

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50341056 (5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kbeta | Bioorg Med Chem Lett 21: 2064-70 (2011) Article DOI: 10.1016/j.bmcl.2011.02.007 BindingDB Entry DOI: 10.7270/Q2FT8MBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50341056 (5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay | Bioorg Med Chem Lett 21: 2064-70 (2011) Article DOI: 10.1016/j.bmcl.2011.02.007 BindingDB Entry DOI: 10.7270/Q2FT8MBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228440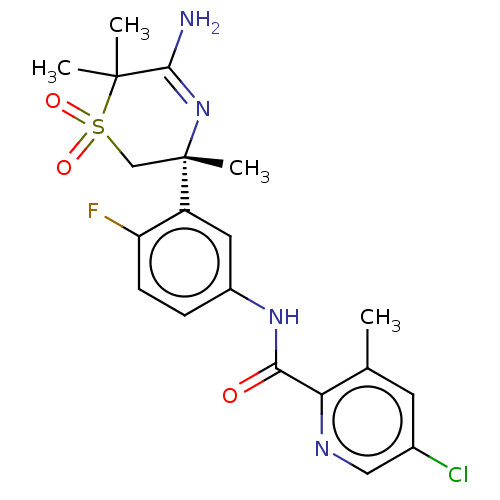 (US9556135, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM229582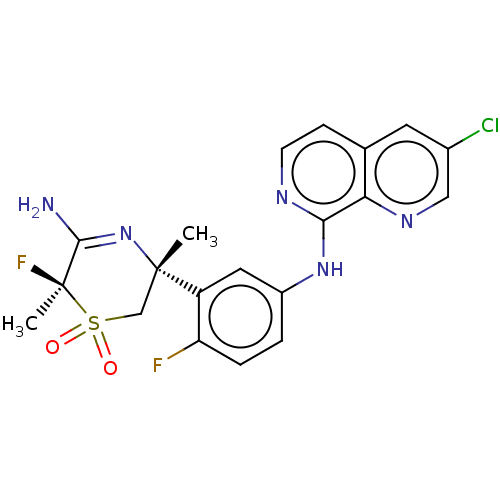 (US9556135, 174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230011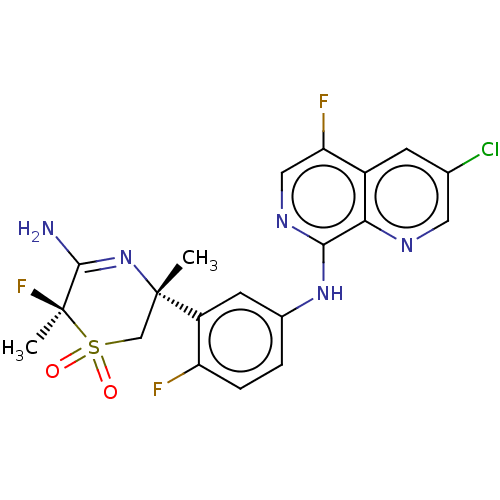 (US9556135, 178) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50341056 (5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta | Bioorg Med Chem Lett 21: 2064-70 (2011) Article DOI: 10.1016/j.bmcl.2011.02.007 