

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | -53.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3414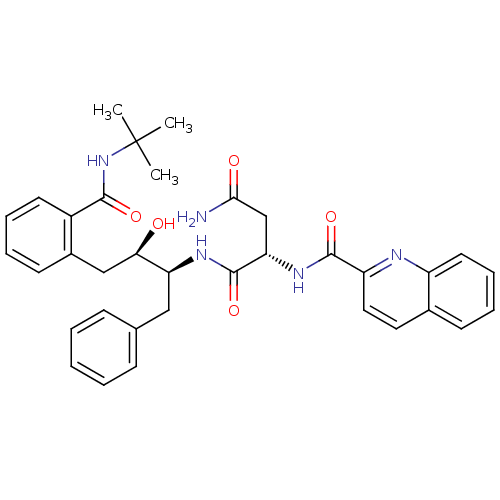 ((2S)-N-[(2S,3R)-4-[2-(tert-butylcarbamoyl)phenyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | -52.2 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3425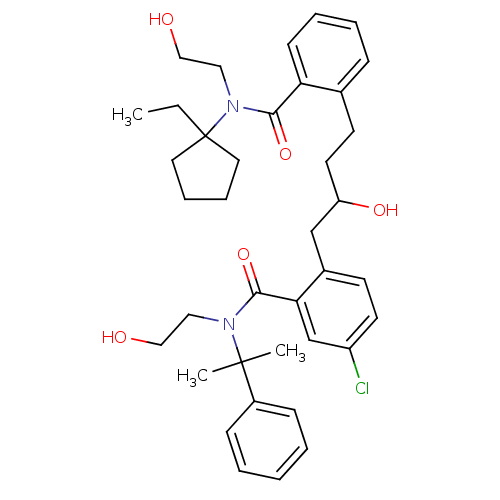 (5-chloro-2-(4-{2-[(1-ethylcyclopentyl)(2-hydroxyet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3424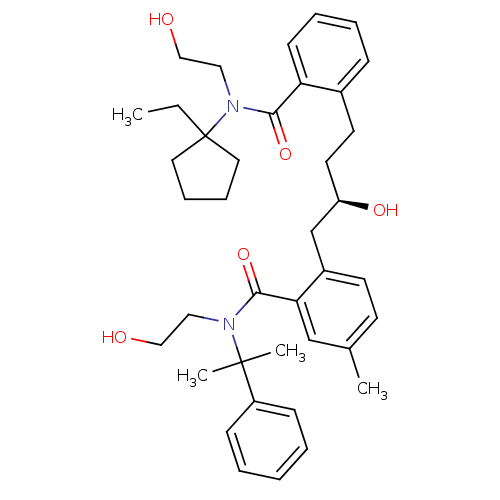 (2-[(2R)-4-{2-[(1-ethylcyclopentyl)(2-hydroxyethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50012244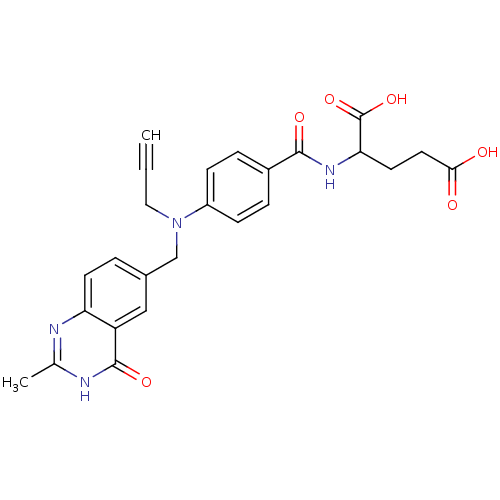 (2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-ylm...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005335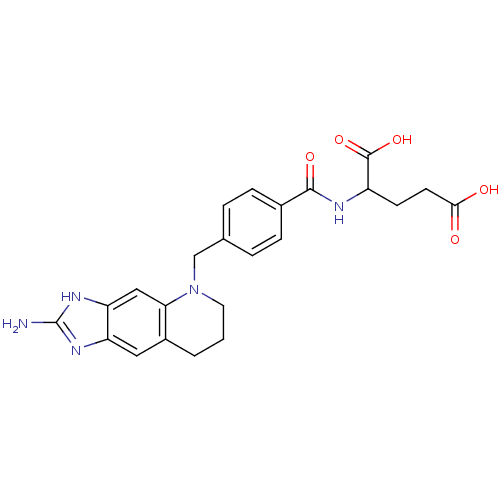 (2-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005329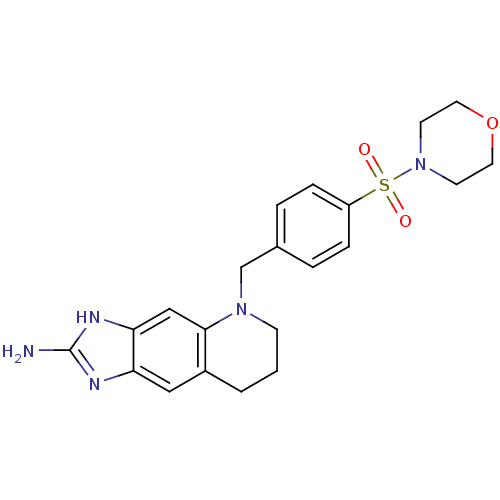 (5-[4-(Morpholine-4-sulfonyl)-benzyl]-5,6,7,8-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005324 (4-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50012244 (2-{4-[(2-Methyl-4-oxo-3,4-dihydro-quinazolin-6-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005335 (2-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005329 (5-[4-(Morpholine-4-sulfonyl)-benzyl]-5,6,7,8-tetra...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005330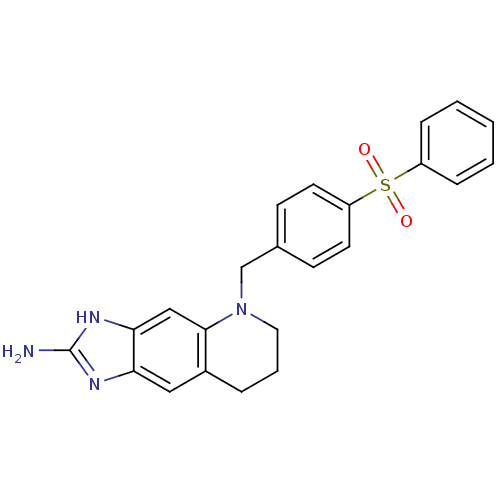 (5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50011244 (6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005324 (4-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3423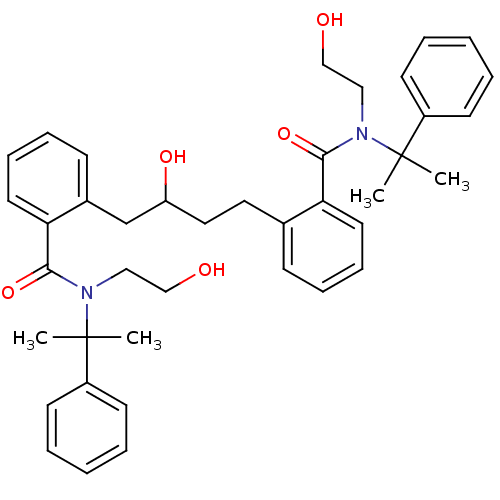 (2-(3-hydroxy-4-{2-[(2-hydroxyethyl)(2-phenylpropan...