

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123599 (ETORPHINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92649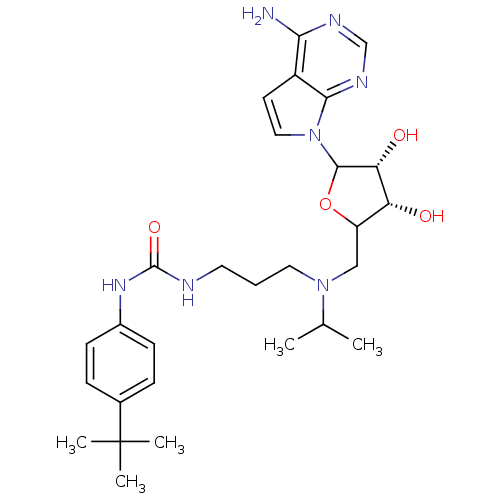 (EPZ004777) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464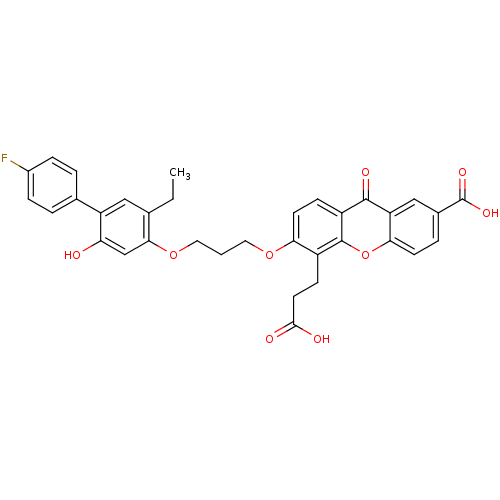 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50123599 (ETORPHINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50189136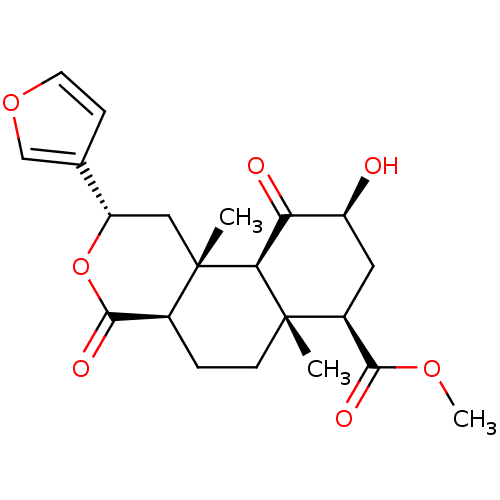 (CHEMBL424698 | Salvinorin B) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052027 ((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037391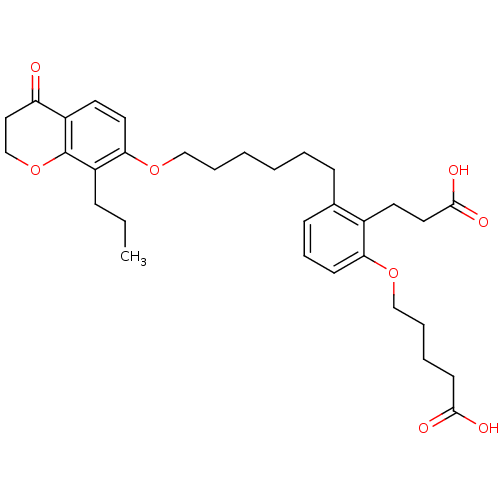 (5-{2-(2-Carboxy-ethyl)-3-[6-(4-oxo-8-propyl-chroma...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042149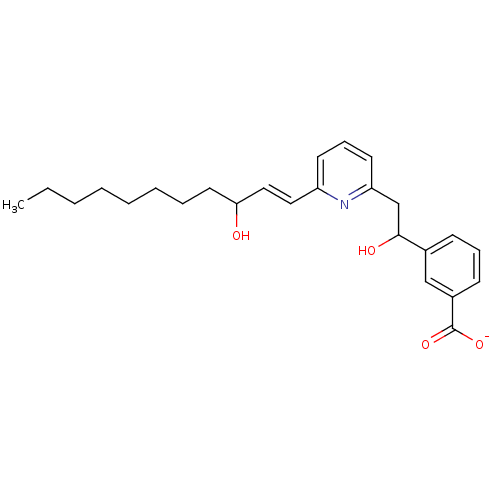 (CHEMBL112520 | Lithium; 3-{1-hydroxy-2-[6-(3-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042180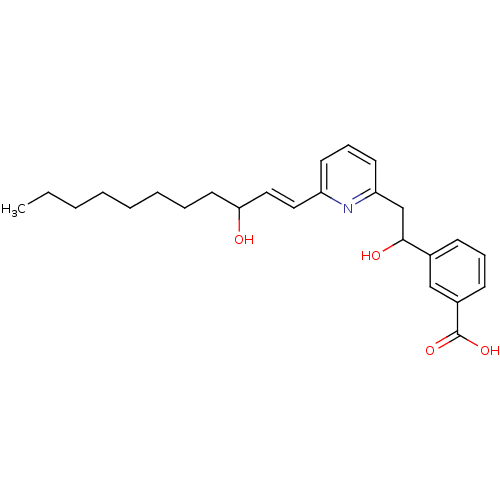 (3-{1-Hydroxy-2-[6-(3-hydroxy-undec-1-enyl)-pyridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity of [3H]-LTB4 to receptors on intact human polymorphonuclear leukocytes | J Med Chem 36: 3321-32 (1993) BindingDB Entry DOI: 10.7270/Q2G73CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053354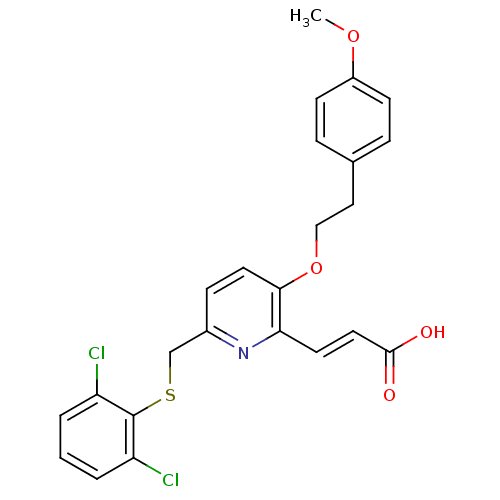 ((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159165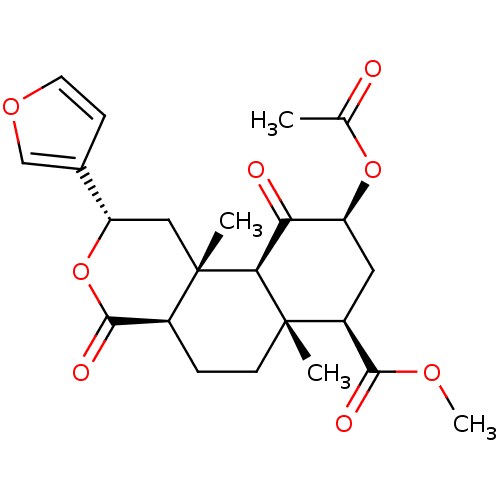 ((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037390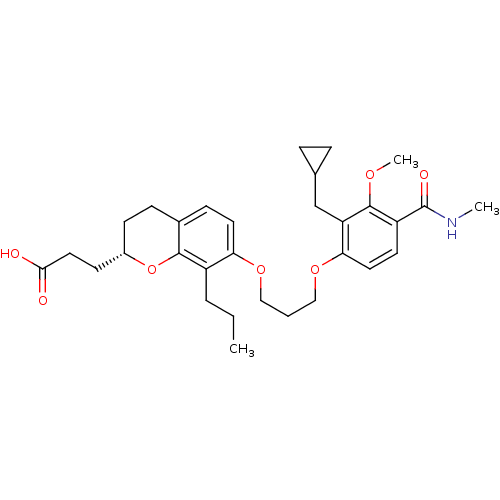 (3-{(S)-2-(2-Carboxy-ethyl)-7-[3-(2-cyclopropylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053350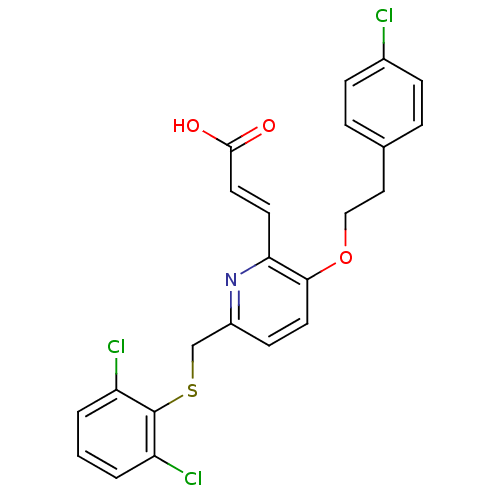 ((E)-3-[3-[2-(4-Chloro-phenyl)-ethoxy]-6-(2,6-dichl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053353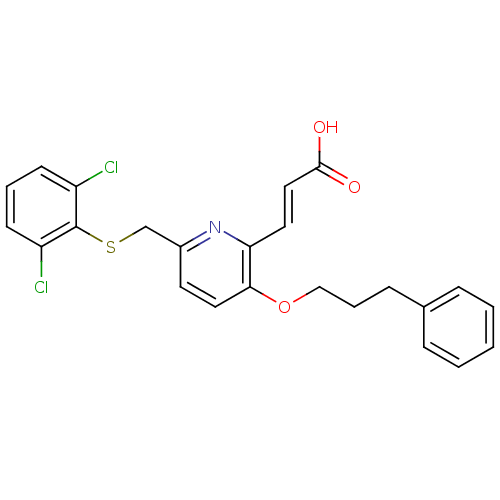 ((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-(3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26399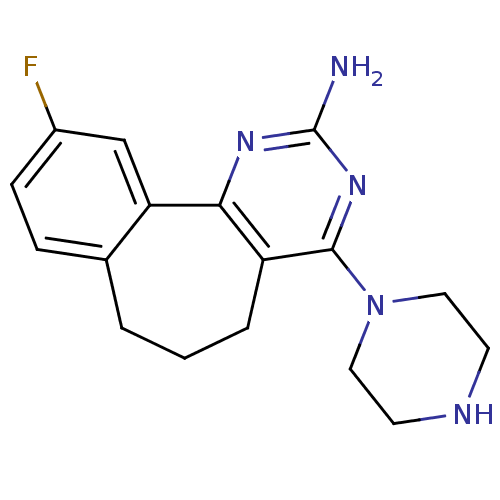 (14-fluoro-6-(piperazin-1-yl)-3,5-diazatricyclo[9.4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.45 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053351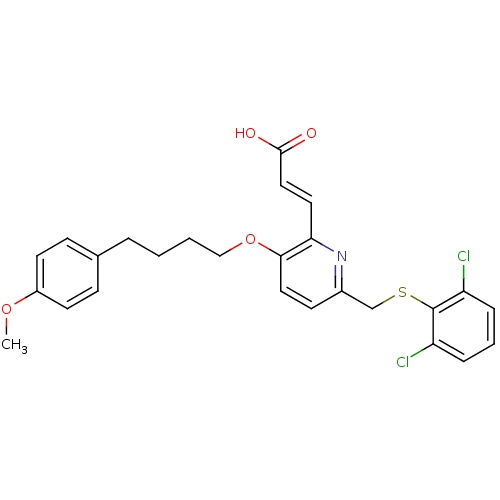 ((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053355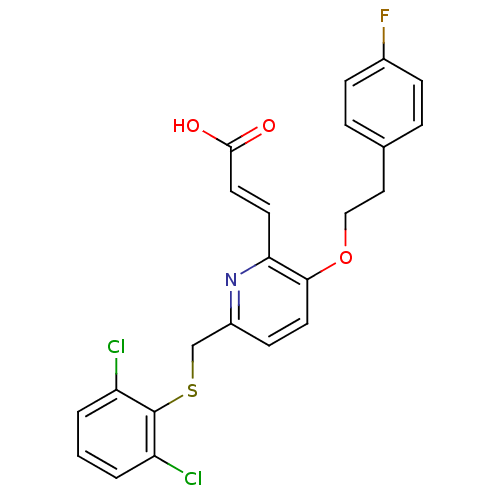 ((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609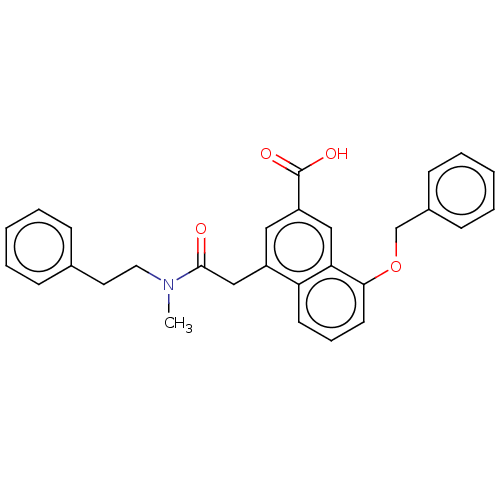 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against LTB4 receptors in human PMNs using [3H]-LTB4 as radioligand | J Med Chem 36: 2703-5 (1993) BindingDB Entry DOI: 10.7270/Q2HQ3XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6571-80 (2008) Article DOI: 10.1021/jm8005959 BindingDB Entry DOI: 10.7270/Q24J0CD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50053356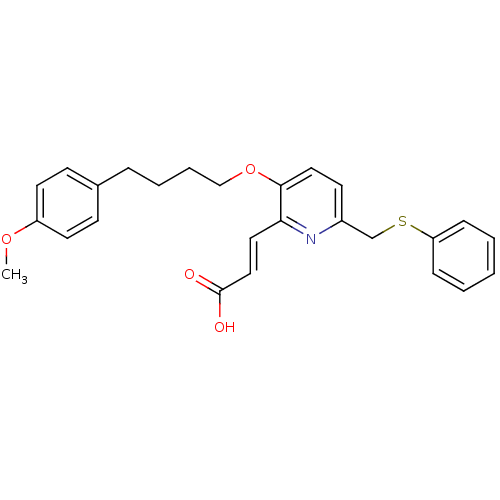 ((E)-3-{3-[4-(4-Methoxy-phenyl)-butoxy]-6-phenylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50123599 (ETORPHINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from FLAG-tagged mouse delta opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042153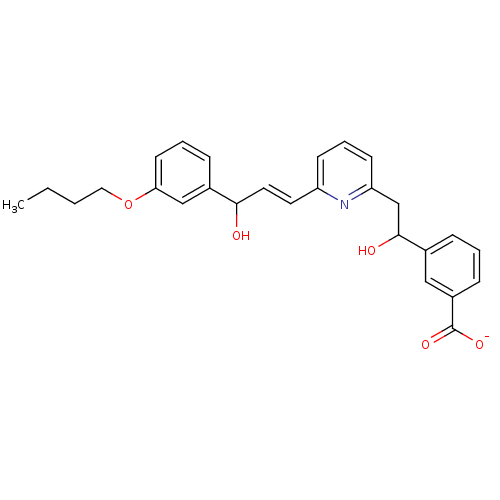 (CHEMBL113288 | Lithium; 3-(2-{7-[3-(3-butoxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26397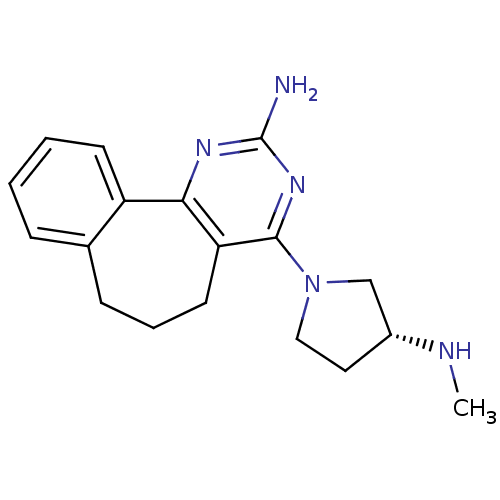 (2,4-diamino-5,6-disubstituted pyrimidine, 11 | 6-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26398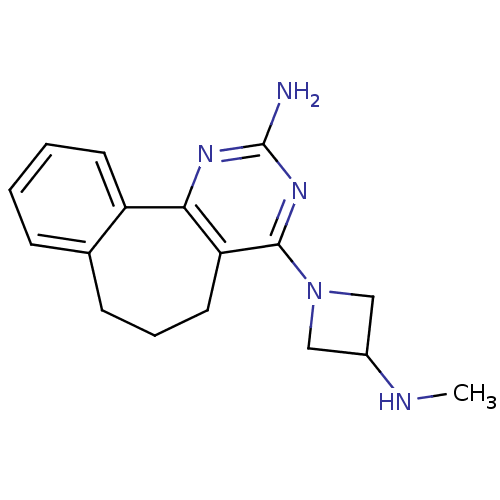 (2,4-diamino-5,6-disubstituted pyrimidine, 12 | 6-[...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26396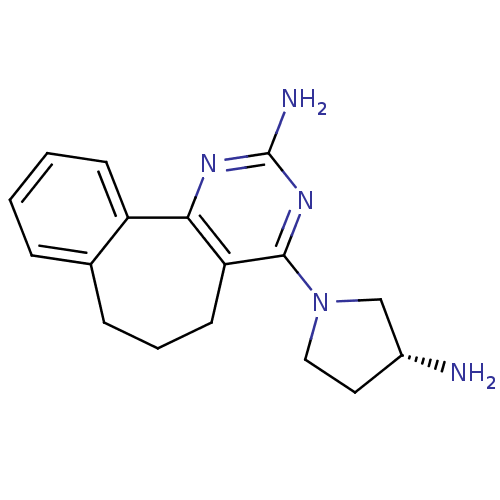 (2,4-diamino-5,6-disubstituted pyrimidine, 10 | 6-[...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037382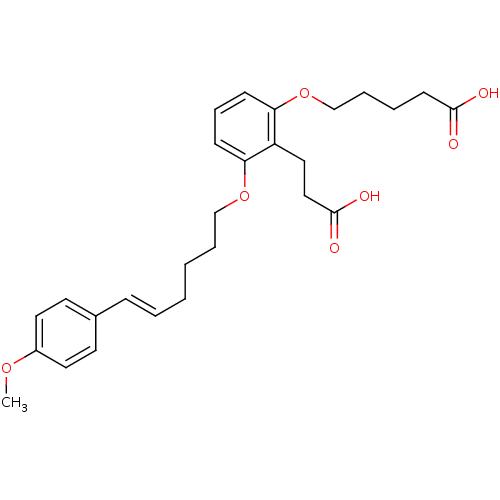 (5-{2-(2-Carboxy-ethyl)-3-[(E)-6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against