

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254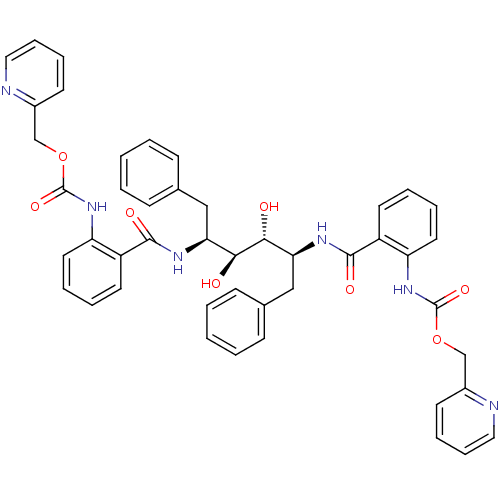 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285701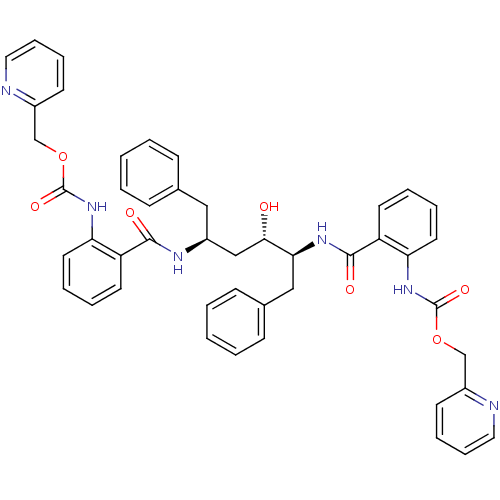 (Anthranilamide derivative | CHEMBL313767) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197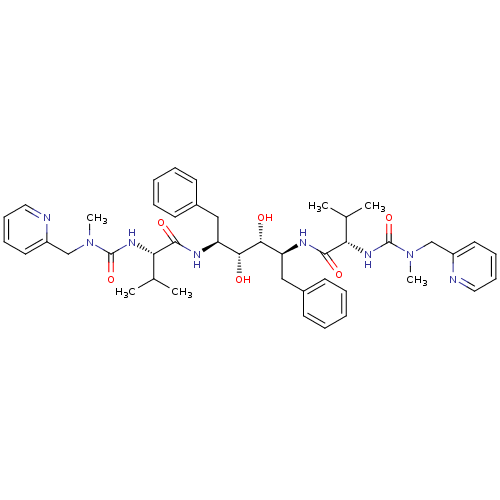 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064201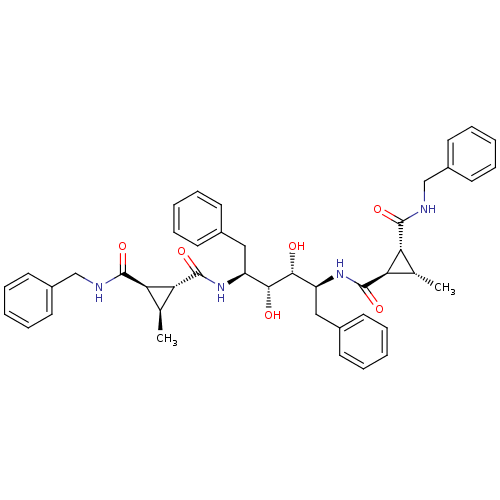 (1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoyl-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064202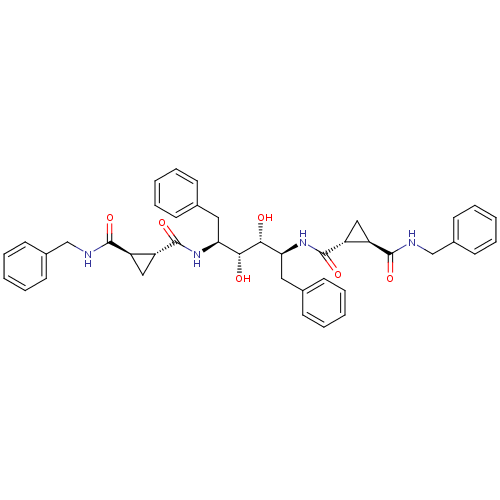 (1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoylcyclopr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285698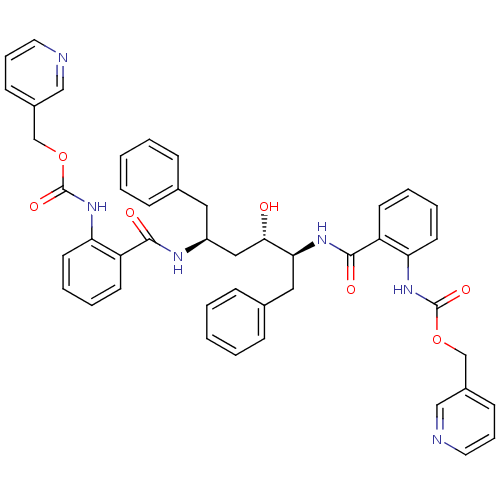 (Anthranilamide derivative | CHEMBL315500) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064199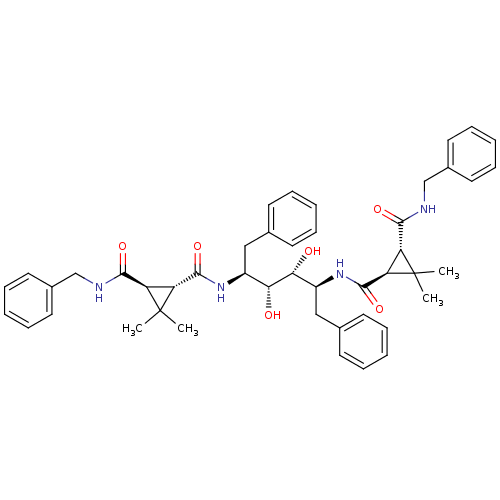 (1N-benzyl-2N-[1-benzyl-4-(3-benzylcarbamoyl-2,2-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285700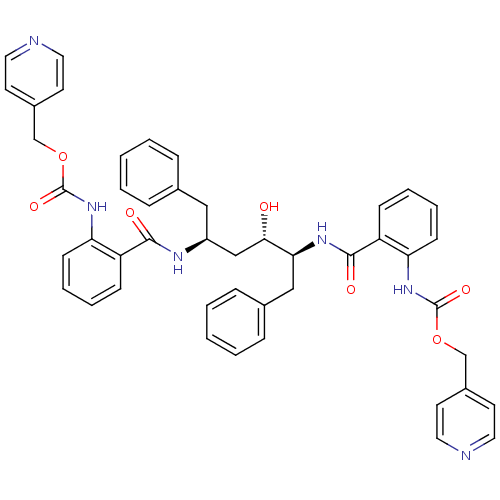 (Anthranilamide derivative | CHEMBL315742) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064203 (1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoyl-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284919 (1N-[1-benzyl-2-hydroxy-4-(3-hydroxy-2-methylphenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284922 (1N-[1-benzyl-2,3-dihydroxy-4-(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285696 (Anthranilamide derivative | CHEMBL85640) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285703 (Anthranilamide derivative | CHEMBL315928) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285691 (Anthranilamide derivative | CHEMBL264622) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284921 (1N-[4-(3-amino-2-methylphenylcarboxamido)-1-benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285695 (Anthranilamide derivative | CHEMBL86263) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284916 (1N-[1-benzyl-2,3-dihydroxy-4-(2-methylphenylcarbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285699 (Anthranilamide derivative | CHEMBL87879) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285690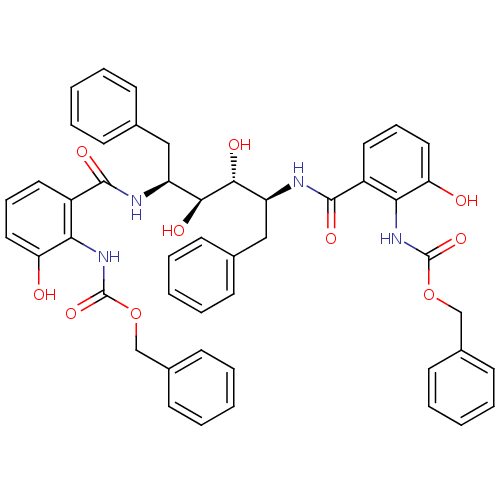 (Anthranilamide derivative | CHEMBL87309) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285702 (Anthranilamide derivative | CHEMBL314408) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284920 (1N-[1-benzyl-2,3-dihydroxy-4-(3-hydroxyphenylcarbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284918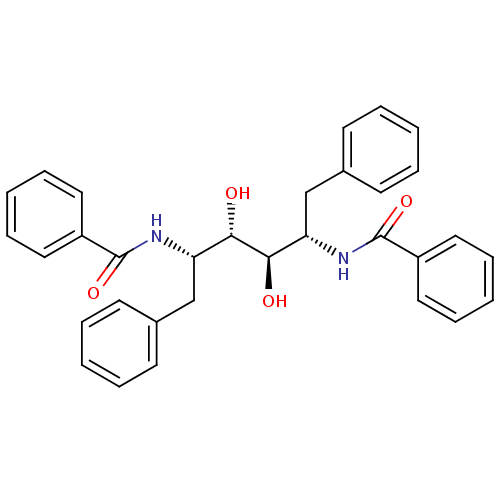 (1N-[1-benzyl-2,3-dihydroxy-5-phenyl-4-phenylcarbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285692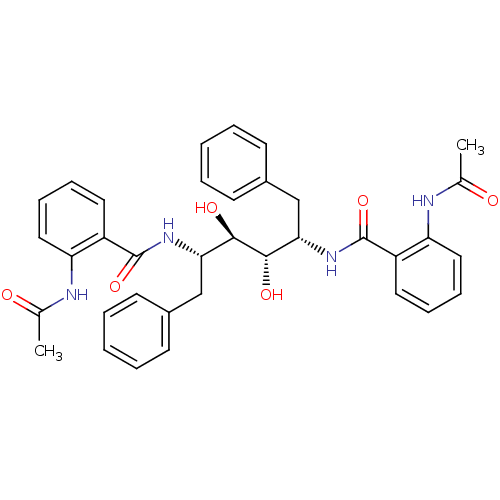 (1N-[1-benzyl-2,3-dihydroxy-4-(2-methylcarboxamidop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16702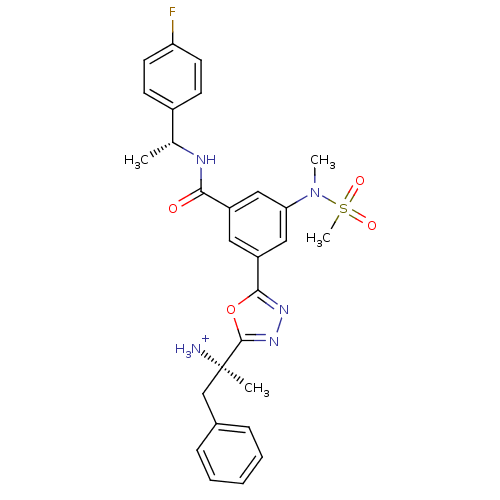 ((2R)-2-[5-(3-{[(1R)-1-(4-fluorophenyl)ethyl]carbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42.4 | -41.7 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 1117-21 (2007) Article DOI: 10.1016/j.bmcl.2006.11.003 BindingDB Entry DOI: 10.7270/Q23X84W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16704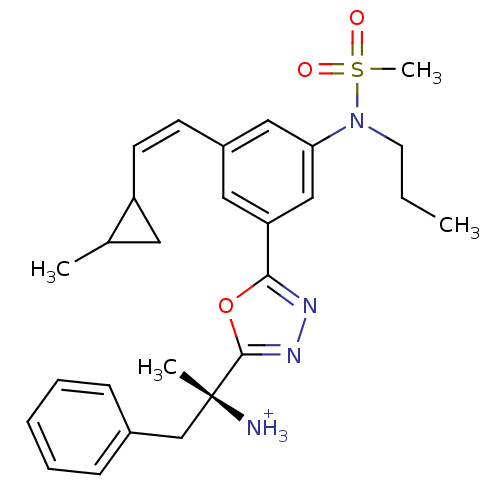 ((2R)-2-(5-{3-[(Z)-2-(2-methylcyclopropyl)ethenyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 51.9 | -41.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 1117-21 (2007) Article DOI: 10.1016/j.bmcl.2006.11.003 BindingDB Entry DOI: 10.7270/Q23X84W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284917 (1N-[1-benzyl-2,3-dihydroxy-4-(5-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064198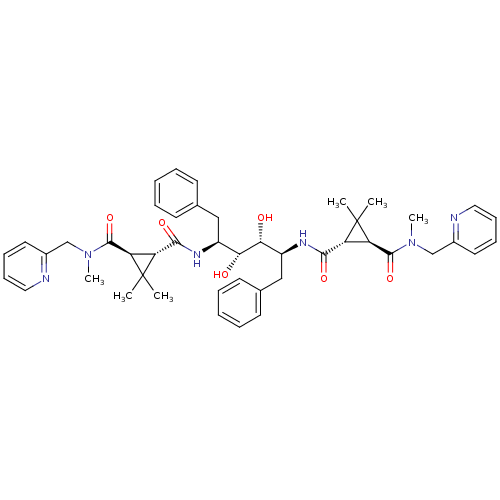 (1N-{1-benzyl-4-[2,2-dimethyl-3-methyl(2-pyridylmet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285693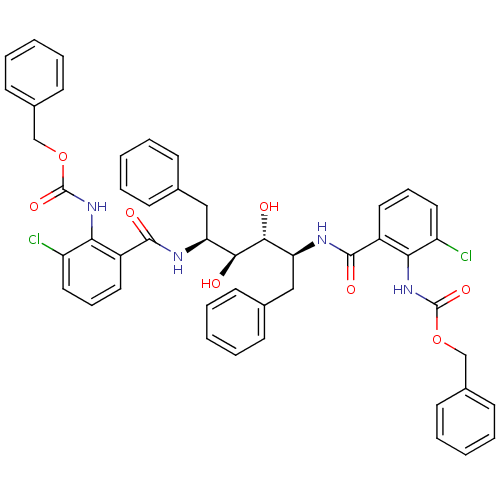 (Anthranilamide derivative | CHEMBL84884) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285697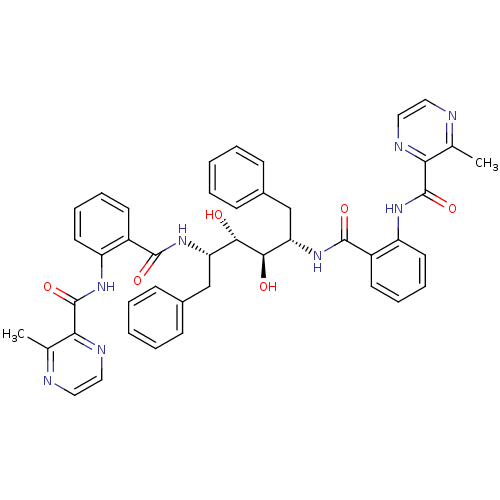 (Anthranilamide derivative | CHEMBL313254) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285694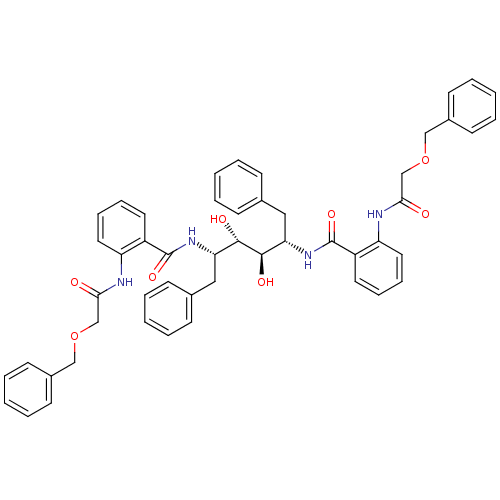 (Anthranilamide