BindingDB Entry DOI: 10.7270/Q2FT8MBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228441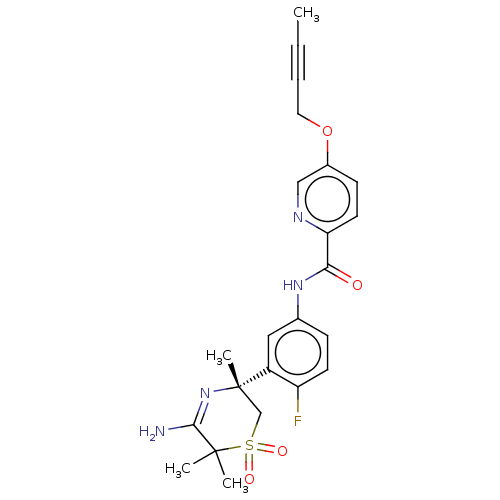 (US9556135, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228479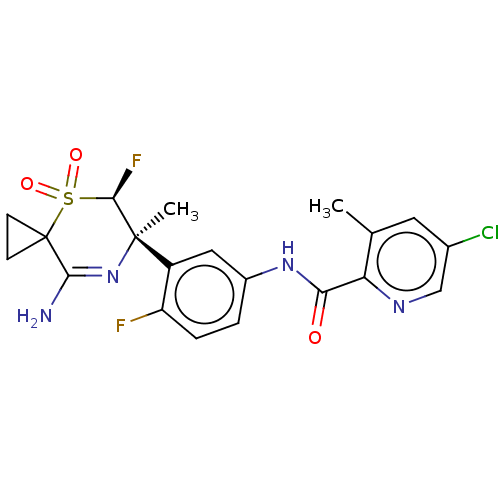 (US9556135, 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230020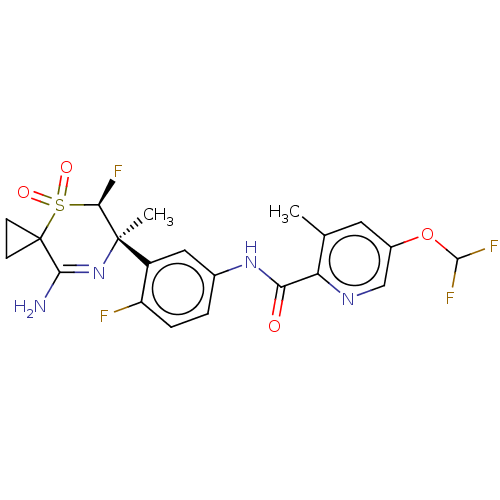 (US9556135, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM229989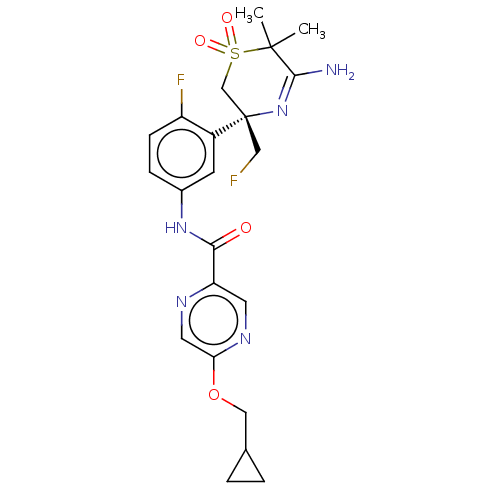 (US9556135, 156) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228404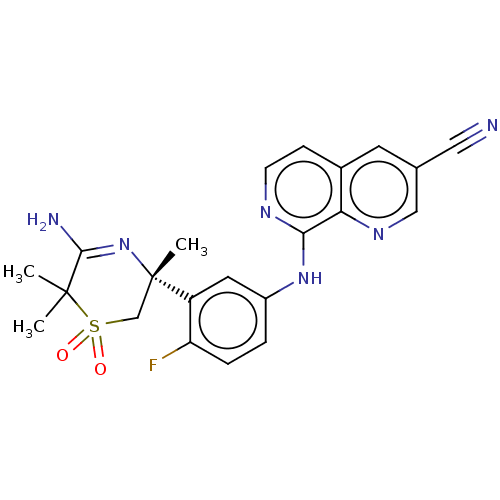 (US9556135, 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230028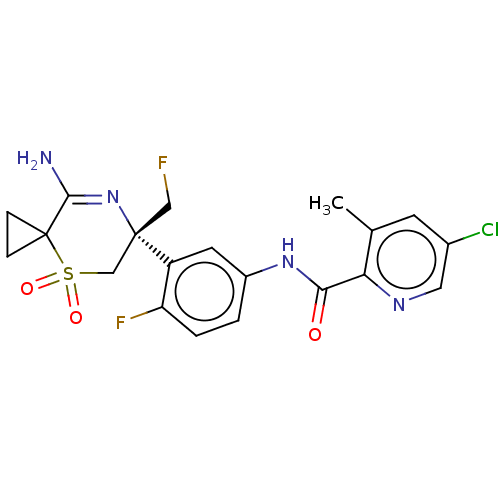 (US9556135, 216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM229992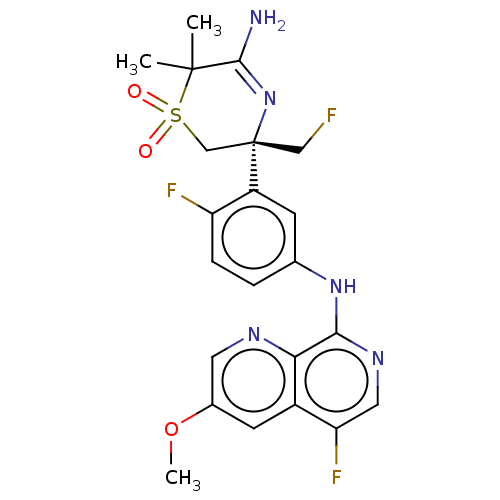 (US9556135, 159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228414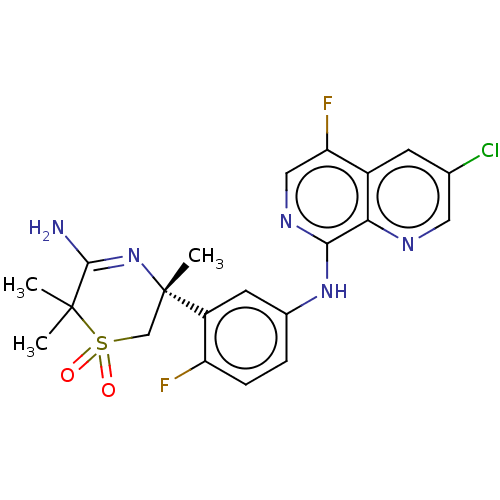 (US9556135, 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM229990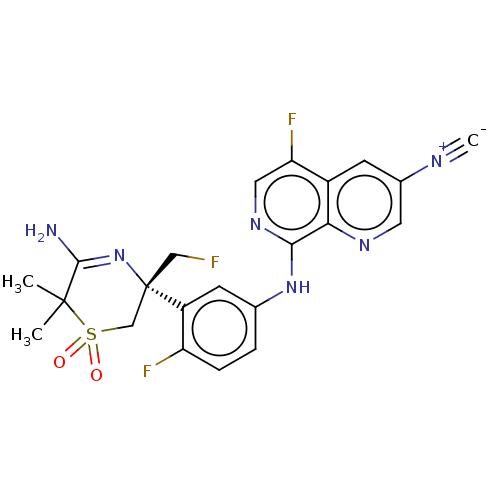 (US9556135, 157) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228399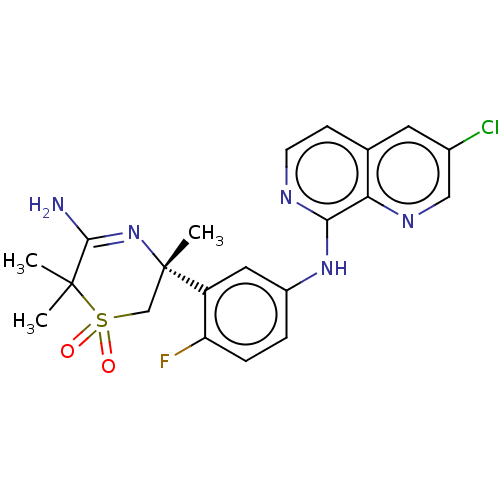 (US9556135, 45) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228473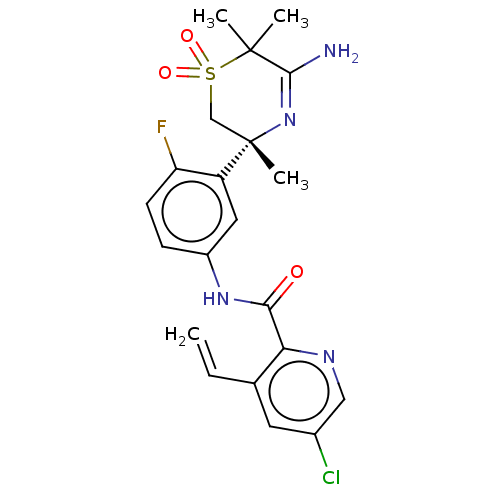 (US9556135, 195) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228439 (US9556135, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228868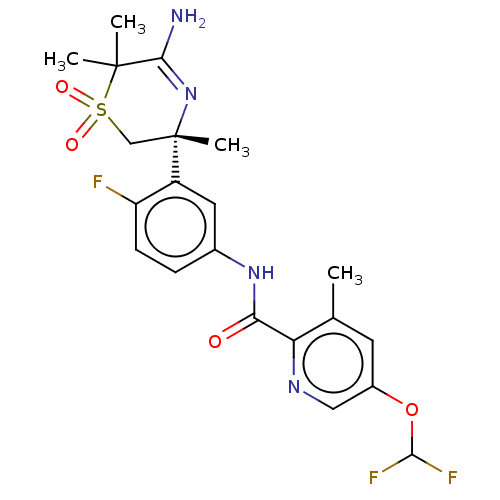 (US9556135, 144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50341056 (5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha | Bioorg Med Chem Lett 21: 2064-70 (2011) Article DOI: 10.1016/j.bmcl.2011.02.007 BindingDB Entry DOI: 10.7270/Q2FT8MBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal GST-tagged JAK3 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... | J Med Chem 54: 8440-50 (2011) Article DOI: 10.1021/jm200911r BindingDB Entry DOI: 10.7270/Q22N52PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230018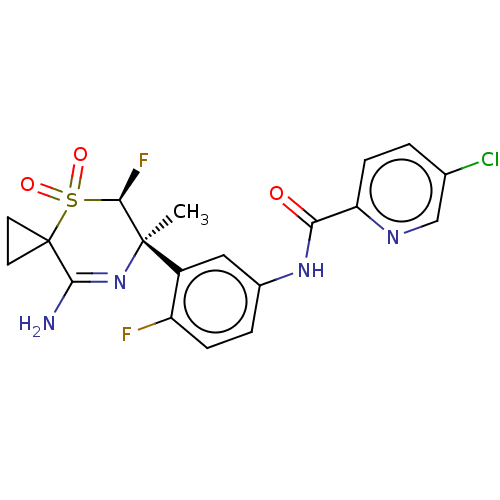 (US9556135, 201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230001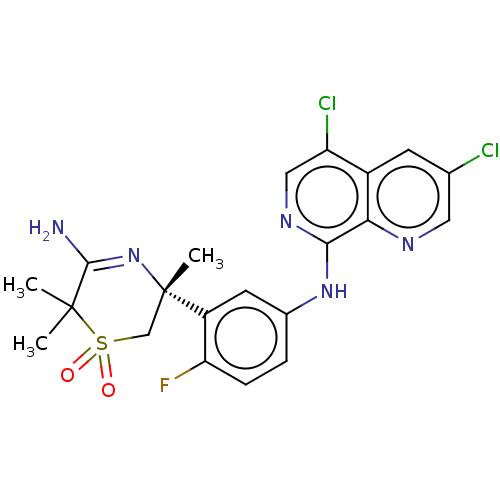 (US9556135, 192) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230025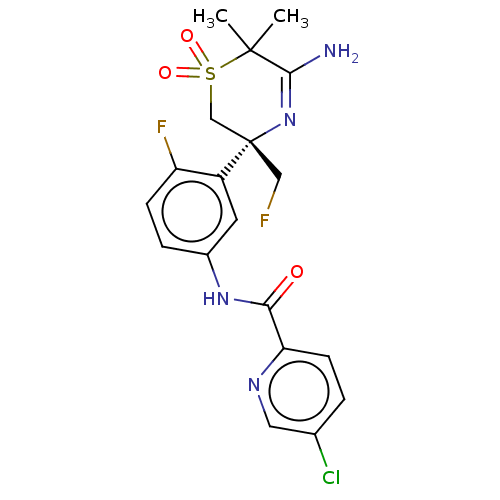 (US9556135, 193) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM229056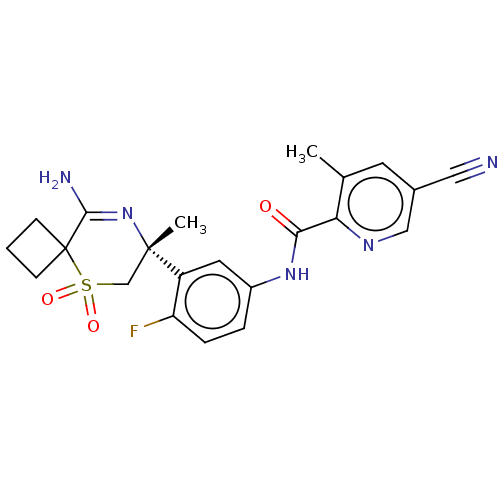 (US9556135, 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230012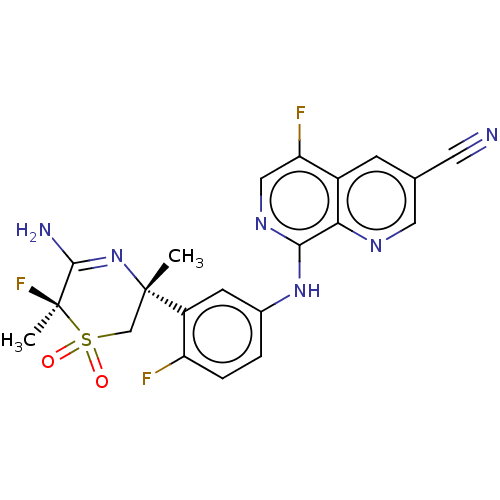 (US9556135, 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230000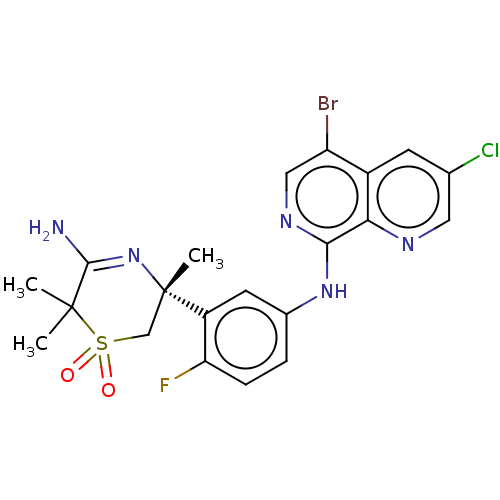 (US9556135, 188) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012660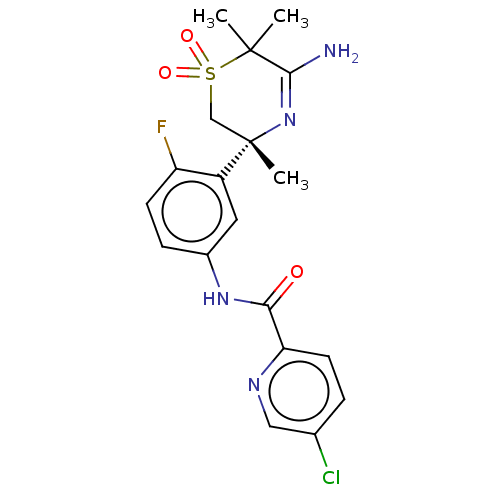 (CHEMBL3261078 | US9273042, 5 | US9556135, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228478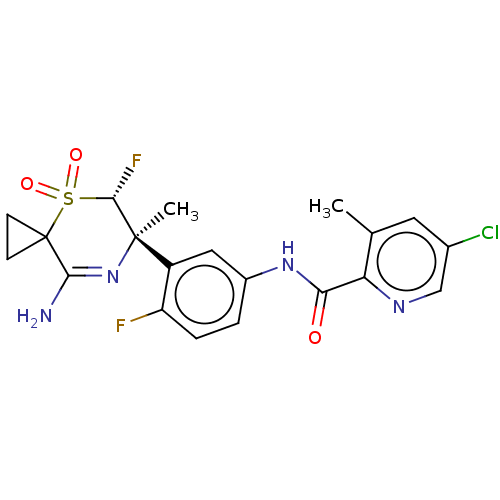 (US9556135, 204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM229508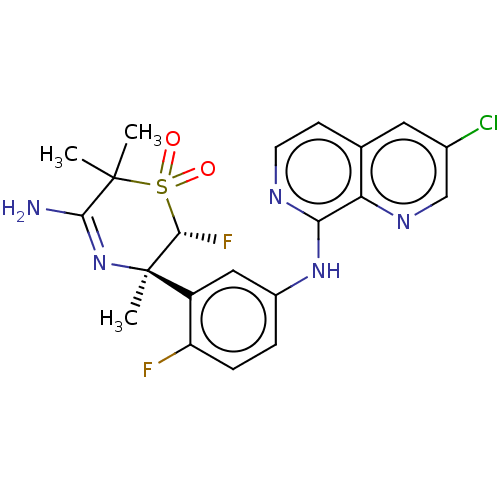 (US9556135, 168) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM230022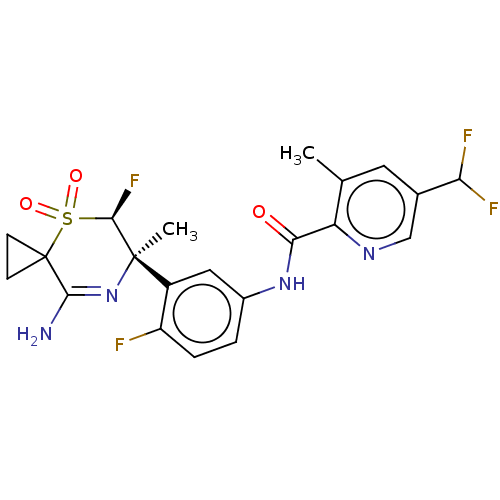 (US9556135, 207) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM14974 (CHEMBL221484 | N-(3-(3-(Dimethylamino)propyl)-5-(t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 611-26 (2007) Article DOI: 10.1021/jm061107l BindingDB Entry DOI: 10.7270/Q2MC8X8S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50266148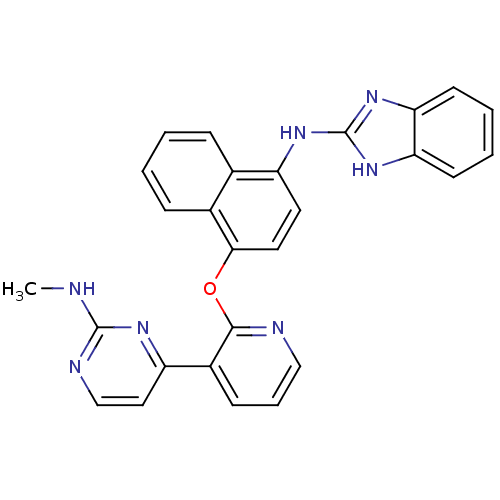 (CHEMBL465793 | N-(4-(3-(2-(methylamino)pyrimidin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Tie2 (unknown origin) | Bioorg Med Chem Lett 19: 424-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.056 BindingDB Entry DOI: 10.7270/Q29P31HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM228963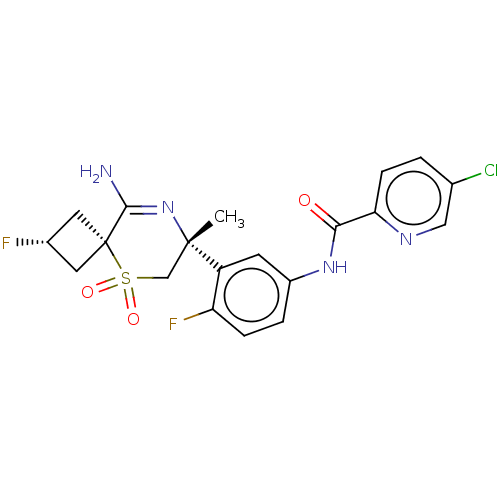 (US9556135, 125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description Several animal models, including mouse, rat, dog, and monkey, may be used to screen for inhibition of beta-secretase activity in vivo following admin... | US Patent US9556135 (2017) BindingDB