(H,106,120)(H,107,124)(H,108,125)(H,109,128)(H,131,132)(H4,88,89,92)(H4,90,91,93)/t43-,44-,45-,46+,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274459
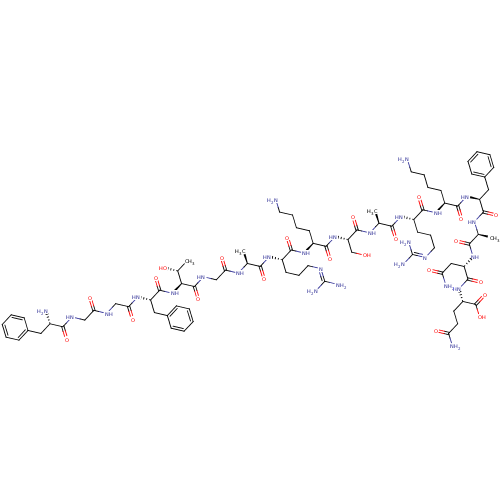
(CHEMBL524339 | FGGFTGARKSARKFANQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C82H127N27O22/c1-44(97-64(115)42-96-79(129)66(47(4)111)109-77(127)57(37-49-22-10-6-11-23-49)100-65(116)41-94-63(114)40-95-70(120)51(85)36-48-20-8-5-9-21-48)67(117)101-54(28-18-34-92-81(88)89)71(121)104-53(27-15-17-33-84)74(124)108-60(43-110)78(128)99-45(2)68(118)102-55(29-19-35-93-82(90)91)72(122)103-52(26-14-16-32-83)73(123)107-58(38-50-24-12-7-13-25-50)75(125)98-46(3)69(119)106-59(39-62(87)113)76(126)105-56(80(130)131)30-31-61(86)112/h5-13,20-25,44-47,51-60,66,110-111H,14-19,26-43,83-85H2,1-4H3,(H2,86,112)(H2,87,113)(H,94,114)(H,95,120)(H,96,129)(H,97,115)(H,98,125)(H,99,128)(H,100,116)(H,101,117)(H,102,118)(H,103,122)(H,104,121)(H,105,126)(H,106,119)(H,107,123)(H,108,124)(H,109,127)(H,130,131)(H4,88,89,92)(H4,90,91,93)/t44-,45-,46-,47+,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274458
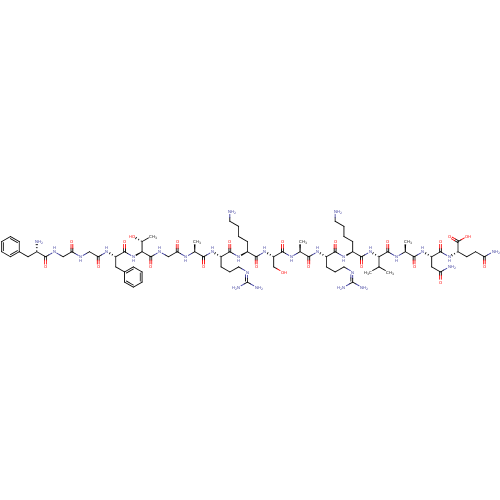
(CHEMBL524873 | FGGFTGARKSARKVANQ)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H127N27O22/c1-40(2)61(75(125)95-43(5)65(115)102-54(35-57(83)109)71(121)101-52(76(126)127)27-28-56(82)108)104-70(120)49(24-14-16-30-80)100-68(118)51(26-18-32-89-78(86)87)98-64(114)42(4)94-73(123)55(39-106)103-69(119)48(23-13-15-29-79)99-67(117)50(25-17-31-88-77(84)85)97-63(113)41(3)93-59(111)38-92-74(124)62(44(6)107)105-72(122)53(34-46-21-11-8-12-22-46)96-60(112)37-90-58(110)36-91-66(116)47(81)33-45-19-9-7-10-20-45/h7-12,19-22,40-44,47-55,61-62,106-107H,13-18,23-39,79-81H2,1-6H3,(H2,82,108)(H2,83,109)(H,90,110)(H,91,116)(H,92,124)(H,93,111)(H,94,123)(H,95,125)(H,96,112)(H,97,113)(H,98,114)(H,99,117)(H,100,118)(H,101,121)(H,102,115)(H,103,119)(H,104,120)(H,105,122)(H,126,127)(H4,84,85,88)(H4,86,87,89)/t41-,42-,43-,44+,47-,48-,49-,50-,51-,52-,53-,54-,55-,61-,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21015
((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 15: 3883-8 (2007)
Article DOI: 10.1016/j.bmc.2007.03.009
BindingDB Entry DOI: 10.7270/Q2QZ2BS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50102837