derivative | CHEMBL314004) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16703 ((2R)-2-(5-{3-[methyl(methylsulfonyl)amino]-5-(2-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.65E+4 | -27.0 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 17: 1117-21 (2007) Article DOI: 10.1016/j.bmcl.2006.11.003 BindingDB Entry DOI: 10.7270/Q23X84W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM724 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM12133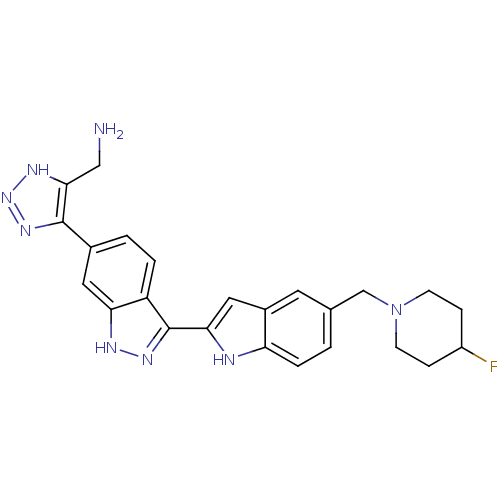 (3-(Indol-2-yl)indazole 23 | [5-(3-{5-[(4-fluoropip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories | Assay Description Kinase activity was measured in a homogeneous assay in a 96-well format. Detection was performed by HTRF using an EuK-labelled anti-phospho(S21)-GSK3... | Bioorg Med Chem Lett 16: 6049-53 (2006) Article DOI: 10.1016/j.bmcl.2006.08.118 BindingDB Entry DOI: 10.7270/Q29P2ZVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM586099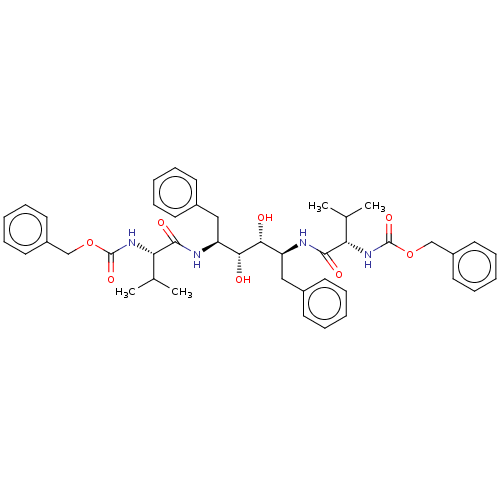 (BDBM50064200 | TL-3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP/microtubule affinity-regulating kinase 3 (Homo sapiens (Human)) | BDBM50536695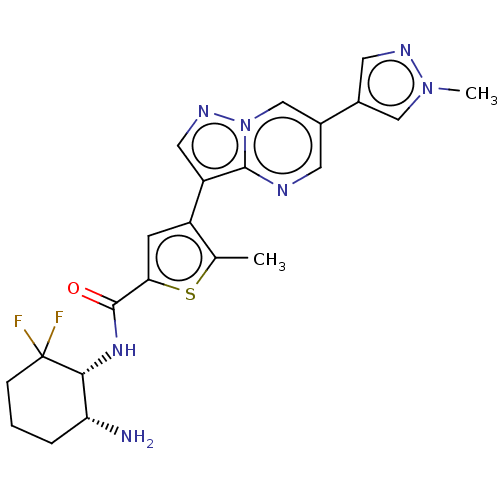 (CHEMBL4565845) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human GST-tagged MARK3 expressed in baculovirus expression system using biotinylated-Cdc25C peptide