 Found 65 hits with Last Name = 'nagamochi' and Initial = 'm'
Found 65 hits with Last Name = 'nagamochi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Oryctolagus cuniculus) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rabbit factor 10a by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Rattus norvegicus (rat)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat factor 10a by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tissue plasminogen activator by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant tissue plasminogen activator by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 7a/soluble tissue factor by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by Lineweaver-Burk plot |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255631
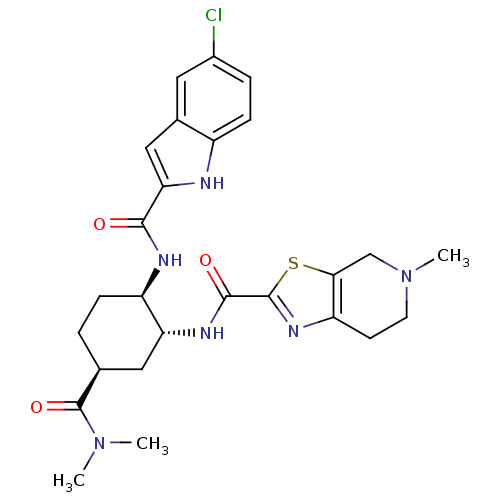
(CHEMBL516922 | N-[(1R,2R,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CN(C)C(=O)[C@H]1CC[C@@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255577

(CHEMBL473243 | N-{(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES COCCN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C28H35ClN6O4S/c1-34-9-8-21-24(15-34)40-27(33-21)26(37)32-22-13-16(28(38)35(2)10-11-39-3)4-6-20(22)31-25(36)23-14-17-12-18(29)5-7-19(17)30-23/h5,7,12,14,16,20,22,30H,4,6,8-11,13,15H2,1-3H3,(H,31,36)(H,32,37)/t16-,20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255205
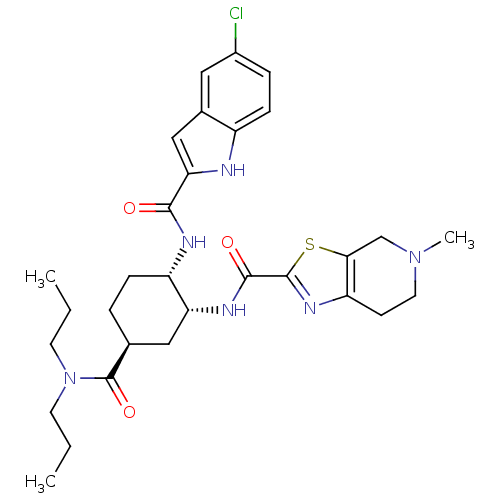
(CHEMBL508906 | N-[(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CCCN(CCC)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C30H39ClN6O3S/c1-4-11-37(12-5-2)30(40)18-6-8-22(33-27(38)25-16-19-14-20(31)7-9-21(19)32-25)24(15-18)34-28(39)29-35-23-10-13-36(3)17-26(23)41-29/h7,9,14,16,18,22,24,32H,4-6,8,10-13,15,17H2,1-3H3,(H,33,38)(H,34,39)/t18-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255207
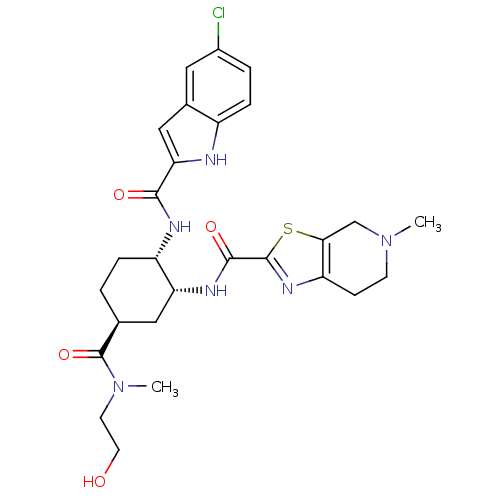
(CHEMBL474459 | N-{(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CN(CCO)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C27H33ClN6O4S/c1-33-8-7-20-23(14-33)39-26(32-20)25(37)31-21-12-15(27(38)34(2)9-10-35)3-5-19(21)30-24(36)22-13-16-11-17(28)4-6-18(16)29-22/h4,6,11,13,15,19,21,29,35H,3,5,7-10,12,14H2,1-2H3,(H,30,36)(H,31,37)/t15-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255159

(CHEMBL515569 | N-{(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CCN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C27H33ClN6O3S/c1-4-34(3)27(37)15-5-7-19(30-24(35)22-13-16-11-17(28)6-8-18(16)29-22)21(12-15)31-25(36)26-32-20-9-10-33(2)14-23(20)38-26/h6,8,11,13,15,19,21,29H,4-5,7,9-10,12,14H2,1-3H3,(H,30,35)(H,31,36)/t15-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255204