(CHEMBL3343948)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CC)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C45H72N14O9/c1-4-26(3)37(43(68)55-31(38(47)63)10-6-7-21-46)59-40(65)33(12-9-23-53-45(50)51)56-41(66)34(24-27-13-17-29(60)18-14-27)58-42(67)35(25-28-15-19-30(61)20-16-28)57-39(64)32(54-36(62)5-2)11-8-22-52-44(48)49/h13-20,26,31-35,37,60-61H,4-12,21-25,46H2,1-3H3,(H2,47,63)(H,54,62)(H,55,68)(H,56,66)(H,57,64)(H,58,67)(H,59,65)(H4,48,49,52)(H4,50,51,53)/t26-,31-,32-,33-,34-,35-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor expressed in African green monkey COS7 cells after 90 mins by topcount analysis |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(RAT) | BDBM50274457
(CHEMBL526352 | FGGFTGARKSARKAANQ)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C76H123N27O22/c1-39(91-58(109)37-90-73(123)60(43(5)105)103-71(121)52(33-45-20-10-7-11-21-45)95-59(110)36-88-57(108)35-89-65(115)46(79)32-44-18-8-6-9-19-44)62(112)96-49(24-16-30-86-75(82)83)68(118)99-48(23-13-15-29-78)69(119)102-54(38-104)72(122)94-42(4)63(113)97-50(25-17-31-87-76(84)85)67(117)98-47(22-12-14-28-77)66(116)93-40(2)61(111)92-41(3)64(114)101-53(34-56(81)107)70(120)100-51(74(124)125)26-27-55(80)106/h6-11,18-21,39-43,46-54,60,104-105H,12-17,22-38,77-79H2,1-5H3,(H2,80,106)(H2,81,107)(H,88,108)(H,89,115)(H,90,123)(H,91,109)(H,92,111)(H,93,116)(H,94,122)(H,95,110)(H,96,112)(H,97,113)(H,98,117)(H,99,118)(H,100,120)(H,101,114)(H,102,119)(H,103,121)(H,124,125)(H4,82,83,86)(H4,84,85,87)/t39-,40-,41-,42-,43+,46-,47-,48-,49-,50-,51-,52-,53-,54-,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from rat ORL1 receptor expressed in african green monkey COS7 cells |
Bioorg Med Chem 16: 9261-7 (2008)
Article DOI: 10.1016/j.bmc.2008.09.014
BindingDB Entry DOI: 10.7270/Q2MC90XH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50103534

(CHEMBL3343949)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)C(C)C)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C46H74N14O9/c1-5-27(4)37(44(69)55-32(38(48)63)10-6-7-21-47)60-41(66)34(12-9-23-54-46(51)52)57-42(67)35(24-28-13-17-30(61)18-14-28)59-43(68)36(25-29-15-19-31(62)20-16-29)58-40(65)33(56-39(64)26(2)3)11-8-22-53-45(49)50/h13-20,26-27,32-37,61-62H,5-12,21-25,47H2,1-4H3,(H2,48,63)(H,55,69)(H,56,64)(H,57,67)(H,58,65)(H,59,68)(H,60,66)(H4,49,50,53)(H4,51,52,54)/t27-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor expressed in African green monkey COS7 cells after 90 mins by topcount analysis |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Rattus norvegicus (Rat)) | BDBM149709
(US8975409, Example 19)Show SMILES CC(C)CC1(O)CN(C1)C(=O)Nc1cc(Oc2ccc(F)cc2)cc(Oc2ccc(cc2)C(C)(C)C(O)=O)c1 Show InChI InChI=1S/C30H33FN2O6/c1-19(2)16-30(37)17-33(18-30)28(36)32-22-13-25(15-26(14-22)39-24-11-7-21(31)8-12-24)38-23-9-5-20(6-10-23)29(3,4)27(34)35/h5-15,19,37H,16-18H2,1-4H3,(H,32,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.2 | 37 |
ONO Pharmaceutical Co., Ltd.