substrate m... | Bioorg Med Chem Lett 26: 4362-6 (2016) Article DOI: 10.1016/j.bmcl.2016.02.003 BindingDB Entry DOI: 10.7270/Q2NP27X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM12134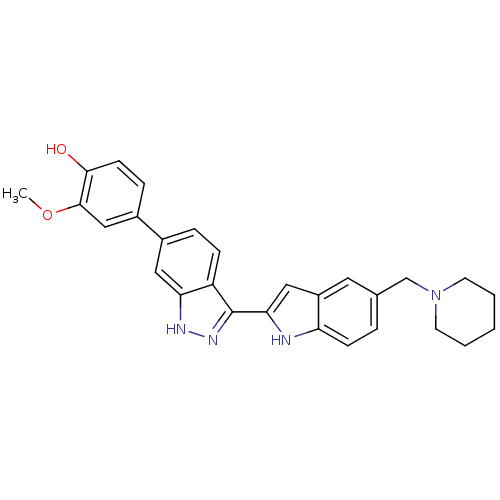 (2-methoxy-4-{3-[5-(piperidin-1-ylmethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories | Assay Description Kinase activity was measured in a homogeneous assay in a 96-well format. Detection was performed by HTRF using an EuK-labelled anti-phospho(S21)-GSK3... | Bioorg Med Chem Lett 16: 6049-53 (2006) Article DOI: 10.1016/j.bmcl.2006.08.118 BindingDB Entry DOI: 10.7270/Q29P2ZVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50223460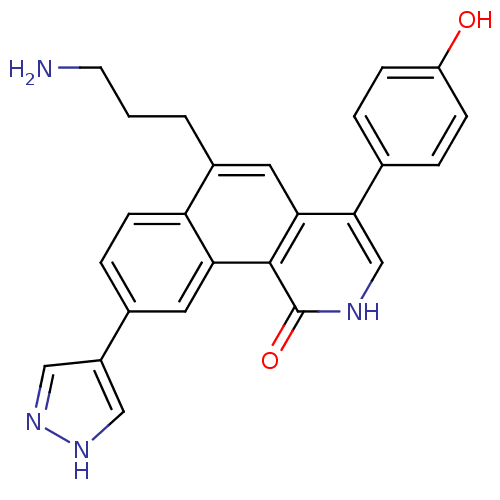 (6-(3-aminopropyl)-4-(4-hydroxyphenyl)-9-(1H-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 17: 6280-5 (2007) Article DOI: 10.1016/j.bmcl.2007.09.007 BindingDB Entry DOI: 10.7270/Q2W958ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM12132 (3-(Indol-2-yl)indazole 22 | [5-(3-{5-[(4-fluoropip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories | Assay Description Kinase activity was measured in a homogeneous assay in a 96-well format. Detection was performed by HTRF using an EuK-labelled anti-phospho(S21)-GSK3... | Bioorg Med Chem Lett 16: 6049-53 (2006) Article DOI: 10.1016/j.bmcl.2006.08.118 BindingDB Entry DOI: 