(CHEMBL515895 | N-[(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C28H35ClN6O3S/c1-4-35(5-2)28(38)16-6-8-20(31-25(36)23-14-17-12-18(29)7-9-19(17)30-23)22(13-16)32-26(37)27-33-21-10-11-34(3)15-24(21)39-27/h7,9,12,14,16,20,22,30H,4-6,8,10-11,13,15H2,1-3H3,(H,31,36)(H,32,37)/t16-,20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255576

(CHEMBL516195 | N-[(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CC(C)NC(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C27H33ClN6O3S/c1-14(2)29-24(35)15-4-6-19(31-25(36)22-12-16-10-17(28)5-7-18(16)30-22)21(11-15)32-26(37)27-33-20-8-9-34(3)13-23(20)38-27/h5,7,10,12,14-15,19,21,30H,4,6,8-9,11,13H2,1-3H3,(H,29,35)(H,31,36)(H,32,37)/t15-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255575

(CHEMBL480637 | N-[(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CCNC(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-3-28-23(34)14-4-6-18(30-24(35)21-12-15-10-16(27)5-7-17(15)29-21)20(11-14)31-25(36)26-32-19-8-9-33(2)13-22(19)37-26/h5,7,10,12,14,18,20,29H,3-4,6,8-9,11,13H2,1-2H3,(H,28,34)(H,30,35)(H,31,36)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255206
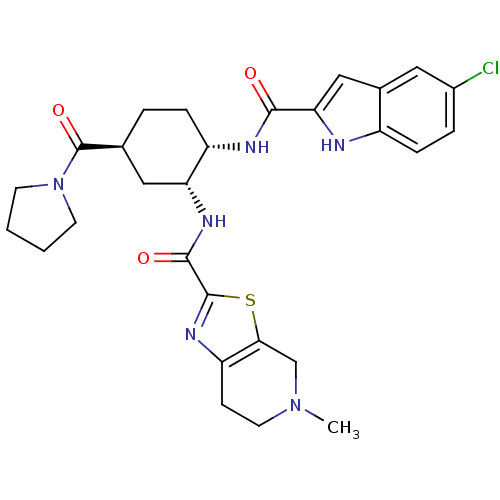
(CHEMBL514798 | N-[(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@H](CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C28H33ClN6O3S/c1-34-11-8-21-24(15-34)39-27(33-21)26(37)32-22-13-16(28(38)35-9-2-3-10-35)4-6-20(22)31-25(36)23-14-17-12-18(29)5-7-19(17)30-23/h5,7,12,14,16,20,22,30H,2-4,6,8-11,13,15H2,1H3,(H,31,36)(H,32,37)/t16-,20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255578

(CHEMBL479661 | N-[(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@@H](CO)CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C24H28ClN5O3S/c1-30-7-6-18-21(11-30)34-24(29-18)23(33)28-19-8-13(12-31)2-4-17(19)27-22(32)20-10-14-9-15(25)3-5-16(14)26-20/h3,5,9-10,13,17,19,26,31H,2,4,6-8,11-12H2,1H3,(H,27,32)(H,28,33)/t13-,17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255158
(CHEMBL473856 | N-[(1R,2S,5S)-5-(tert-Butylcarbamoy...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@H](CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C28H35ClN6O3S/c1-28(2,3)34-24(36)15-5-7-19(31-25(37)22-13-16-11-17(29)6-8-18(16)30-22)21(12-15)32-26(38)27-33-20-9-10-35(4)14-23(20)39-27/h6,8,11,13,15,19,21,30H,5,7,9-10,12,14H2,1-4H3,(H,31,37)(H,32,38)(H,34,36)/t15-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM35743