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -45.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005332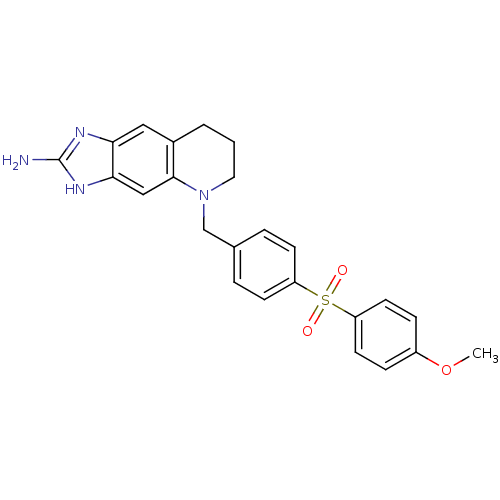 (5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-5,6,7,8-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50011244 (6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005203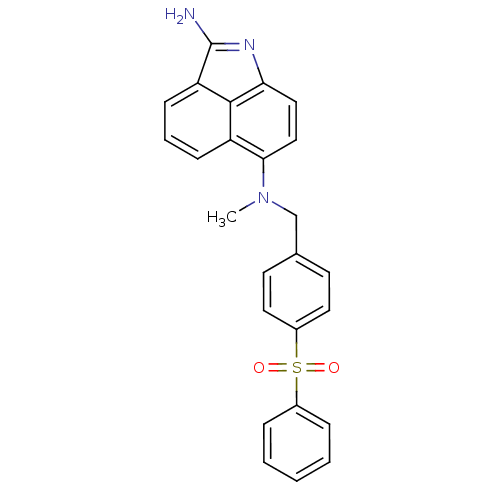 ((4-Benzenesulfonyl-benzyl)-(2-imino-1-methyl-1,2-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005330 (5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50012245 (6-{[(4-Benzenesulfonyl-3-trifluoromethyl-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005322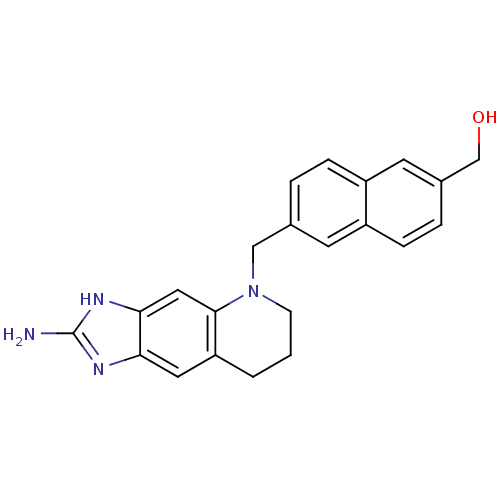 (CHEMBL353813 | [6-(2-Amino-1,6,7,8-tetrahydro-imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005322 (CHEMBL353813 | [6-(2-Amino-1,6,7,8-tetrahydro-imid...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005332 (5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-5,6,7,8-t...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005330 (5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005330 (5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50012245 (6-{[(4-Benzenesulfonyl-3-trifluoromethyl-phenyl)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005327 (5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-1-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005323 (5-[4-(Piperazine-1-sulfonyl)-benzyl]-5,6,7,8-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005323 (5-[4-(Piperazine-1-sulfonyl)-benzyl]-5,6,7,8-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50012240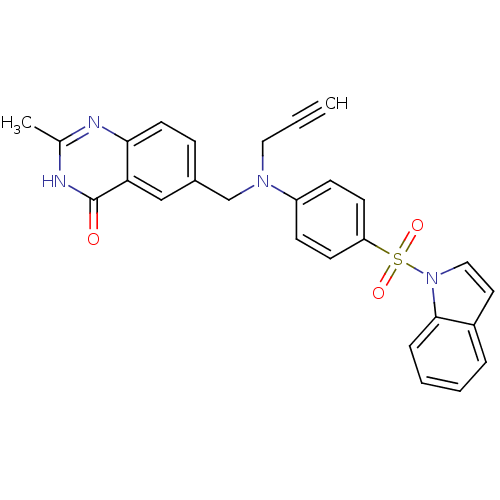 (6-({[4-(Indole-1-sulfonyl)-phenyl]-prop-2-ynyl-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3422 (N-tert-butyl-2-(3-hydroxy-4-{2-[(2-hydroxyethyl)(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | -41.