10.7270/Q29P2ZVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM12131 ((5-{3-[5-(piperidin-1-ylmethyl)-1H-indol-2-yl]-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories | Assay Description Kinase activity was measured in a homogeneous assay in a 96-well format. Detection was performed by HTRF using an EuK-labelled anti-phospho(S21)-GSK3... | Bioorg Med Chem Lett 16: 6049-53 (2006) Article DOI: 10.1016/j.bmcl.2006.08.118 BindingDB Entry DOI: 10.7270/Q29P2ZVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM723 (CHEMBL277908 | Pyrrolinone inhibitor 4 | tert-buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50195211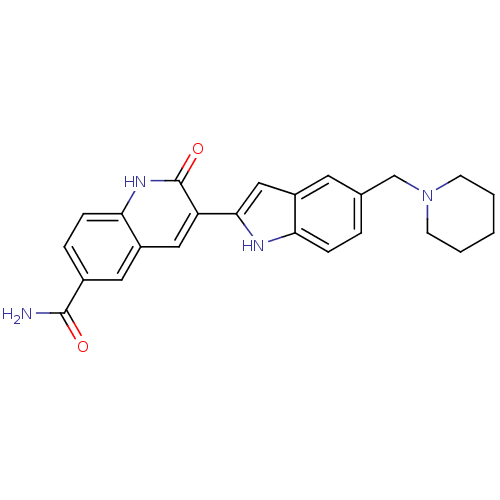 (2-oxo-3-(5-(piperidin-1-ylmethyl)-1H-indol-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CHEK1 | Bioorg Med Chem Lett 16: 5907-12 (2006) Article DOI: 10.1016/j.bmcl.2006.08.053 BindingDB Entry DOI: 10.7270/Q27H1J73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8530 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-4-[(3aR,8aS)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50223478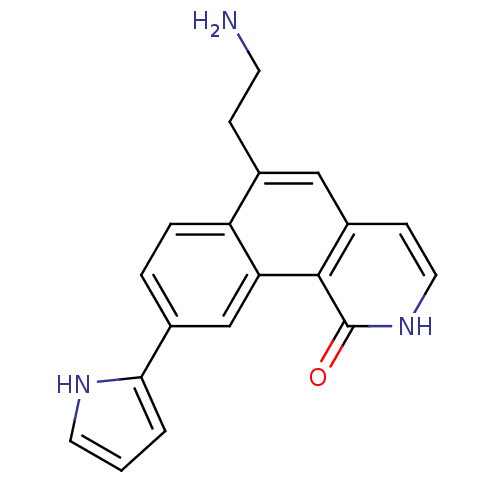 (6-(2-aminoethyl)-9-(1H-pyrrol-2-yl)benzo[h]isoquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 17: 6280-5 (2007) Article DOI: 10.1016/j.bmcl.2007.09.007 BindingDB Entry DOI: 10.7270/Q2W958ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50223480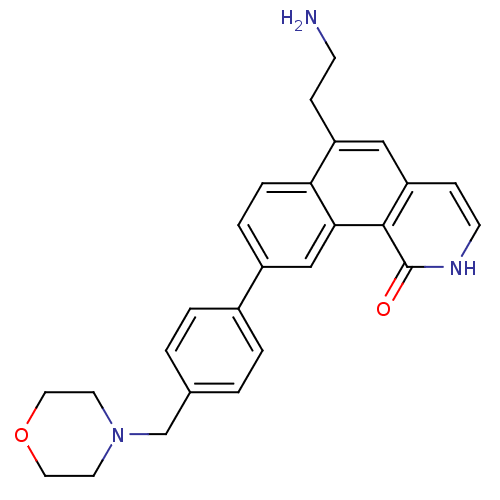 (6-(2-aminoethyl)-9-(4-(morpholinomethyl)phenyl)ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Chk1 expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 17: 6280-5 (2007) Article DOI: 10.1016/j.bmcl.2007.09.007 BindingDB Entry DOI: 10.7270/Q2W958ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM751 (CHEMBL289195 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50195197 (6-(isothiazol-4-yl)-3-(5-(piperidin-1-ylmethyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CHEK1 | Bioorg Med Chem Lett 16: 5907-12 (2006) Article DOI: 10.1016/j.bmcl.2006.08.053 BindingDB Entry DOI: 10.7270/Q27H1J73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50195213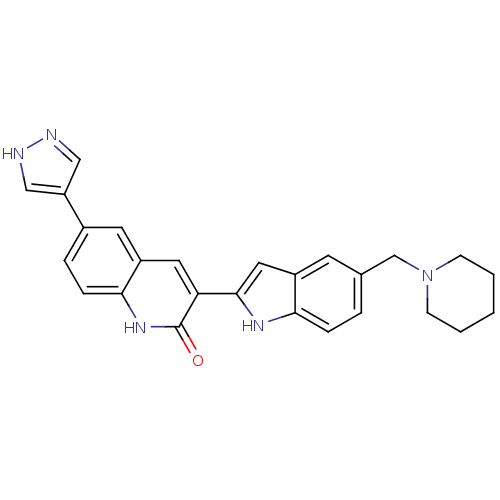 (3-(5-(piperidin-1-ylmethyl)-1H-indol-2-yl)-6-(1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CHEK1 | Bioorg Med Chem Lett 16: 5907-12 (2006) Article DOI: 10.1016/j.bmcl.2006.08.053 BindingDB Entry DOI: 10.7270/Q27H1J73 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50195198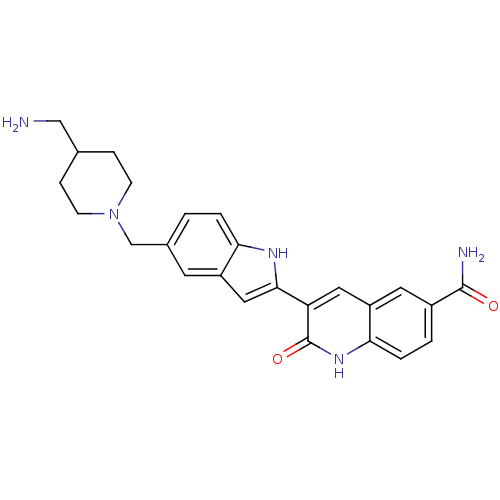 (3-(5-((4-(aminomethyl)piperidin-1-yl)methyl)-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CHEK1 | Bioorg Med Chem Lett 16: 5907-12 (2006) Article DOI: 10.1016/j.bmcl.2006.08.053 BindingDB Entry DOI: 10.7270/Q27H1J73 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 1083 total ) | Next | Last >> |