US Patent
| Assay Description
Chinese hamster ovary (CHO) cells overexpressing the human S1P2 gene were cultured in a Ham's F12 medium containing 10% fetal bovine serum (FBS), an ... |
US Patent US8975409 (2015)
BindingDB Entry DOI: 10.7270/Q2X34W60 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50372277
(CHEMBL403807)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C45H72N14O8/c1-4-27(3)37(43(67)55-31(38(47)62)15-9-10-22-46)59-40(64)33(17-12-24-53-45(50)51)56-41(65)35(26-29-18-20-30(60)21-19-29)58-42(66)34(25-28-13-7-6-8-14-28)57-39(63)32(54-36(61)5-2)16-11-23-52-44(48)49/h6-8,13-14,18-21,27,31-35,37,60H,4-5,9-12,15-17,22-26,46H2,1-3H3,(H2,47,62)(H,54,61)(H,55,67)(H,56,65)(H,57,63)(H,58,66)(H,59,64)(H4,48,49,52)(H4,50,51,53)/t27-,31-,32-,33-,34-,35-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human ORL1-Galpha fusion receptor in COS7 cells |
Bioorg Med Chem 16: 2635-44 (2008)
Article DOI: 10.1016/j.bmc.2007.11.043
BindingDB Entry DOI: 10.7270/Q2416XW9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]isoVa-RYYRIK-NH2 from human ORL1 receptor low affinity binding site expressed in African green monkey COS7 cells after 90 mins b... |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50372282
(CHEMBL428076)Show SMILES [#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C46H74N14O8/c1-4-13-37(62)55-33(17-11-24-53-45(49)50)40(64)58-35(26-29-14-7-6-8-15-29)43(67)59-36(27-30-19-21-31(61)22-20-30)42(66)57-34(18-12-25-54-46(51)52)41(65)60-38(28(3)5-2)44(68)56-32(39(48)63)16-9-10-23-47/h6-8,14-15,19-22,28,32-36,38,61H,4-5,9-13,16-18,23-27,47H2,1-3H3,(H2,48,63)(H,55,62)(H,56,68)(H,57,66)(H,58,64)(H,59,67)(H,60,65)(H4,49,50,53)(H4,51,52,54)/t28-,32-,33-,34-,35-,36-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human ORL1-Galpha fusion receptor in COS7 cells |
Bioorg Med Chem 16: 2635-44 (2008)
Article DOI: 10.1016/j.bmc.2007.11.043
BindingDB Entry DOI: 10.7270/Q2416XW9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155
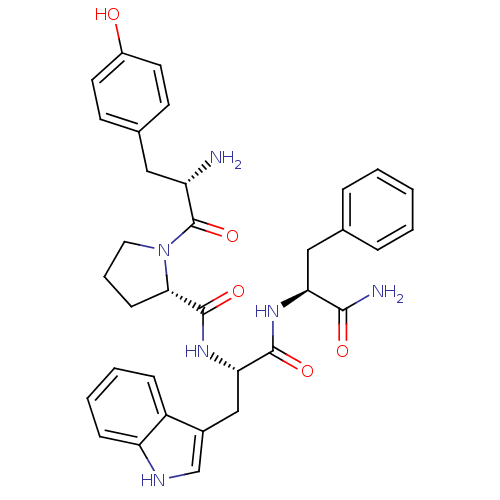
((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAGO from mu opioid receptor in rat brain membrane |
Bioorg Med Chem 15: 3883-8 (2007)
Article DOI: 10.1016/j.bmc.2007.03.009
BindingDB Entry DOI: 10.7270/Q2QZ2BS0 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Oryctolagus cuniculus) | BDBM50268433
((S)-2-((S)-2-amino-N-methyl-3-phenylpropanamido)-N...)Show SMILES CC(C)[C@H](N(C)C(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@H](C)Cc1ccc(O)c(c1)C(C)(C)C |r| Show InChI InChI=1S/C28H41N3O3/c1-18(2)25(31(7)27(34)23(29)17-20-11-9-8-10-12-20)26(33)30-19(3)15-21-13-14-24(32)22(16-21)28(4,5)6/h8-14,16,18-19,23,25,32H,15,17,29H2,1-7H3,(H,30,33)/t19-,23+,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]motilin from MTL receptor in rabbit duodenum homogenate |
Bioorg Med Chem Lett 19: 3426-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.059
BindingDB Entry DOI: 10.7270/Q2445MCH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data