LTB4 receptors in human PMNs using [3H]-LTB4 as radioligand | J Med Chem 36: 2703-5 (1993) BindingDB Entry DOI: 10.7270/Q2HQ3XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26391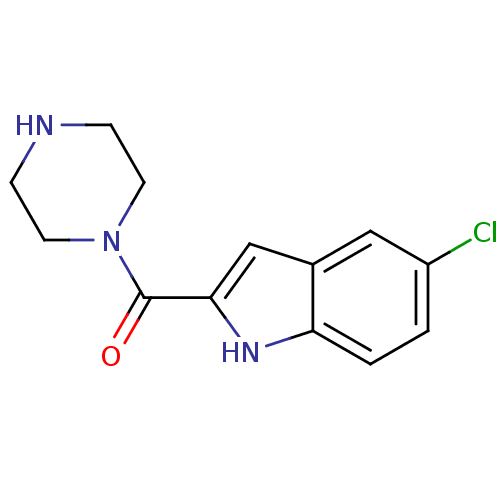 (5-chloro-2-(piperazin-1-ylcarbonyl)-1H-indole | JN...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037382 (5-{2-(2-Carboxy-ethyl)-3-[(E)-6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM92648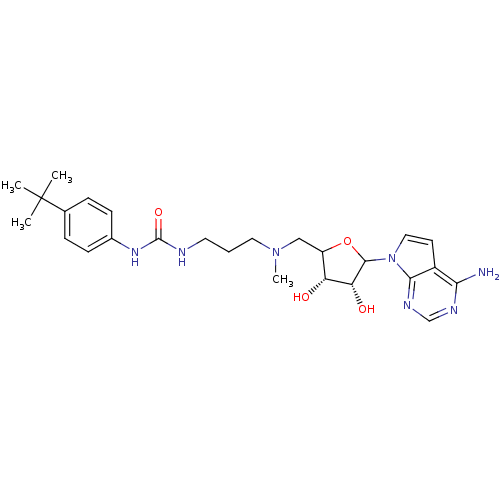 (EPZ004450) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc. | Assay Description Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. | Chem Biol Drug Des 80: 971-80 (2012) Article DOI: 10.1111/cbdd.12050 BindingDB Entry DOI: 10.7270/Q2Z89B12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26400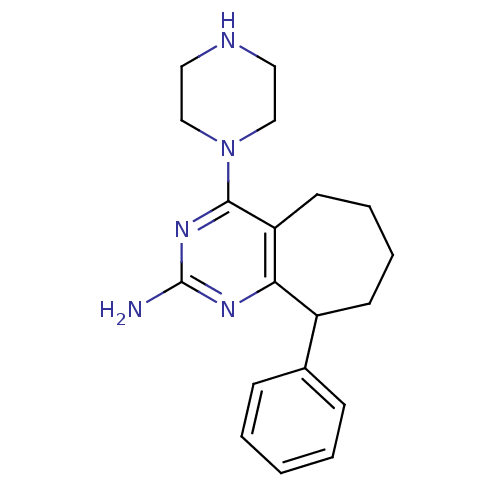 (2,4-diamino-5,6-disubstituted pyrimidine, 14 | 9-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042182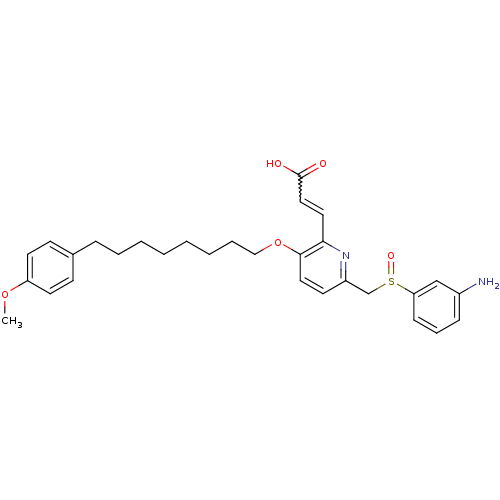 ((E)-3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50123598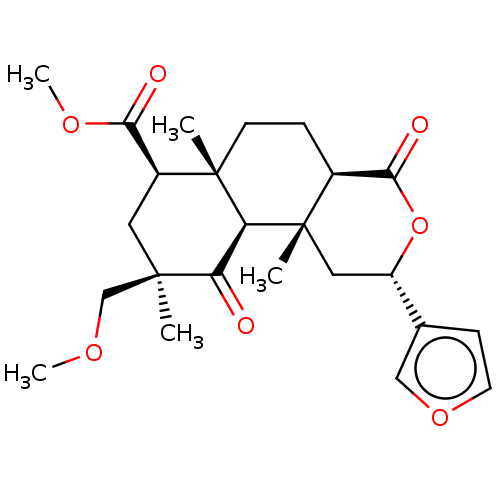 (CHEMBL3622713) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042182 ((E)-3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity of [3H]-LTB4 to receptors on intact human polymorphonuclear leukocytes | J Med Chem 36: 3321-32 (1993) BindingDB Entry DOI: 10.7270/Q2G73CRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26396 (2,4-diamino-5,6-disubstituted pyrimidine, 10 | 6-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26394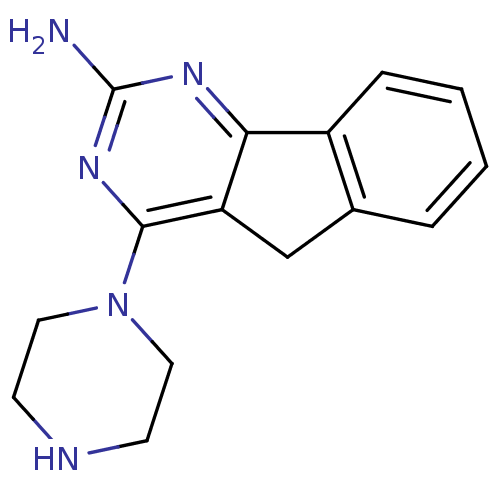 (2,4-diamino-5,6-disubstituted pyrimidine, 8 | 4-(p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037376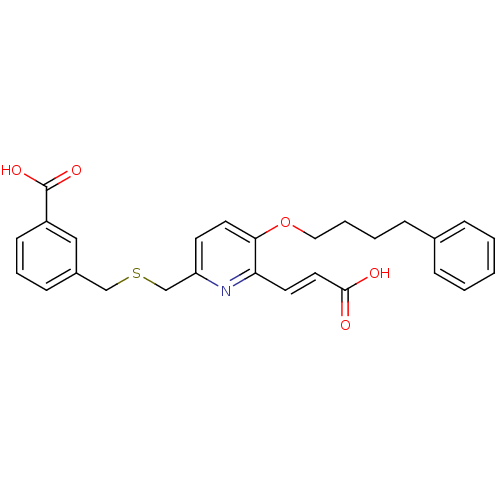 (3-[6-((E)-2-Carboxy-vinyl)-5-(4-phenyl-butoxy)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26390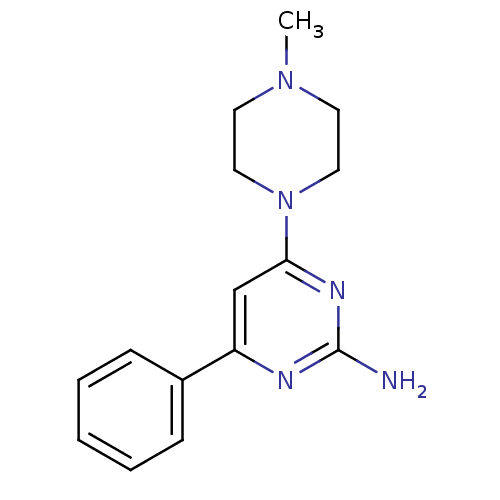 (4-(4-methylpiperazin-1-yl)-6-phenylpyrimidin-2-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26397 (2,4-diamino-5,6-disubstituted pyrimidine, 11 | 6-[...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26399 (14-fluoro-6-(piperazin-1-yl)-3,5-diazatricyclo[9.4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26392 (2,4-diamino-5,6-disubstituted pyrimidine, 6 | 6-(4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.40 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037389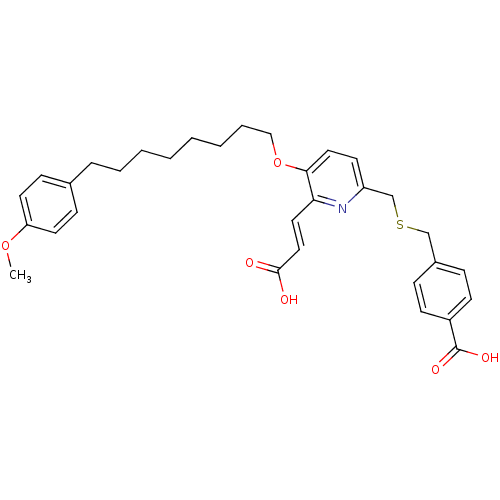 (4-{6-((E)-2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037385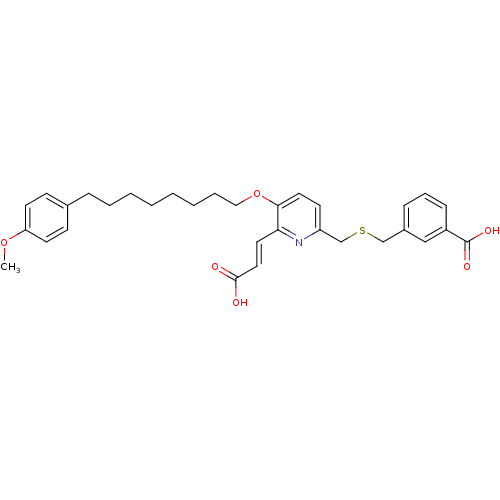 (3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037385 (3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to human neutrophils | J Med Chem 39: 3837-41 (1996) Article DOI: 10.1021/jm960248s BindingDB Entry DOI: 10.7270/Q2VX0FKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037385 (3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against LTB4 receptors in human PMNs | J Med Chem 36: 2703-5 (1993) BindingDB Entry DOI: 10.7270/Q2HQ3XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26395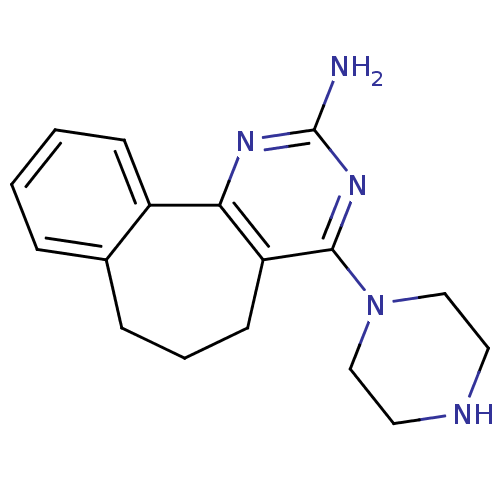 (2,4-diamino-5,6-disubstituted pyrimidine, 9 | 6-(p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.60 | -46.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037386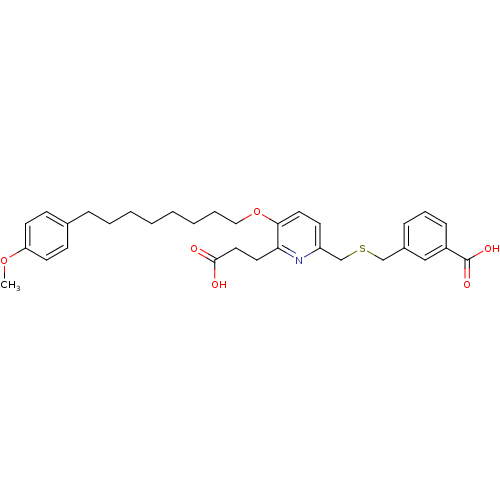 (3-{6-(2-Carboxy-ethyl)-5-[8-(4-methoxy-phenyl)-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037381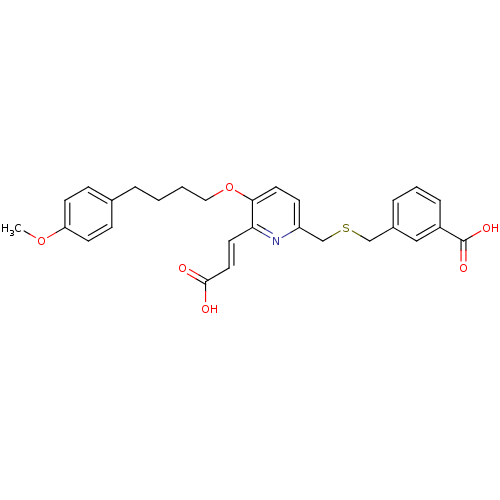 (3-{6-((E)-2-Carboxy-vinyl)-5-[4-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at leukotriene B4 receptor on intact human PMNs by displacement of [3H]-LTB4. | J Med Chem 37: 3327-36 (1994) BindingDB Entry DOI: 10.7270/Q2HT2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 393 total ) | Next | Last >> |