

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50059414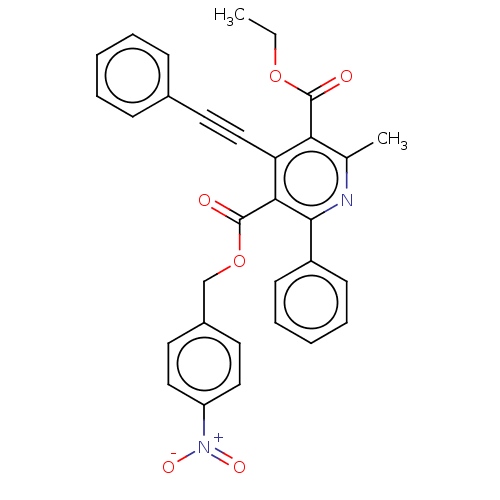 (2-Methyl-6-phenyl-4-phenylethynyl-1,4-dihydro-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM85776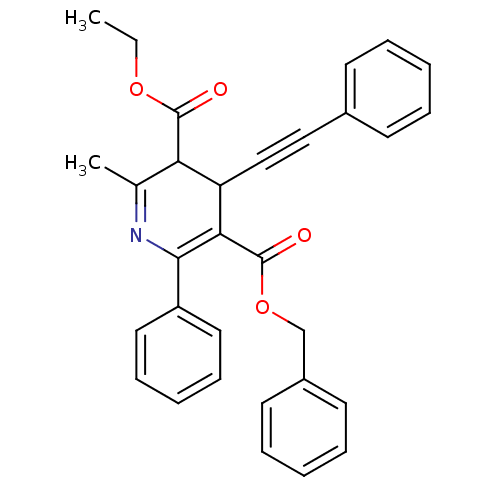 (CAS_393594 | CHEMBL89852 | MRS1191 | NSC_393594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50053927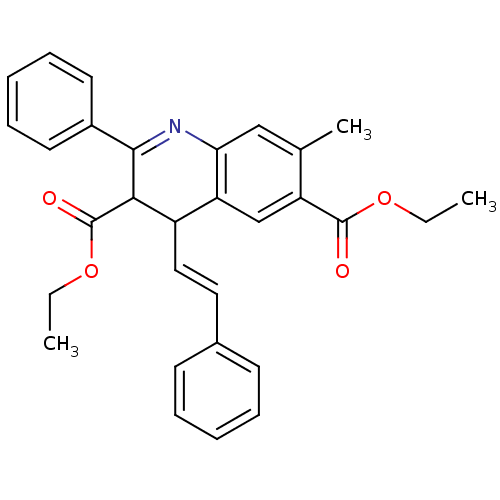 (7-Methyl-2-phenyl-4-((E)-styryl)-1,4-dihydro-quino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro binding affinity at human Adenosine A3 receptor from HEK-293 cells by [125I]-AB-MECA displacement. | J Med Chem 39: 4142-8 (1996) Article DOI: 10.1021/jm960482i BindingDB Entry DOI: 10.7270/Q2FQ9VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17B (Homo sapiens (Human)) | BDBM50166121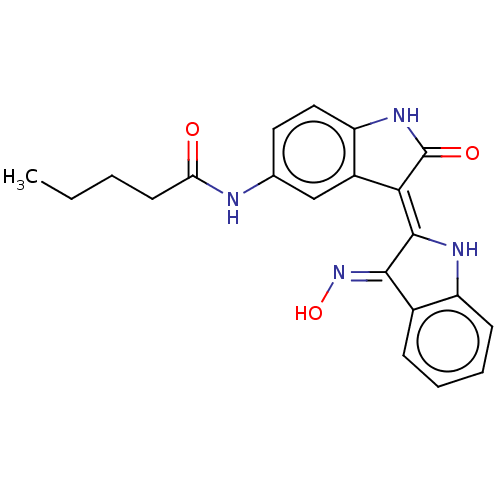 (CHEMBL3797480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065757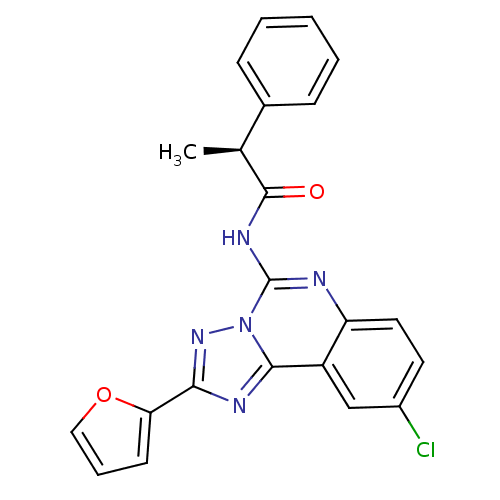 ((S)-N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.362 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065774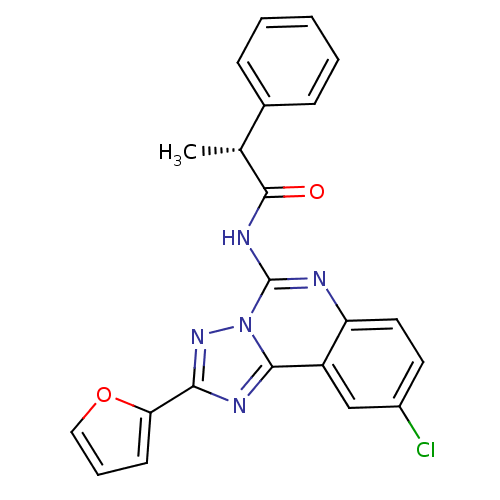 ((R)-N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50053924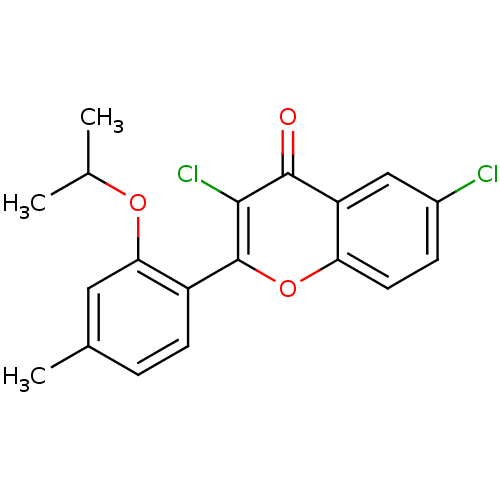 (3,6-Dichloro-2-(2-isopropoxy-4-methyl-phenyl)-chro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro binding affinity at human Adenosine A3 receptor from HEK-293 cells by [125I]-AB-MECA displacement. | J Med Chem 39: 4142-8 (1996) Article DOI: 10.1021/jm960482i BindingDB Entry DOI: 10.7270/Q2FQ9VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065756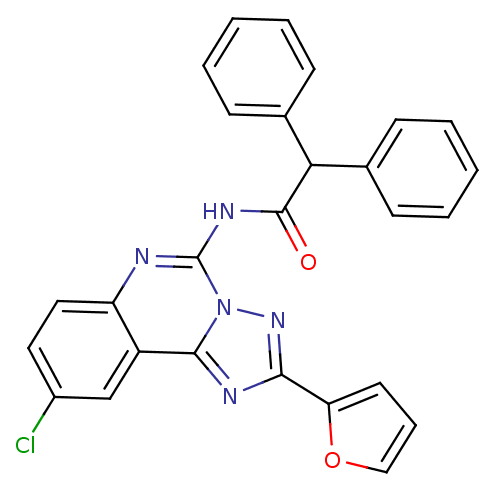 (CHEMBL317580 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.586 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50053929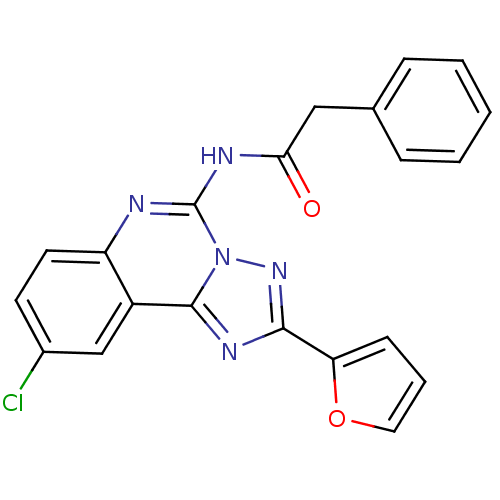 (CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tria...