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005323 (5-[4-(Piperazine-1-sulfonyl)-benzyl]-5,6,7,8-tetra...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005323 (5-[4-(Piperazine-1-sulfonyl)-benzyl]-5,6,7,8-tetra...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50012240 (6-({[4-(Indole-1-sulfonyl)-phenyl]-prop-2-ynyl-ami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005331 (5-(4-Benzenesulfonyl-phenylsulfanyl)-5,6,7,8-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005331 (5-(4-Benzenesulfonyl-phenylsulfanyl)-5,6,7,8-tetra...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50012243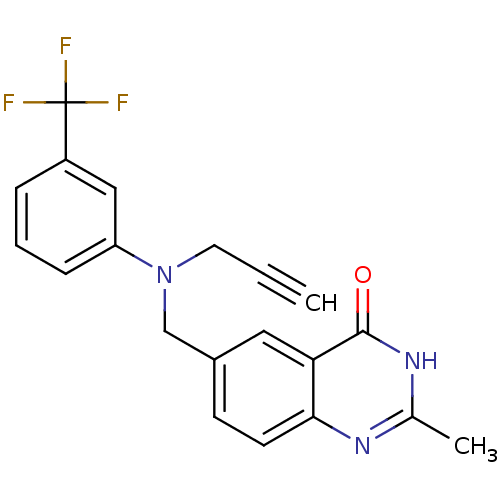 (2-Methyl-6-{[prop-2-ynyl-(3-trifluoromethyl-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005325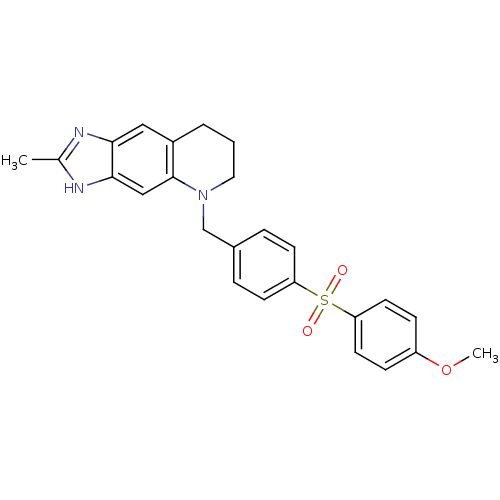 (5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-2-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50012243 (2-Methyl-6-{[prop-2-ynyl-(3-trifluoromethyl-phenyl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005326 (5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-1,2-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3410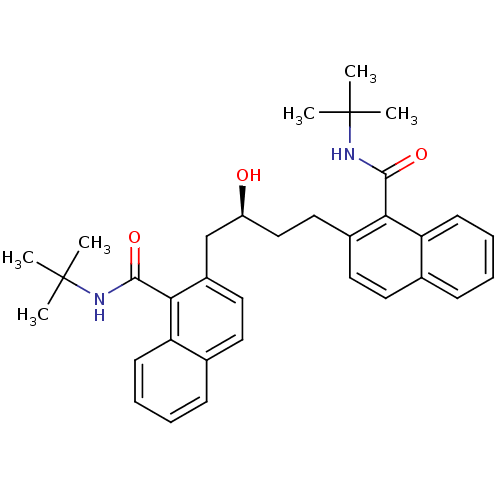 (2-Butanol deriv. 18 | N-tert-butyl-2-[(3S)-4-[1-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 480 | -37.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | J Med Chem 39: 2781-94 (1996) Article DOI: 10.1021/jm960093o BindingDB Entry DOI: 10.7270/Q2K072F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3410 (2-Butanol deriv. 