(CHEMBL479863 | cis-1,2-diaminocyclohexane derivati...)Show SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4587-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.031
BindingDB Entry DOI: 10.7270/Q2X92B3B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255579
(CHEMBL479662 | N-[(1R,2S,5S)-5-Acetyl-2-{[(5-chlor...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@H](CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1)C(C)=O |r| Show InChI InChI=1S/C25H28ClN5O3S/c1-13(32)14-3-5-18(28-23(33)21-11-15-9-16(26)4-6-17(15)27-21)20(10-14)29-24(34)25-30-19-7-8-31(2)12-22(19)35-25/h4,6,9,11,14,18,20,27H,3,5,7-8,10,12H2,1-2H3,(H,28,33)(H,29,34)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255574
((1S,3R,4S)-4-(5-chloro-1H-indole-2-carboxamido)-3-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@H](CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1)C(O)=O |r| Show InChI InChI=1S/C24H26ClN5O4S/c1-30-7-6-17-20(11-30)35-23(29-17)22(32)28-18-9-12(24(33)34)2-4-16(18)27-21(31)19-10-13-8-14(25)3-5-15(13)26-19/h3,5,8,10,12,16,18,26H,2,4,6-7,9,11H2,1H3,(H,27,31)(H,28,32)(H,33,34)/t12-,16-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255574

((1S,3R,4S)-4-(5-chloro-1H-indole-2-carboxamido)-3-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@H](CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1)C(O)=O |r| Show InChI InChI=1S/C24H26ClN5O4S/c1-30-7-6-17-20(11-30)35-23(29-17)22(32)28-18-9-12(24(33)34)2-4-16(18)27-21(31)19-10-13-8-14(25)3-5-15(13)26-19/h3,5,8,10,12,16,18,26H,2,4,6-7,9,11H2,1H3,(H,27,31)(H,28,32)(H,33,34)/t12-,16-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4587-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.031
BindingDB Entry DOI: 10.7270/Q2X92B3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50255580
(CHEMBL479860 | N-{(1R,2S,5S)-2-{[(5-Chloroindol-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@@H](CS(C)(=O)=O)CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C25H30ClN5O4S2/c1-31-8-7-19-22(12-31)36-25(30-19)24(33)29-20-9-14(13-37(2,34)35)3-5-18(20)28-23(32)21-11-15-10-16(26)4-6-17(15)27-21/h4,6,10-11,14,18,20,27H,3,5,7-9,12-13H2,1-2H3,(H,28,32)(H,29,33)/t14-,18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50272340
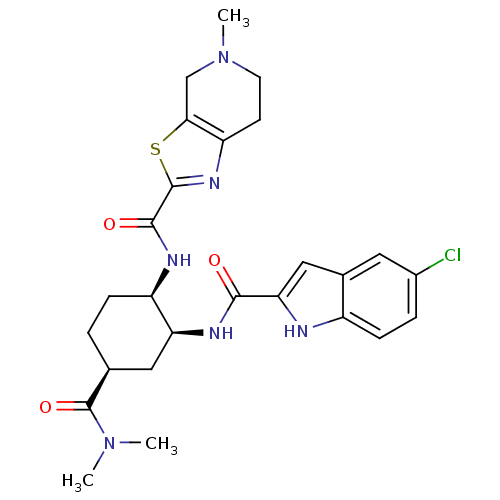
(CHEMBL501184 | cis-N-((4S)-2-(5-chloro-1H-indole-2...)Show SMILES CN(C)C(=O)[C@H]1CC[C@@H](NC(=O)c2nc3CCN(C)Cc3s2)[C@H](C1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-24(35)25-31-19-8-9-33(3)13-22(19)37-25)20(11-14)30-23(34)21-12-15-10-16(27)5-7-17(15)28-21/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,35)(H,30,34)/t14-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4587-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.031
BindingDB Entry DOI: 10.7270/Q2X92B3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214995
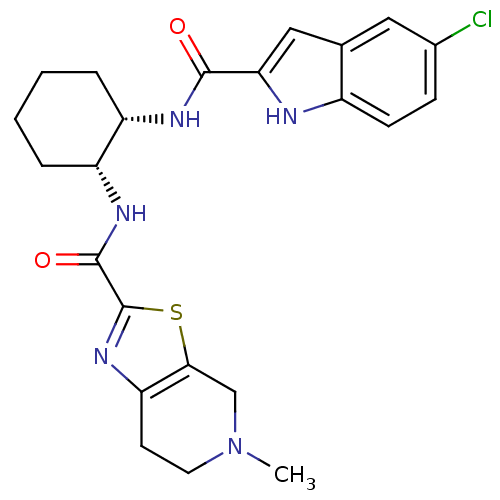
(CHEMBL245678 | N-((1R,2S)-2-(5-CHLORO-1H-INDOLE-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H26ClN5O2S/c1-29-9-8-18-20(12-29)32-23(28-18)22(31)27-17-5-3-2-4-16(17)26-21(30)19-11-13-10-14(24)6-7-15(13)25-19/h6-7,10-11,16-17,25H,2-5,8-9,12H2,1H3,(H,26,30)(H,27,31)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4587-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.031
BindingDB Entry DOI: 10.7270/Q2X92B3B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50272339
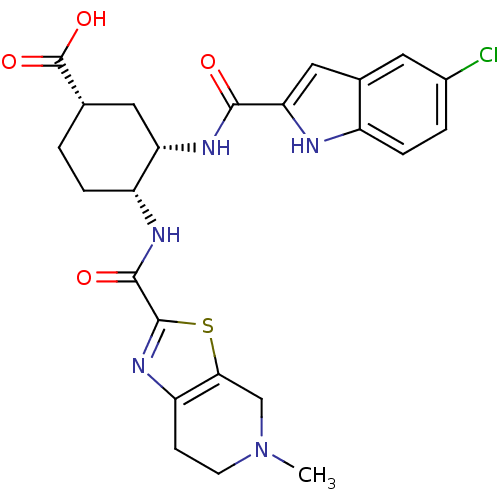
(CHEMBL501183 | cis-(4R)-3-(5-chloro-1H-indole-2-ca...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CC[C@@H](C[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1)C(O)=O |r| Show InChI InChI=1S/C24H26ClN5O4S/c1-30-7-6-17-20(11-30)35-23(29-17)22(32)27-16-4-2-12(24(33)34)9-18(16)28-21(31)19-10-13-8-14(25)3-5-15(13)26-19/h3,5,8,10,12,16,18,26H,2,4,6-7,9,11H2,1H3,(H,27,32)(H,28,31)(H,33,34)/t12-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4587-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.031
BindingDB Entry DOI: 10.7270/Q2X92B3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50214995

(CHEMBL245678 | N-((1R,2S)-2-(5-CHLORO-1H-INDOLE-2-...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H26ClN5O2S/c1-29-9-8-18-20(12-29)32-23(28-18)22(31)27-17-5-3-2-4-16(17)26-21(30)19-11-13-10-14(24)6-7-15(13)25-19/h6-7,10-11,16-17,25H,2-5,8-9,12H2,1H3,(H,26,30)(H,27,31)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50600479
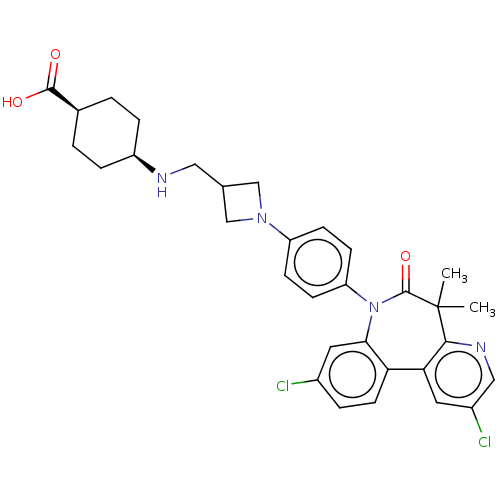
(CHEMBL5171680)Show SMILES CC1(C)c2ncc(Cl)cc2-c2ccc(Cl)cc2N(c2ccc(cc2)N2CC(CN[C@H]3CC[C@H](CC3)C(O)=O)C2)C1=O |r,wU:29.31,32.38,(3.5,-4,;2.01,-3.61,;3.1,-2.52,;1.35,-5,;2.4,-6.12,;1.96,-7.6,;.46,-7.96,;.06,-9.45,;-.6,-6.84,;-.16,-5.35,;-1.38,-4.39,;-2.7,-5.16,;-4.04,-4.4,;-4.04,-2.85,;-5.37,-2.08,;-2.71,-2.08,;-1.38,-2.85,;-.18,-1.89,;-.58,-.4,;-2.06,0,;-2.46,1.48,;-1.37,2.57,;.11,2.18,;.52,.7,;-1.77,4.06,;-1,5.4,;-2.36,6.18,;-2.75,7.67,;-1.67,8.75,;-.18,8.36,;.91,9.45,;2.4,9.05,;2.8,7.56,;1.71,6.47,;.22,6.87,;4.28,7.16,;5.37,8.25,;4.68,5.67,;-3.13,4.84,;1.32,-2.21,;2.41,-1.13,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50600480