Entry DOI: 10.7270/Q2X0691F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14967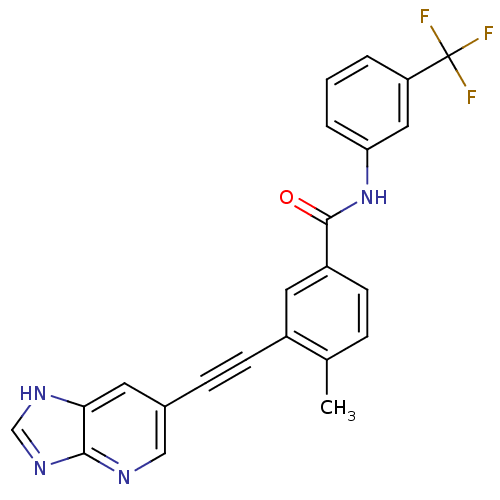 (3-(2-(3H-Imidazo[4,5-b]pyridin-6-yl)ethynyl)-4-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14966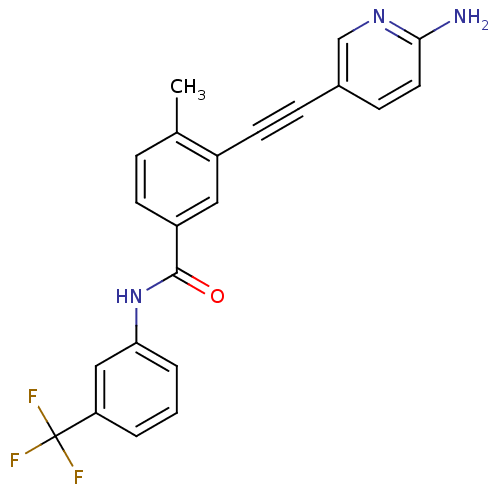 (3-(2-(6-Aminopyridin-3-yl)ethynyl)-4-methyl-N-(3-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14950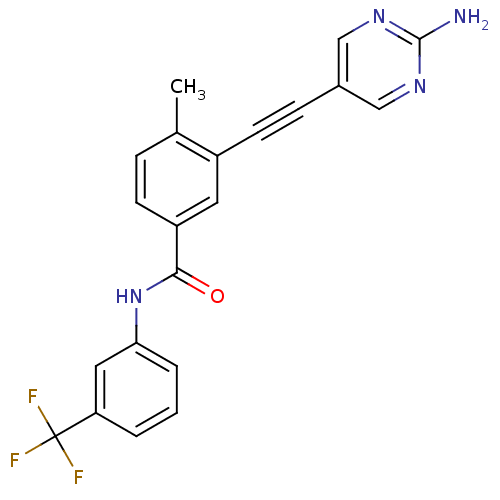 (3-(2-(2-Aminopyrimidin-5-yl)ethynyl)-4-methyl-N-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14949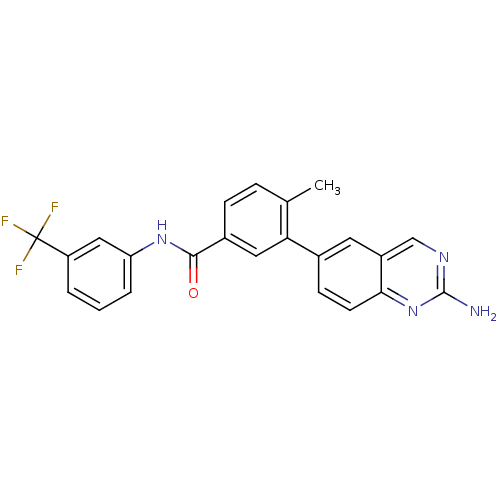 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14948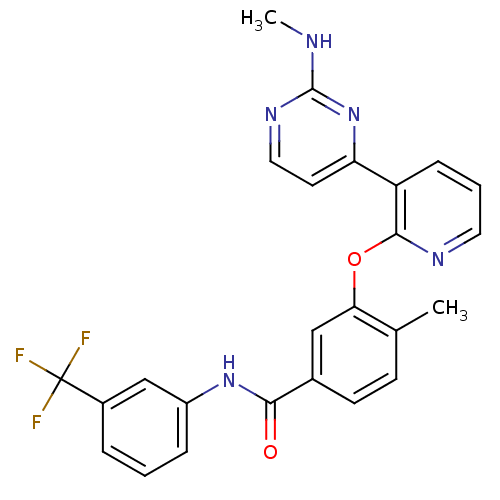 (4-Methyl-3-(3-(2-(methylamino)pyrimidin-4-yl)pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM14969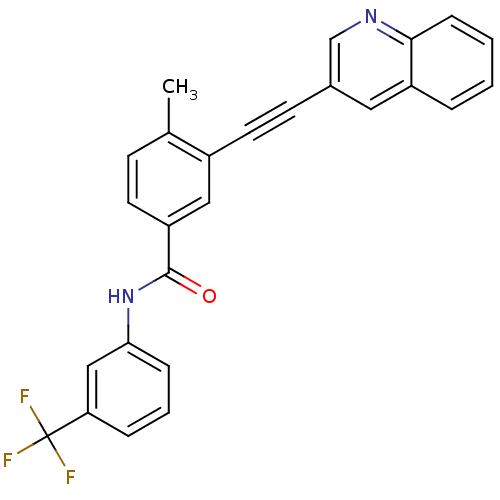 (4-Methyl-3-(2-(quinolin-3-yl)ethynyl)-N-(3-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck [245-498] (Homo sapiens (Human)) | BDBM14969 (4-Methyl-3-(2-(quinolin-3-yl)ethynyl)-N-(3-(triflu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM14967 (3-(2-(3H-Imidazo[4,5-b]pyridin-6-yl)ethynyl)-4-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM14966 (3-(2-(6-Aminopyridin-3-yl)ethynyl)-4-methyl-N-(3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM14951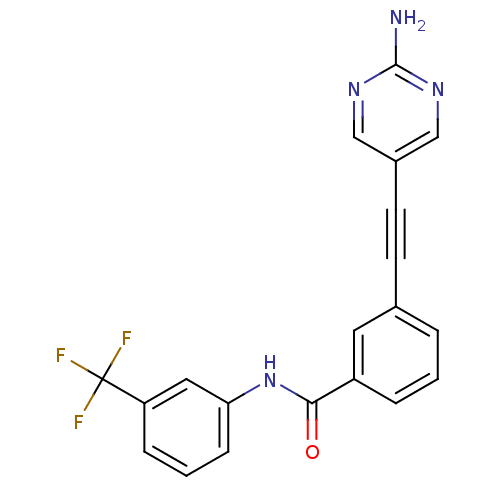 (3-(2-(2-Aminopyrimidin-5-yl)ethynyl)-N-(3-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM14949 (2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM14948 (4-Methyl-3-(3-(2-(methylamino)pyrimidin-4-yl)pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 50: 627-40 (2007) Article DOI: 10.1021/jm061112p BindingDB Entry DOI: 10.7270/Q2R49P1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50207852 (3-isopropyl-N-(4-methyl-3-(3-(4-(methylamino)-1,3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Tie2 after 90 mins by HTRF assay | Bioorg Med Chem Lett 17: 2886-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.067 BindingDB Entry DOI: 10.7270/Q2445M4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50207856 (3-isopropyl-N-(4-methyl-3-(3-(2-(methylamino)pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Tie2 after 90 mins by HTRF assay | Bioorg Med Chem Lett 17: 2886-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.067 BindingDB Entry DOI: 10.7270/Q2445M4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50207855 (CHEMBL245744 | N-(3-isopropylphenyl)-4-methyl-3-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Tie2 after 90 mins by HTRF assay | Bioorg Med Chem Lett 17: 2886-9 (2007) Article DOI: 10.1016/j.bmcl.2007.02.067 BindingDB Entry DOI: 10.7270/Q2445M4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50361821 (CHEMBL1938654) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal GST-tagged JAK2 expressed in Sf9 cells using Biotin-LC-EQEDEPEGDYFEWLE as substrate after 90 mins by TR-FR... | J Med Chem 54: 8440-50 (2011) Article DOI: 10.1021/jm200911r BindingDB Entry DOI: 10.7270/Q22N52PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1610 total ) | Next | Last >> |