18 | N-tert-butyl-2-[(3S)-4-[1-(t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 481 | -37.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005327 (5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-1-methyl-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005202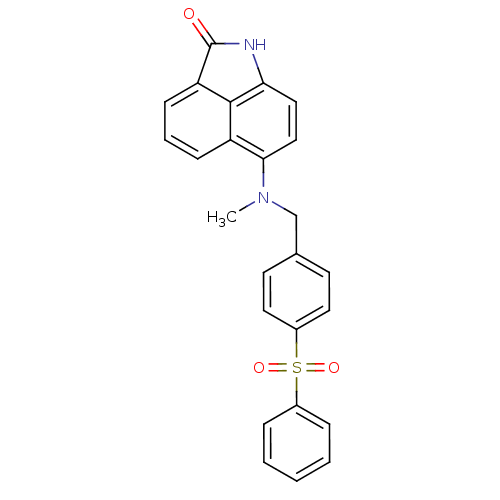 (6-[(4-Benzenesulfonyl-benzyl)-methyl-amino]-1-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005333 (CHEMBL169726 | [5-(4-Benzenesulfonyl-benzyl)-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (TS) | J Med Chem 35: 847-58 (1992) BindingDB Entry DOI: 10.7270/Q2X0660R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005183 (1-Methyl-6-{methyl-[4-(piperazine-1-sulfonyl)-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human Thymidylate synthase (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005202 (6-[(4-Benzenesulfonyl-benzyl)-methyl-amino]-1-meth...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Thymidylate synthase of E. coli (Ki) | J Med Chem 34: 1925-34 (1991) BindingDB Entry DOI: 10.7270/Q25Q4V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM3421 (N-tert-Butyl-N-(2-hydroxyethyl)-2-[3-hydroxy-4-[2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | -35.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
Agouron Pharmaceuticals, Inc. | Assay Description Protease activity was measured by continuous chromogenic assay. The chromogenic peptide His-Lys-Ala-Arg-Val-Leu- (p-NO2-Phe)-Glu-Ala-Nleu-Ser (Bache... | Proc Natl Acad Sci U S A 92: 3298-302 (1995) Article DOI: 10.1073/pnas.92.8.3298 BindingDB Entry DOI: 10.7270/Q2F769RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |