

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50262557 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50244419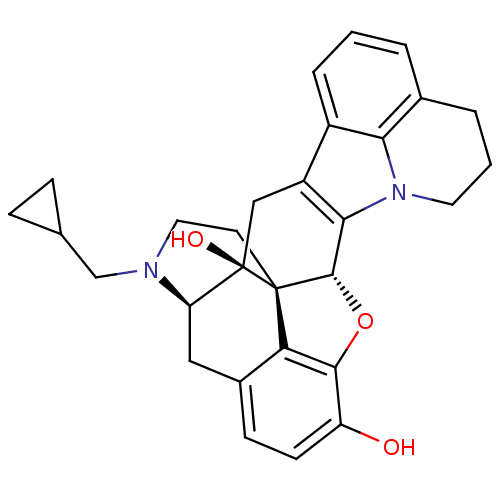 ((4bS,8R,8aS,16bR)-7-(cyclopropylmethyl)-5,6,7,8,8a...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50262557 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Binding affinity to guinea pig mu opioid receptor | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 30.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Binding affinity to guinea pig kappa opioid receptor | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50261726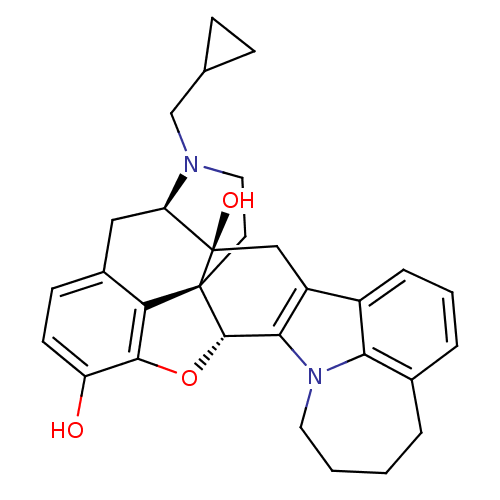 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50261791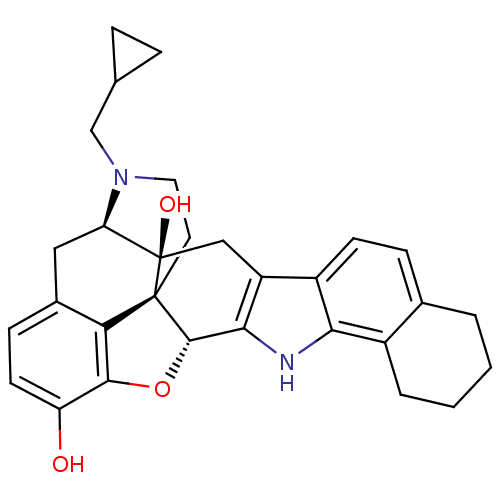 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50244419 ((4bS,8R,8aS,16bR)-7-(cyclopropylmethyl)-5,6,7,8,8a...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6-phosphogluconate dehydrogenase, decarboxylating (Rattus norvegicus) | BDBM50590158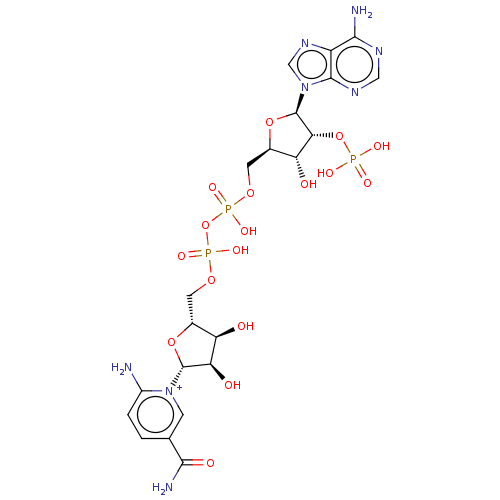 (CHEMBL5174714) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00404 BindingDB Entry DOI: 10.7270/Q2280CK5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50261726 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50261791 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50262499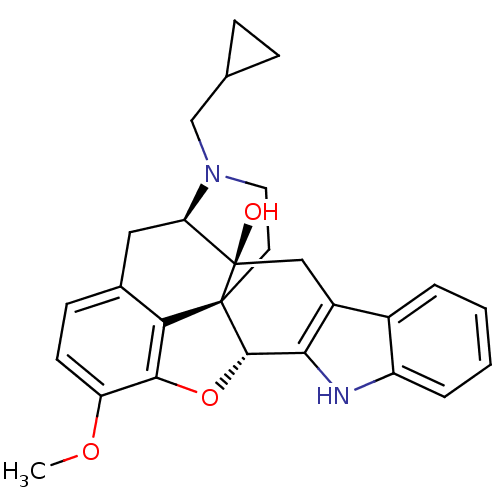 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Binding affinity to guinea pig mu opioid receptor | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50245093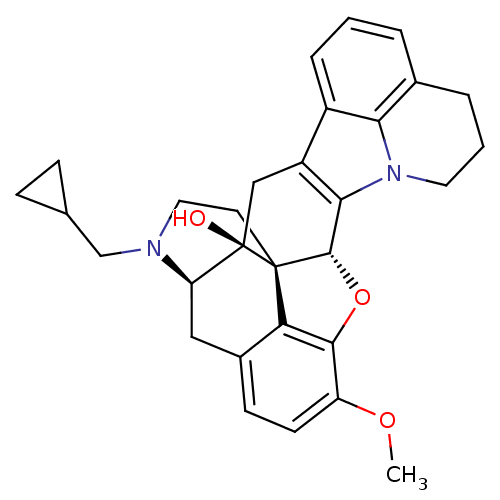 ((5R,9R,13S,14S)-17-cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50261727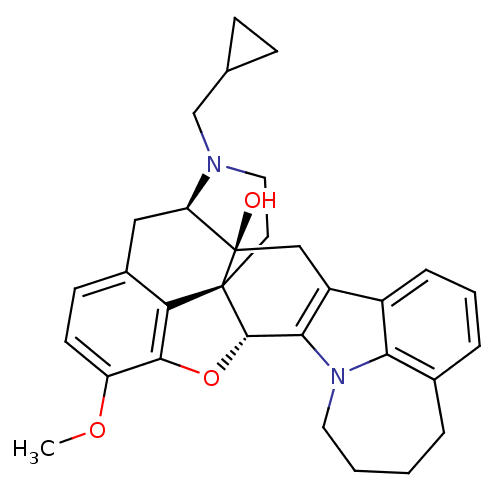 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50261727 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50245093 ((5R,9R,13S,14S)-17-cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50262556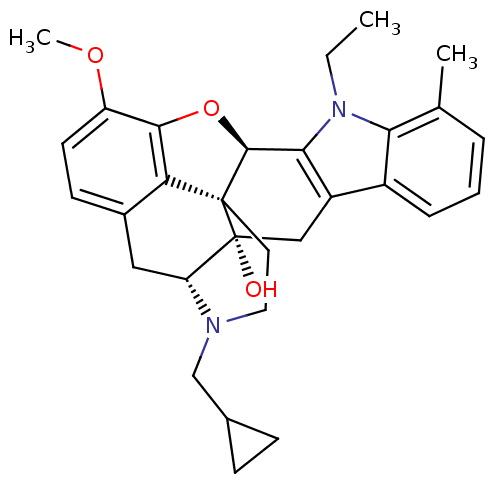 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50262499 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Binding affinity to guinea pig kappa opioid receptor | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50262556 ((5R,9R,13S,14S)-17-Cyclopropylmethyl-6,7-didehydro...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in Hartley guinea pig brain membrane | J Med Chem 51: 4404-11 (2008) Article DOI: 10.1021/jm701440h BindingDB Entry DOI: 10.7270/Q2PG1SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Rattus norvegicus) | BDBM50367068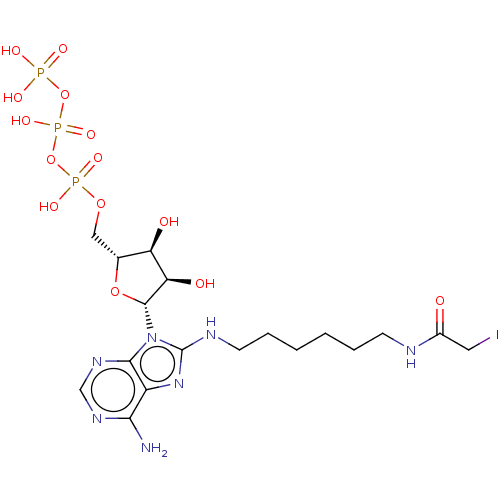 (CHEMBL3350524 | CHEMBL609795) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTP:AMP phosphotransferase AK3, mitochondrial (Rattus norvegicus) | BDBM50027422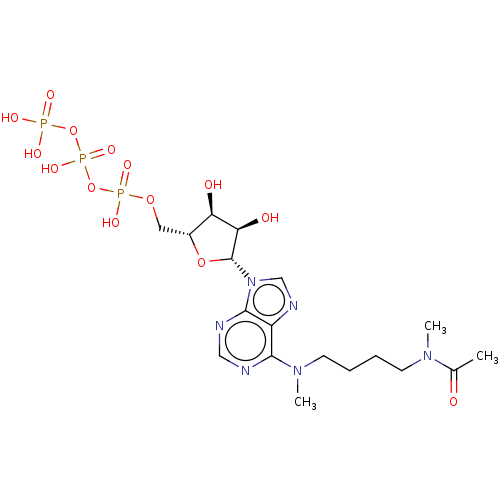 (1N-{4-[9-(3,4-dihydroxy-5-hydroxymethyltetrahydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of adenylate kinase II in rat liver with respect to ATP | J Med Chem 25: 373-81 (1982) BindingDB Entry DOI: 10.7270/Q28P5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Rattus norvegicus) | BDBM50027422 (1N-{4-[9-(3,4-dihydroxy-5-hydroxymethyltetrahydro-...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of adenylate kinase III in rat liver with respect to ATP | J Med Chem 25: 373-81 (1982) BindingDB Entry DOI: 10.7270/Q28P5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Rattus norvegicus) | BDBM50367067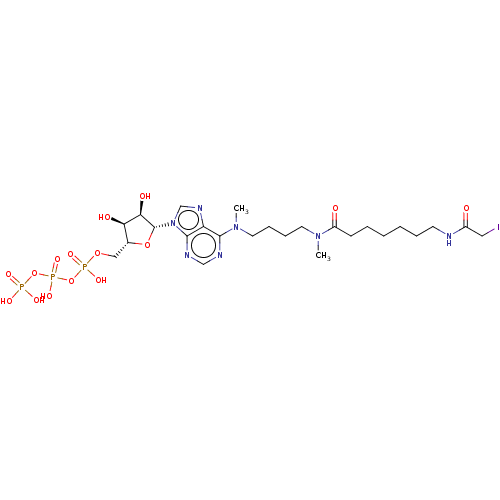 (CHEMBL3350514 | CHEMBL612174) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Rattus norvegicus) | BDBM50367066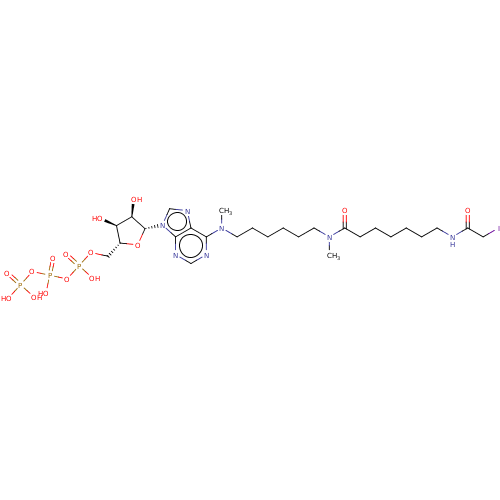 (CHEMBL3350509 | CHEMBL610373) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Rattus norvegicus) | BDBM50367073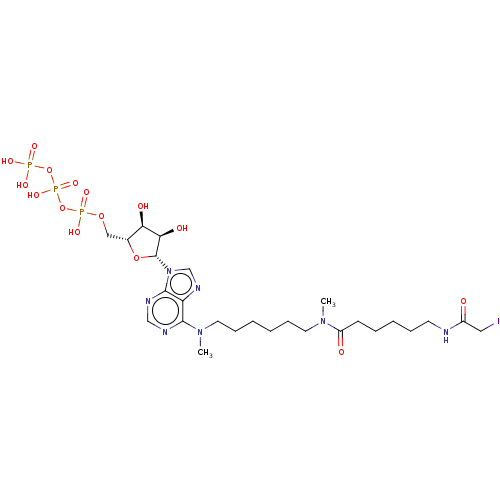 (CHEMBL3350528 | CHEMBL609819) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTP:AMP phosphotransferase AK3, mitochondrial (Rattus norvegicus) | BDBM50367067 (CHEMBL3350514 | CHEMBL612174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase III, activity expressed as Inhibition constant; Competitive inhibition | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTP:AMP phosphotransferase AK3, mitochondrial (Rattus norvegicus) | BDBM50367066 (CHEMBL3350509 | CHEMBL610373) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase III, activity expressed as Inhibition constant; Competitive inhibition | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTP:AMP phosphotransferase AK3, mitochondrial (Rattus norvegicus) | BDBM50367073 (CHEMBL3350528 | CHEMBL609819) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase III, activity expressed as Inhibition constant; Competitive inhibition | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50367071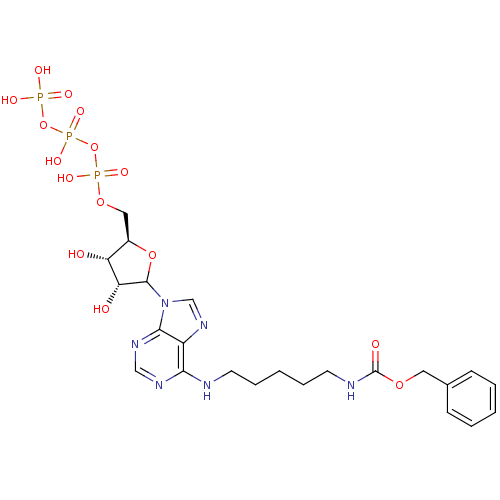 (CHEMBL607736) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Escherichia coli adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 4, mitochondrial (Homo sapiens (Human)) | BDBM50010316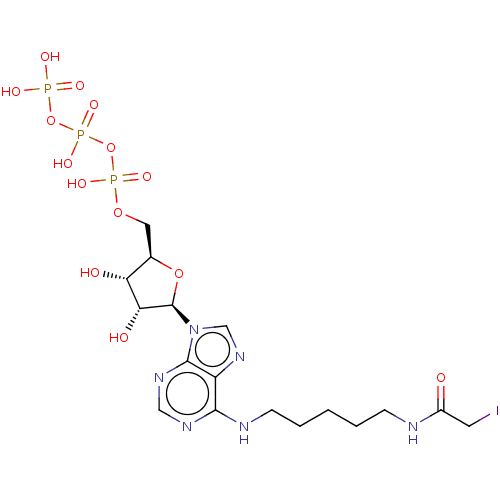 (CHEMBL3251364) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 4.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Escherichia coli adenylate kinase III, activity expressed as Inhibition constant; Non competitive inhibition | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTP:AMP phosphotransferase AK3, mitochondrial (Rattus norvegicus) | BDBM50367075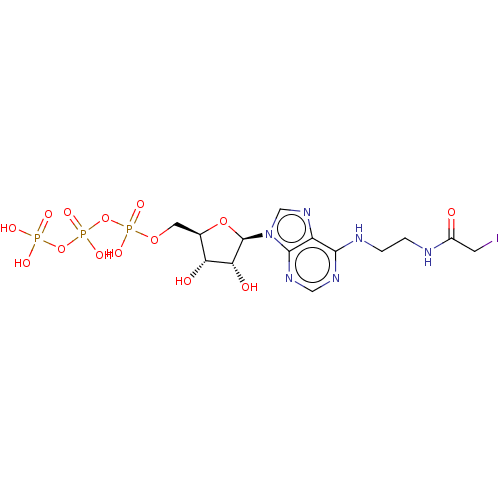 (CHEMBL3350513 | CHEMBL611859) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase III, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTP:AMP phosphotransferase AK3, mitochondrial (Rattus norvegicus) | BDBM50010316 (CHEMBL3251364) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase III, activity expressed as Inhibition constant; Competitive inhibition | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50367065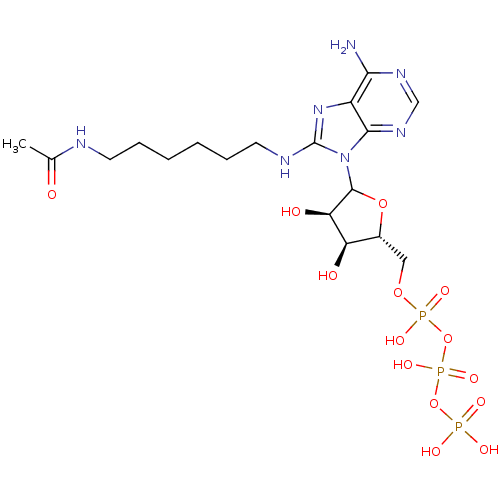 (CHEMBL608643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Escherichia coli adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50367072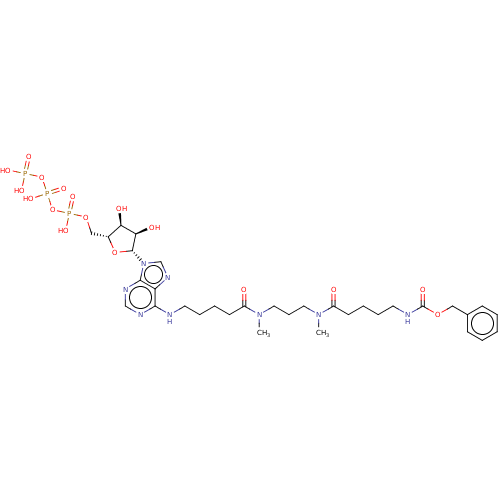 (CHEMBL3350534 | CHEMBL610134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Escherichia coli adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50367070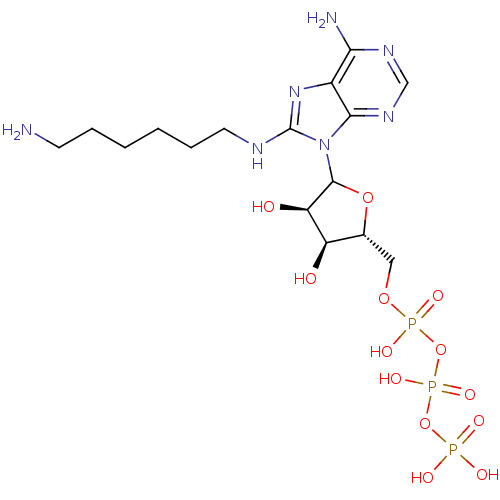 (CHEMBL609238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Escherichia coli adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate kinase 2, mitochondrial (Rattus norvegicus) | BDBM50367069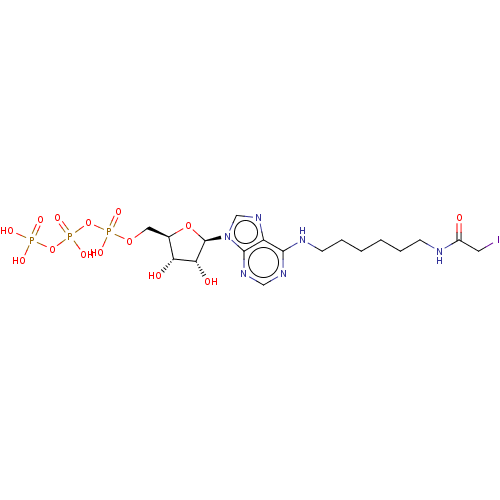 (CHEMBL3350522 | CHEMBL609792) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit rat adenylate kinase II, activity expressed as Inhibition constant | J Med Chem 25: 382-6 (1982) BindingDB Entry DOI: 10.7270/Q24F1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072905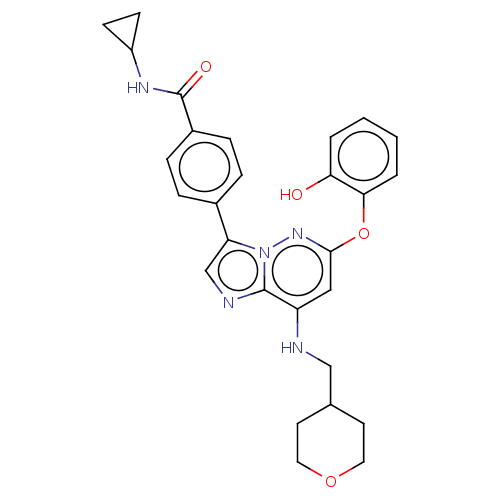 (CHEMBL3410078) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072911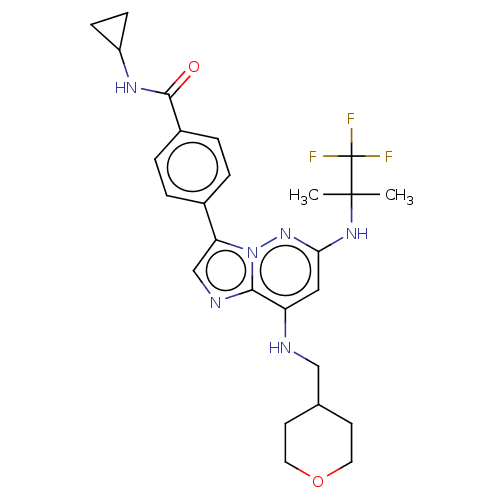 (CHEMBL3410084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072900 (CHEMBL3410073) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50386816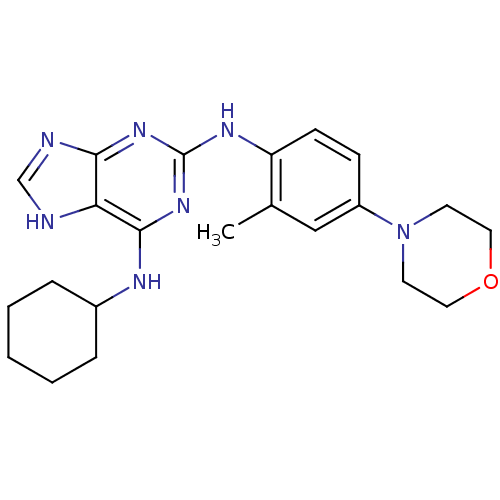 (CHEMBL2047943) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of human MPS1 expressed in Escherichia coli | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072900 (CHEMBL3410073) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072905 (CHEMBL3410078) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072911 (CHEMBL3410084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assay | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433907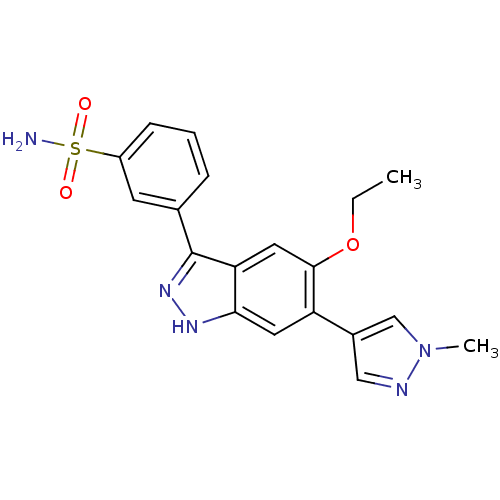 (CHEMBL2380582) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072910 (CHEMBL3410083) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072902 (CHEMBL3410075) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50433906 (CHEMBL2380583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIA | J Med Chem 56: 4343-56 (2013) Article DOI: 10.1021/jm4000215 BindingDB Entry DOI: 10.7270/Q2SF2XJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50072904 (CHEMBL3410077) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblotting | J Med Chem 58: 1760-75 (2015) Article DOI: 10.1021/jm501599u BindingDB Entry DOI: 10.7270/Q2FF3V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 233 total ) | Next | Last >> |