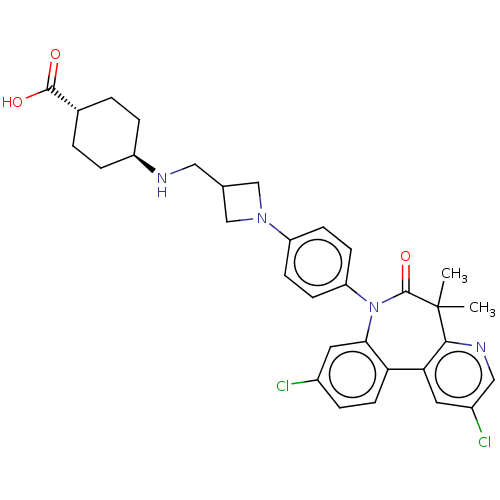
(CHEMBL5192194)Show SMILES CC1(C)c2ncc(Cl)cc2-c2ccc(Cl)cc2N(c2ccc(cc2)N2CC(CN[C@H]3CC[C@@H](CC3)C(O)=O)C2)C1=O |r,wU:32.38,wD:29.31,(3.5,-4,;2.01,-3.61,;3.1,-2.52,;1.35,-5,;2.4,-6.12,;1.96,-7.6,;.46,-7.96,;.06,-9.45,;-.6,-6.84,;-.16,-5.35,;-1.38,-4.39,;-2.7,-5.16,;-4.04,-4.4,;-4.04,-2.85,;-5.37,-2.08,;-2.71,-2.08,;-1.38,-2.85,;-.18,-1.89,;-.58,-.4,;-2.06,0,;-2.46,1.48,;-1.37,2.57,;.11,2.18,;.52,.7,;-1.77,4.06,;-1,5.4,;-2.36,6.18,;-2.75,7.67,;-1.67,8.75,;-.18,8.36,;.22,6.87,;1.71,6.47,;2.8,7.56,;2.4,9.05,;.91,9.45,;4.28,7.16,;5.37,8.25,;4.68,5.67,;-3.13,4.84,;1.32,-2.21,;2.41,-1.13,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 17: 1193-206 (2009)
Article DOI: 10.1016/j.bmc.2008.12.037
BindingDB Entry DOI: 10.7270/Q2V69JGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50272337
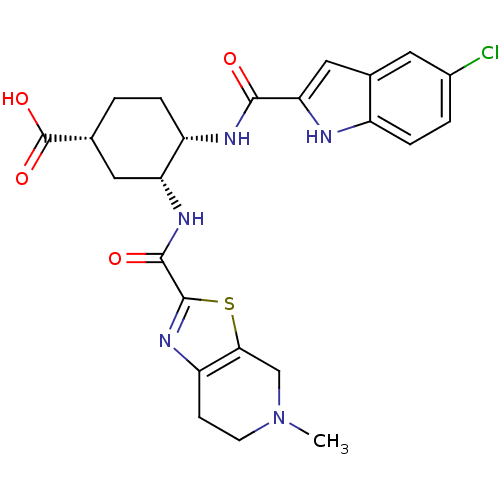
(CHEMBL503078 | cis-(1R)-4-(5-chloro-1H-indole-2-ca...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1C[C@@H](CC[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1)C(O)=O |r| Show InChI InChI=1S/C24H26ClN5O4S/c1-30-7-6-17-20(11-30)35-23(29-17)22(32)28-18-9-12(24(33)34)2-4-16(18)27-21(31)19-10-13-8-14(25)3-5-15(13)26-19/h3,5,8,10,12,16,18,26H,2,4,6-7,9,11H2,1H3,(H,27,31)(H,28,32)(H,33,34)/t12-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4587-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.031
BindingDB Entry DOI: 10.7270/Q2X92B3B |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50600483
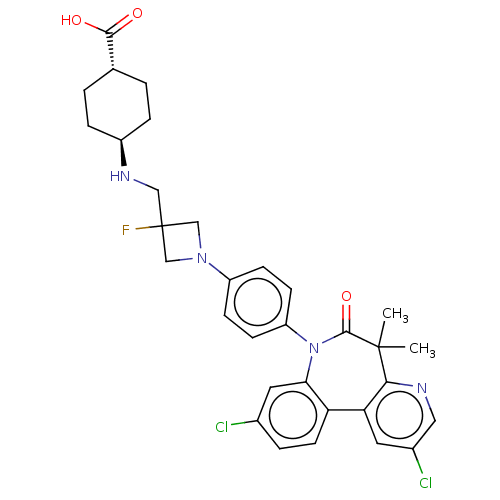
(CHEMBL5201424)Show SMILES CC1(C)c2ncc(Cl)cc2-c2ccc(Cl)cc2N(c2ccc(cc2)N2CC(F)(CN[C@H]3CC[C@@H](CC3)C(O)=O)C2)C1=O |r,wU:30.32,wD:33.39,(3.5,-3.32,;2.01,-2.92,;3.1,-1.83,;1.35,-4.31,;2.4,-5.43,;1.96,-6.91,;.46,-7.27,;.06,-8.76,;-.6,-6.15,;-.16,-4.66,;-1.38,-3.7,;-2.7,-4.47,;-4.04,-3.71,;-4.04,-2.16,;-5.37,-1.39,;-2.71,-1.39,;-1.38,-2.16,;-.18,-1.2,;-.58,.29,;-2.06,.69,;-2.46,2.17,;-1.37,3.26,;.11,2.87,;.52,1.38,;-1.77,4.75,;-1,6.08,;-2.36,6.87,;-3.69,7.64,;-1.96,8.36,;-.47,8.76,;.62,7.67,;2.11,8.07,;3.2,6.98,;2.8,5.49,;1.31,5.09,;.22,6.18,;3.89,4.4,;3.49,2.91,;5.37,4.8,;-3.13,5.53,;1.32,-1.53,;2.41,-.44,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50272338
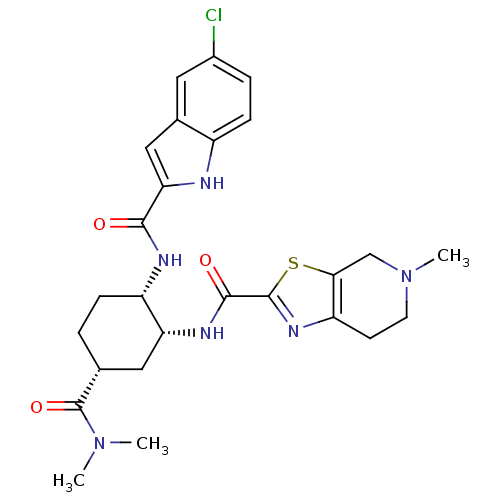
(CHEMBL500916 | cis-N-(5R)-2-(5-chloro-1H-indole-2-...)Show SMILES CN(C)C(=O)[C@@H]1CC[C@H](NC(=O)c2cc3cc(Cl)ccc3[nH]2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1 |r| Show InChI InChI=1S/C26H31ClN6O3S/c1-32(2)26(36)14-4-6-18(29-23(34)21-12-15-10-16(27)5-7-17(15)28-21)20(11-14)30-24(35)25-31-19-8-9-33(3)13-22(19)37-25/h5,7,10,12,14,18,20,28H,4,6,8-9,11,13H2,1-3H3,(H,29,34)(H,30,35)/t14-,18+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4587-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.031
BindingDB Entry DOI: 10.7270/Q2X92B3B |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50522126
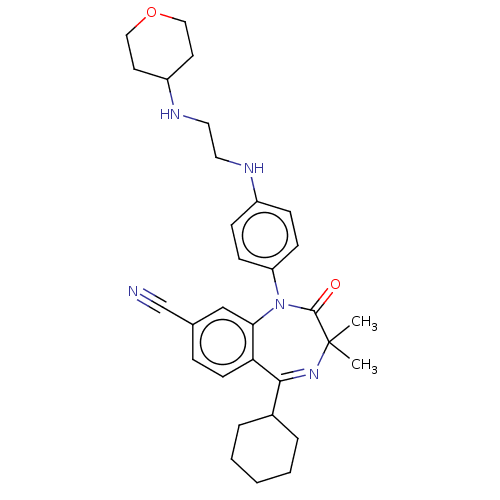
(CHEMBL4465123)Show SMILES CC1(C)N=C(C2CCCCC2)c2ccc(cc2N(c2ccc(NCCNC3CCOCC3)cc2)C1=O)C#N |t:3| Show InChI InChI=1S/C31H39N5O2/c1-31(2)30(37)36(26-11-9-24(10-12-26)33-16-17-34-25-14-18-38-19-15-25)28-20-22(21-32)8-13-27(28)29(35-31)23-6-4-3-5-7-23/h8-13,20,23,25,33-34H,3-7,14-19H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at PTHR1 (unknown origin) expressed in CHOK1 cells co-expressing Gs/Gq assessed as reduction in human PTH (1 to 34 residues)-indu... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115524
BindingDB Entry DOI: 10.7270/Q2H135J0 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50600486
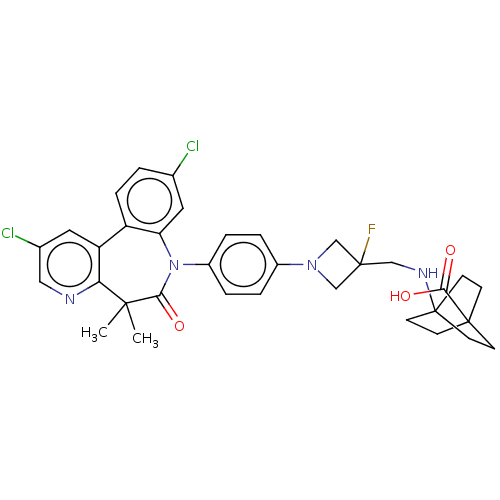
(CHEMBL5201635)Show SMILES CC1(C)c2ncc(Cl)cc2-c2ccc(Cl)cc2N(c2ccc(cc2)N2CC(F)(CNC34CCC(CC3)(CC4)C(O)=O)C2)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50600485
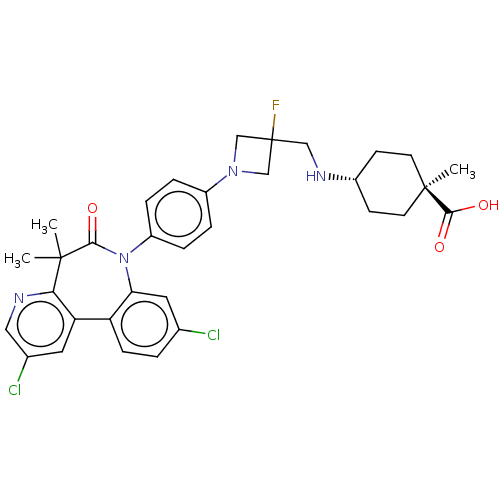
(CHEMBL5202067)Show SMILES C[C@@]1(CC[C@@H](CC1)NCC1(F)CN(C1)c1ccc(cc1)N1c2cc(Cl)ccc2-c2cc(Cl)cnc2C(C)(C)C1=O)C(O)=O |r,wU:4.7,wD:1.45,(4.63,5.09,;3.14,5.49,;3.54,6.98,;2.45,8.07,;.96,7.67,;.57,6.18,;1.65,5.09,;-.12,8.76,;-1.61,8.36,;-2.01,6.87,;-3.35,7.64,;-.66,6.08,;-1.43,4.75,;-2.78,5.53,;-1.03,3.26,;-2.12,2.17,;-1.72,.69,;-.23,.29,;.86,1.38,;.45,2.87,;.17,-1.2,;-1.03,-2.16,;-2.36,-1.39,;-3.69,-2.16,;-5.03,-1.39,;-3.69,-3.71,;-2.36,-4.47,;-1.03,-3.7,;.19,-4.66,;-.26,-6.15,;.81,-7.27,;.41,-8.76,;2.3,-6.91,;2.75,-5.43,;1.69,-4.31,;2.35,-2.92,;3.84,-3.32,;3.44,-1.83,;1.67,-1.53,;2.76,-.44,;3.54,4,;5.03,3.6,;2.45,2.91,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50542604
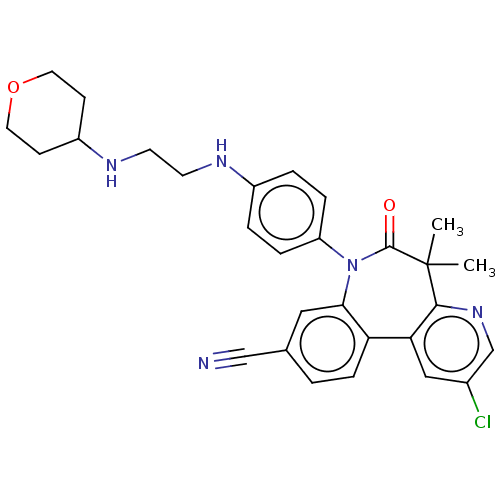
(CHEMBL4642072)Show SMILES CC1(C)c2ncc(Cl)cc2-c2ccc(cc2N(c2ccc(NCCNC3CCOCC3)cc2)C1=O)C#N Show InChI InChI=1S/C29H30ClN5O2/c1-29(2)27-25(16-20(30)18-34-27)24-8-3-19(17-31)15-26(24)35(28(29)36)23-6-4-21(5-7-23)32-11-12-33-22-9-13-37-14-10-22/h3-8,15-16,18,22,32-33H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at PTHR1 (unknown origin) expressed in CHOK1 cells co-expressing Gs/Gq assessed as reduction in human PTH (1 to 34 residues)-indu... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115524
BindingDB Entry DOI: 10.7270/Q2H135J0 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50600475
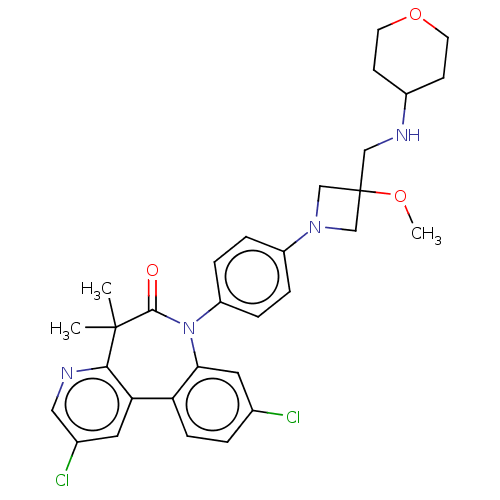
(CHEMBL5190633)Show SMILES COC1(CNC2CCOCC2)CN(C1)c1ccc(cc1)N1c2cc(Cl)ccc2-c2cc(Cl)cnc2C(C)(C)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50600477
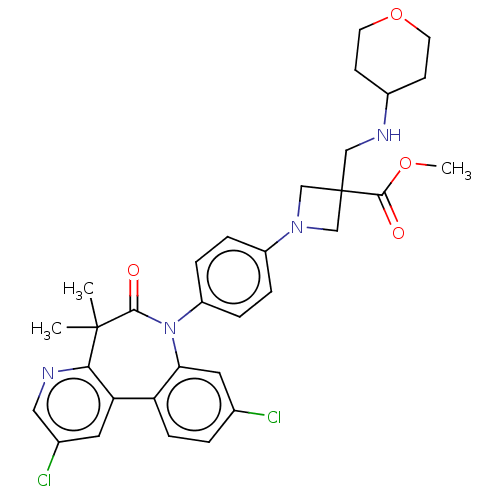
(CHEMBL5191139)Show SMILES COC(=O)C1(CNC2CCOCC2)CN(C1)c1ccc(cc1)N1c2cc(Cl)ccc2-c2cc(Cl)cnc2C(C)(C)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
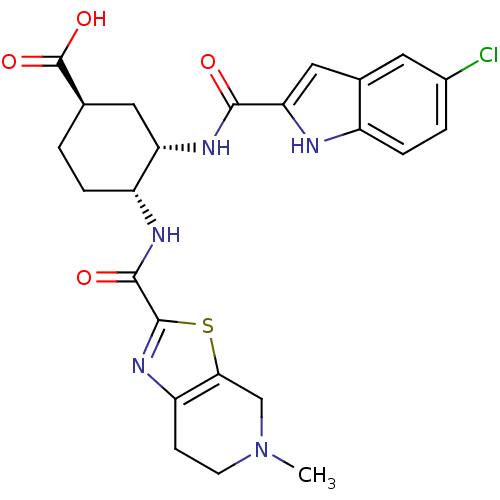
(Homo sapiens (Human)) | BDBM50272305
(CHEMBL496083 | cis-(4R)-3-(5-chloro-1H-indole-2-ca...)Show SMILES CN1CCc2nc(sc2C1)C(=O)N[C@@H]1CC[C@H](C[C@@H]1NC(=O)c1cc2cc(Cl)ccc2[nH]1)C(O)=O |r| Show InChI InChI=1S/C24H26ClN5O4S/c1-30-7-6-17-20(11-30)35-23(29-17)22(32)27-16-4-2-12(24(33)34)9-18(16)28-21(31)19-10-13-8-14(25)3-5-15(13)26-19/h3,5,8,10,12,16,18,26H,2,4,6-7,9,11H2,1H3,(H,27,32)(H,28,31)(H,33,34)/t12-,16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 4587-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.031
BindingDB Entry DOI: 10.7270/Q2X92B3B |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
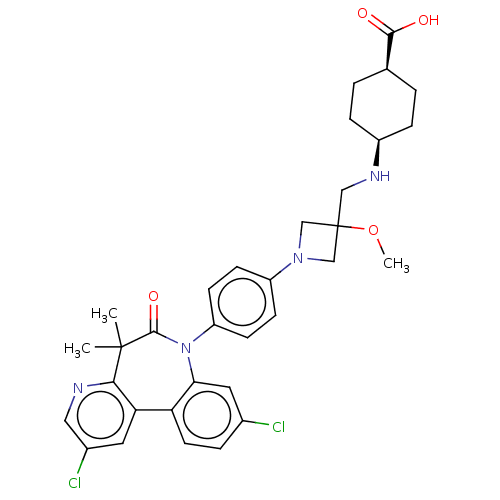
(Homo sapiens (Human)) | BDBM50600481
(CHEMBL5189957)Show SMILES COC1(CN[C@H]2CC[C@H](CC2)C(O)=O)CN(C1)c1ccc(cc1)N1c2cc(Cl)ccc2-c2cc(Cl)cnc2C(C)(C)C1=O |r,wD:5.4,8.11,(-4.93,7.56,;-3.44,7.96,;-2.36,6.87,;-1.96,8.36,;-.47,8.76,;.62,7.67,;.22,6.18,;1.31,5.09,;2.8,5.49,;3.2,6.98,;2.11,8.07,;3.89,4.4,;3.49,2.91,;5.37,4.8,;-1,6.08,;-1.77,4.75,;-3.13,5.53,;-1.37,3.26,;-2.46,2.17,;-2.06,.69,;-.58,.29,;.52,1.38,;.11,2.87,;-.18,-1.2,;-1.38,-2.16,;-2.71,-1.39,;-4.04,-2.16,;-5.37,-1.39,;-4.04,-3.71,;-2.7,-4.47,;-1.38,-3.7,;-.16,-4.66,;-.6,-6.15,;.46,-7.27,;.06,-8.76,;1.96,-6.91,;2.4,-5.43,;1.35,-4.31,;2.01,-2.92,;3.5,-3.32,;3.1,-1.83,;1.32,-1.53,;2.41,-.44,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
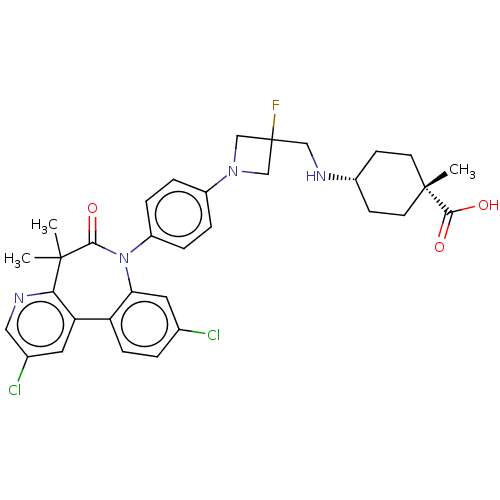
(Homo sapiens (Human)) | BDBM50600484
(CHEMBL5171324)Show SMILES C[C@]1(CC[C@@H](CC1)NCC1(F)CN(C1)c1ccc(cc1)N1c2cc(Cl)ccc2-c2cc(Cl)cnc2C(C)(C)C1=O)C(O)=O |r,wU:4.7,1.45,(4.63,5.09,;3.14,5.49,;3.54,6.98,;2.45,8.07,;.96,7.67,;.57,6.18,;1.65,5.09,;-.12,8.76,;-1.61,8.36,;-2.01,6.87,;-3.35,7.64,;-.66,6.08,;-1.43,4.75,;-2.78,5.53,;-1.03,3.26,;-2.12,2.17,;-1.72,.69,;-.23,.29,;.86,1.38,;.45,2.87,;.17,-1.2,;-1.03,-2.16,;-2.36,-1.39,;-3.69,-2.16,;-5.03,-1.39,;-3.69,-3.71,;-2.36,-4.47,;-1.03,-3.7,;.19,-4.66,;-.26,-6.15,;.81,-7.27,;.41,-8.76,;2.3,-6.91,;2.75,-5.43,;1.69,-4.31,;2.35,-2.92,;3.84,-3.32,;3.44,-1.83,;1.67,-1.53,;2.76,-.44,;3.54,4,;5.03,3.6,;2.45,2.91,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50542598
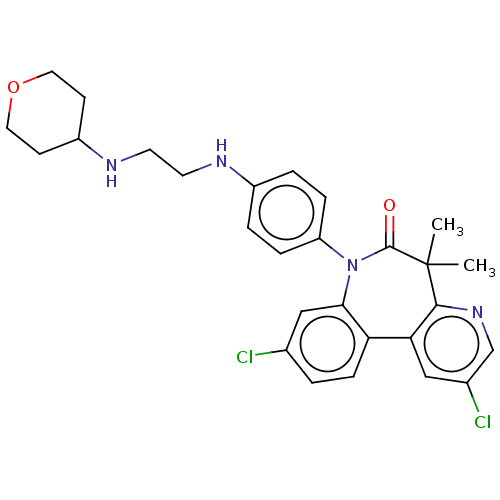
(CHEMBL4647898)Show SMILES CC1(C)c2ncc(Cl)cc2-c2ccc(Cl)cc2N(c2ccc(NCCNC3CCOCC3)cc2)C1=O Show InChI InChI=1S/C28H30Cl2N4O2/c1-28(2)26-24(15-19(30)17-33-26)23-8-3-18(29)16-25(23)34(27(28)35)22-6-4-20(5-7-22)31-11-12-32-21-9-13-36-14-10-21/h3-8,15-17,21,31-32H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at PTHR1 (unknown origin) expressed in CHOK1 cells co-expressing Gs/Gq assessed as reduction in human PTH (1 to 34 residues)-indu... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115524
BindingDB Entry DOI: 10.7270/Q2H135J0 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
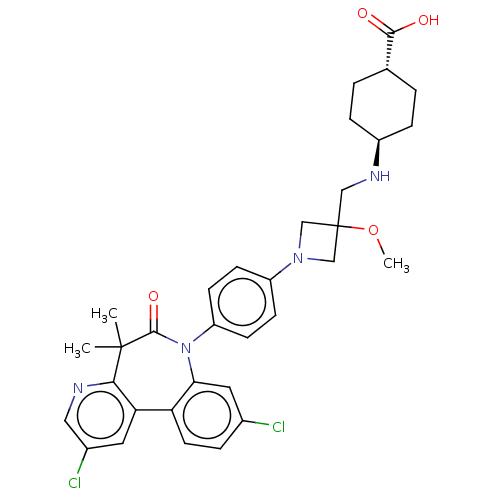
(Homo sapiens (Human)) | BDBM50600482
(CHEMBL5199789)Show SMILES COC1(CN[C@H]2CC[C@@H](CC2)C(O)=O)CN(C1)c1ccc(cc1)N1c2cc(Cl)ccc2-c2cc(Cl)cnc2C(C)(C)C1=O |r,wU:5.4,wD:8.11,(-4.93,7.56,;-3.44,7.96,;-2.36,6.87,;-1.96,8.36,;-.47,8.76,;.62,7.67,;2.11,8.07,;3.2,6.98,;2.8,5.49,;1.31,5.09,;.22,6.18,;3.89,4.4,;3.49,2.91,;5.37,4.8,;-1,6.08,;-1.77,4.75,;-3.13,5.53,;-1.37,3.26,;-2.46,2.17,;-2.06,.69,;-.58,.29,;.52,1.38,;.11,2.87,;-.18,-1.2,;-1.38,-2.16,;-2.71,-1.39,;-4.04,-2.16,;-5.37,-1.39,;-4.04,-3.71,;-2.7,-4.47,;-1.38,-3.7,;-.16,-4.66,;-.6,-6.15,;.46,-7.27,;.06,-8.76,;1.96,-6.91,;2.4,-5.43,;1.35,-4.31,;2.01,-2.92,;3.5,-3.32,;3.1,-1.83,;1.32,-1.53,;2.41,-.44,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116763
BindingDB Entry DOI: 10.7270/Q2280CPH |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50542602
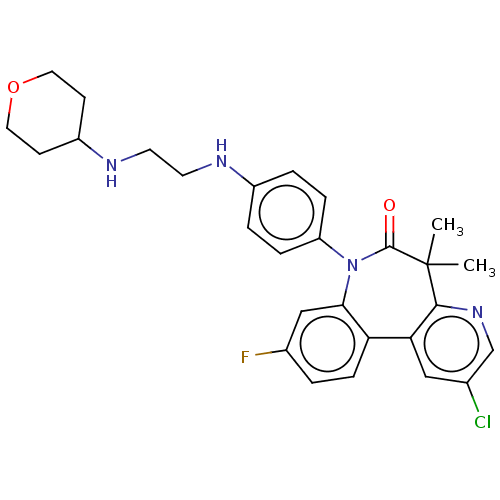
(CHEMBL4633101)Show SMILES CC1(C)c2ncc(Cl)cc2-c2ccc(F)cc2N(c2ccc(NCCNC3CCOCC3)cc2)C1=O Show InChI InChI=1S/C28H30ClFN4O2/c1-28(2)26-24(15-18(29)17-33-26)23-8-3-19(30)16-25(23)34(27(28)35)22-6-4-20(5-7-22)31-11-12-32-21-9-13-36-14-10-21/h3-8,15-17,21,31-32H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at PTHR1 (unknown origin) expressed in CHOK1 cells co-expressing Gs/Gq assessed as reduction in human PTH (1 to 34 residues)-indu... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115524
BindingDB Entry DOI: 10.7270/Q2H135J0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data