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50053929 (CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tria...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro binding affinity at human Adenosine A3 receptor from HEK-293 cells by [125I]-AB-MECA displacement. | J Med Chem 39: 4142-8 (1996) Article DOI: 10.1021/jm960482i BindingDB Entry DOI: 10.7270/Q2FQ9VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity for rat Adenosine A3 receptor in CHO cells using [125I]iodo-AB-MECA | J Med Chem 46: 3775-7 (2003) Article DOI: 10.1021/jm034098e BindingDB Entry DOI: 10.7270/Q2W959XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50065775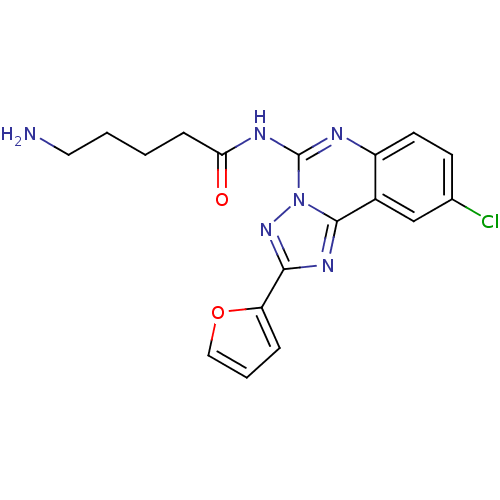 (5-Amino-pentanoic acid (9-chloro-2-furan-2-yl-[1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat striatal Adenosine A2A receptor vs [3H]-CGS- 21680 | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50065771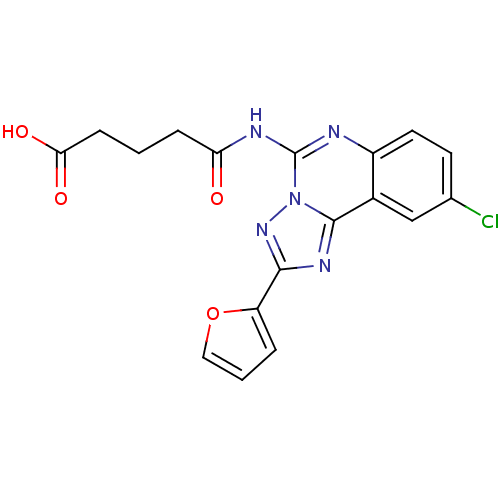 (4-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat striatal Adenosine A2A receptor vs [3H]-CGS- 21680 | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50207816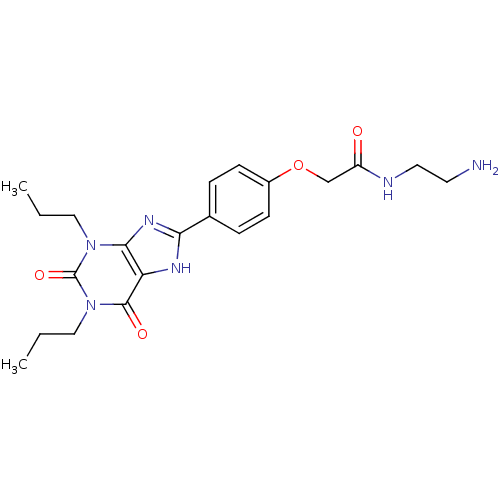 (CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against adenosine A1 receptor in rat brain membrane in presence of [3H]-R-PIA radioligand. | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50053936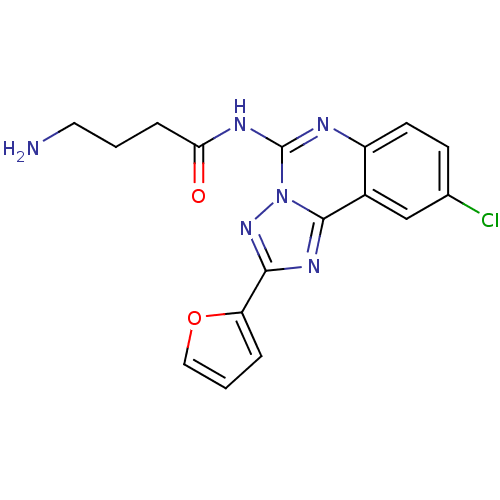 (4-Amino-N-(9-chloro-2-furan-2-yl-[1,2,4]triazolo[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat striatal Adenosine A2A receptor vs [3H]-CGS- 21680 | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086173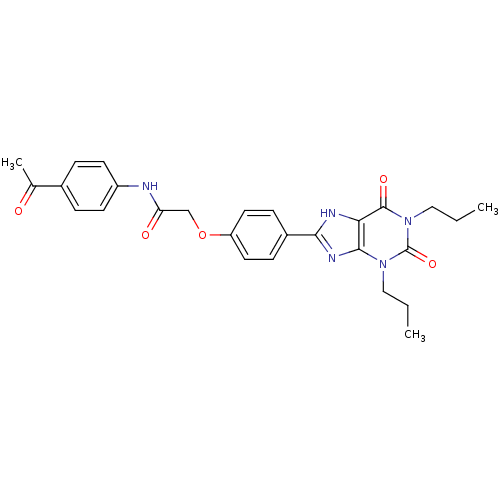 (CHEMBL17002 | N-(4-Acetyl-phenyl)-2-[4-(2,6-dioxo-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086168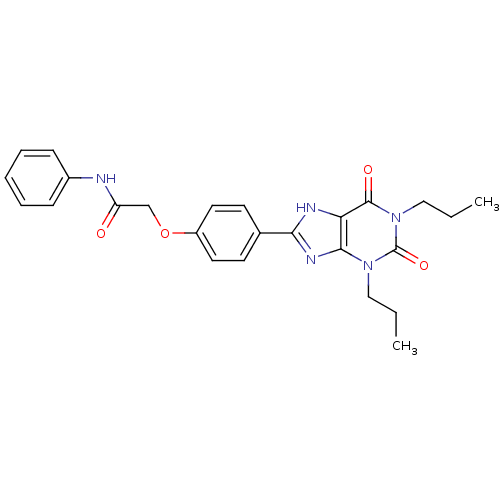 (2-(4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086167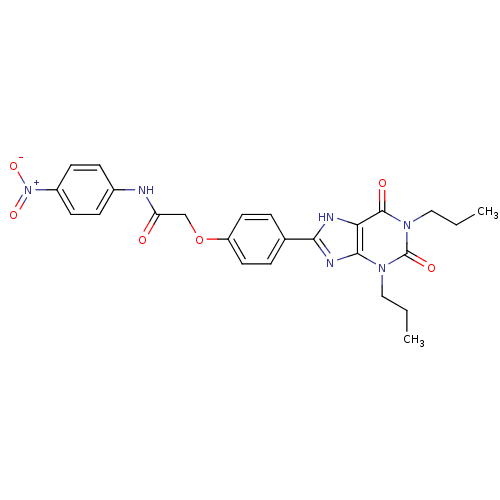 (2-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50004566 (9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity for human Adenosine A2A receptor from HEK-293 cells | J Med Chem 39: 4142-8 (1996) Article DOI: 10.1021/jm960482i BindingDB Entry DOI: 10.7270/Q2FQ9VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50025687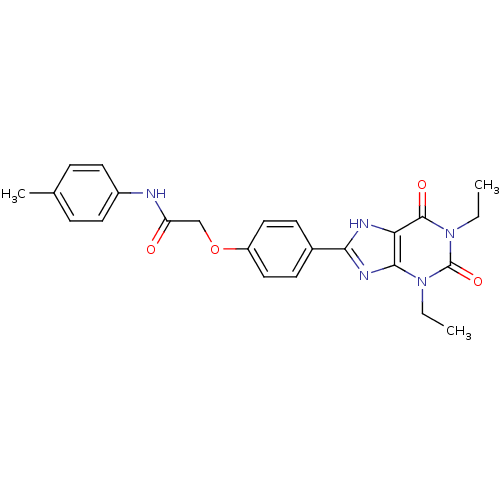 (2-[4-(1,3-Diethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50021080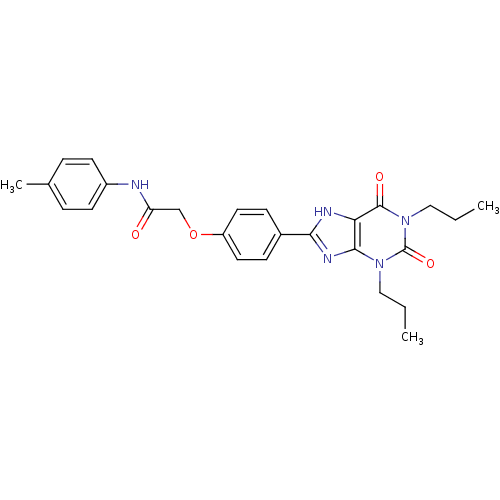 (2-(4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086170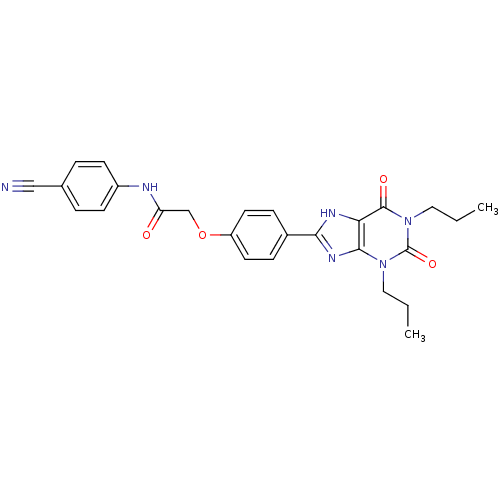 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086166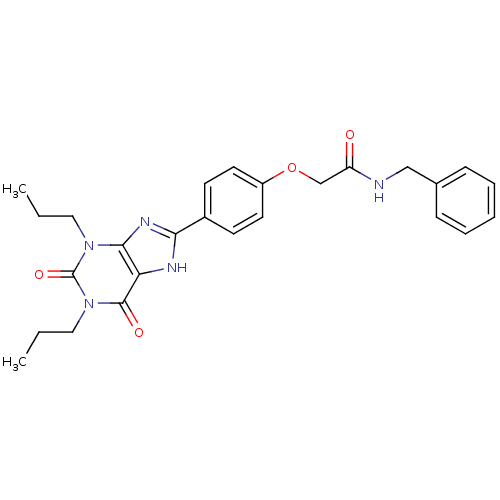 (CHEMBL275605 | N-Benzyl-2-[4-(2,6-dioxo-1,3-diprop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086165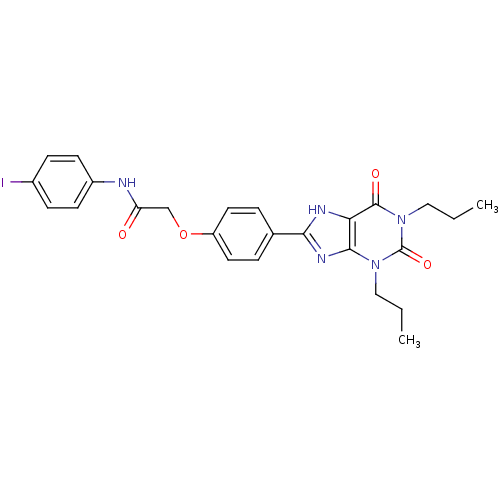 (2-(4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086174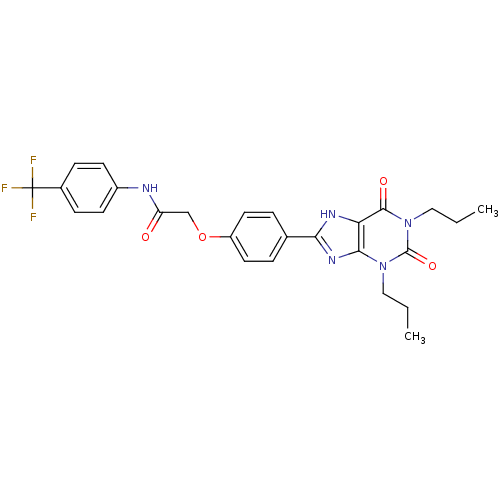 (2-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086182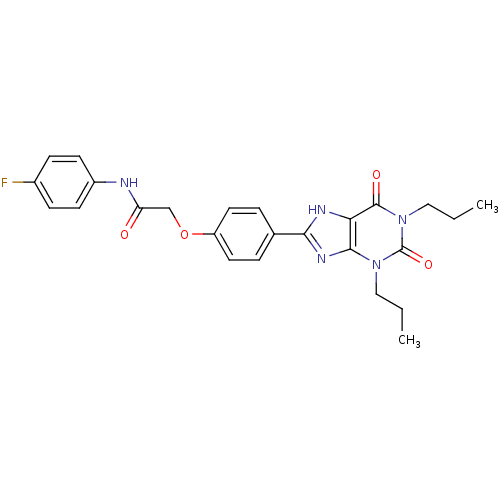 (2-(4-(2,4-dioxo-1,3-dipropyl-2,3,4,5-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086184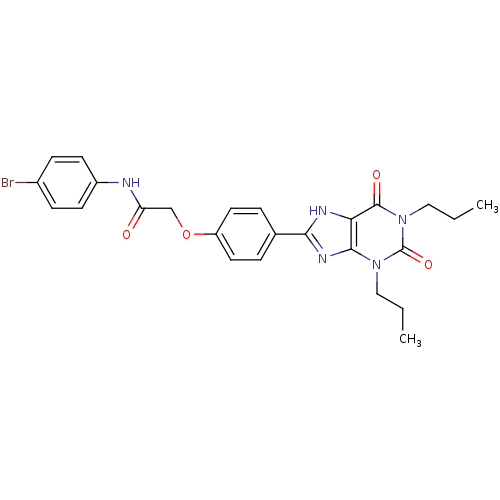 (CHEMBL17003 | N-(4-Bromo-phenyl)-2-[4-(2,6-dioxo-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086164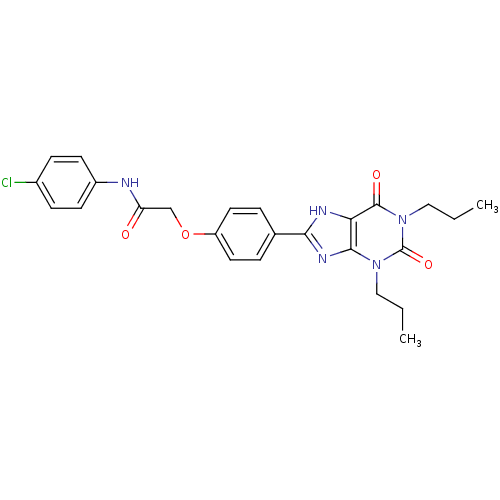 (CHEMBL275427 | N-(4-Chloro-phenyl)-2-[4-(2,6-dioxo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086186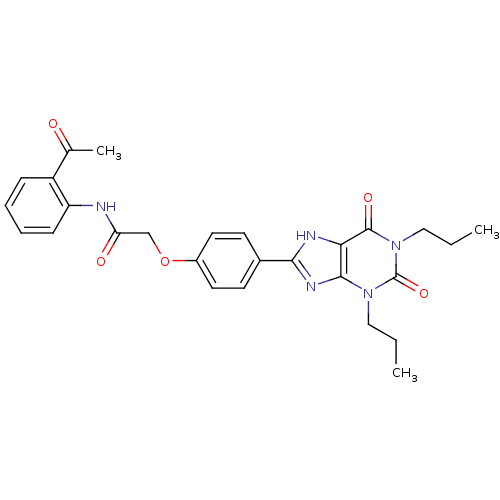 (CHEMBL17341 | N-(2-Acetyl-phenyl)-2-[4-(2,6-dioxo-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50053925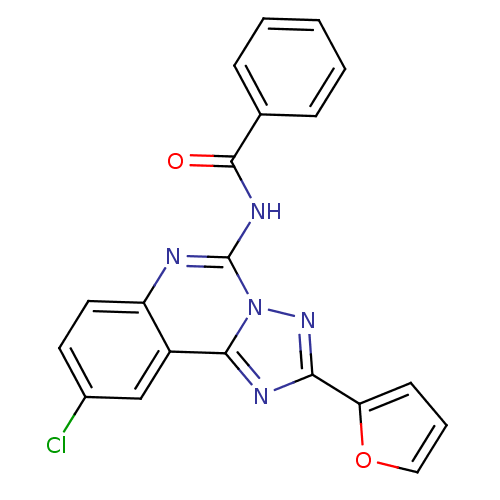 (CHEMBL317382 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro binding affinity at human Adenosine A3 receptor from HEK-293 cells by [125I]-AB-MECA displacement. | J Med Chem 39: 4142-8 (1996) Article DOI: 10.1021/jm960482i BindingDB Entry DOI: 10.7270/Q2FQ9VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50004566 (9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat striatal Adenosine A2A receptor vs [3H]-CGS- 21680 | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086177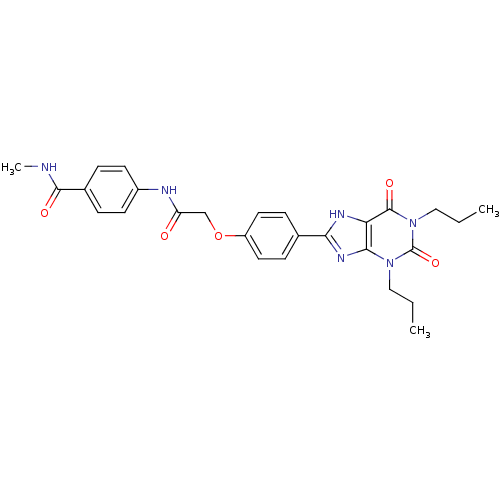 (4-{2-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065766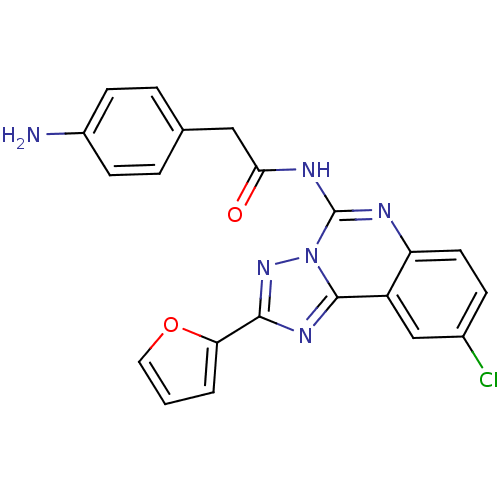 (2-(4-Amino-phenyl)-N-(9-chloro-2-furan-2-yl-[1,2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50065758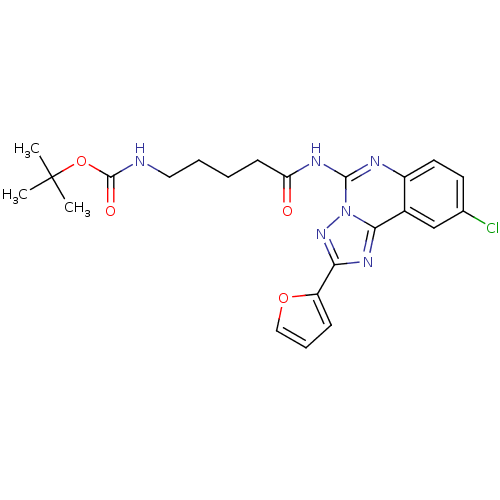 (CHEMBL98570 | [4-(9-Chloro-2-furan-2-yl-[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat striatal Adenosine A2A receptor vs [3H]-CGS- 21680 | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086181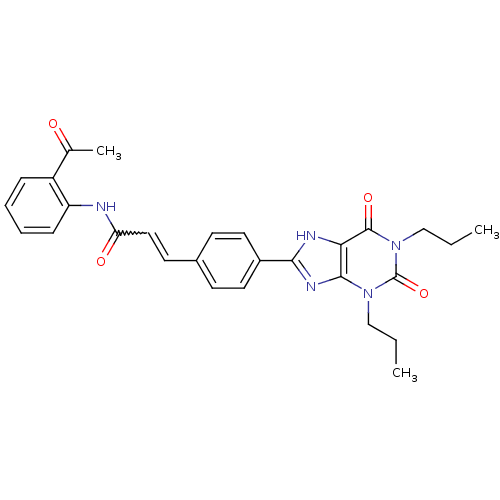 (CHEMBL17202 | N-(2-Acetyl-phenyl)-3-[4-(2,6-dioxo-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50021085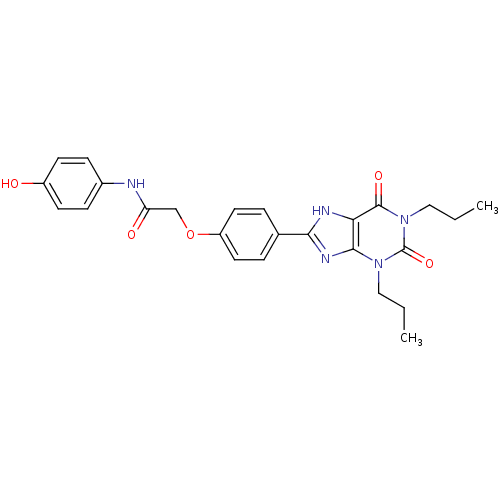 (2-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086172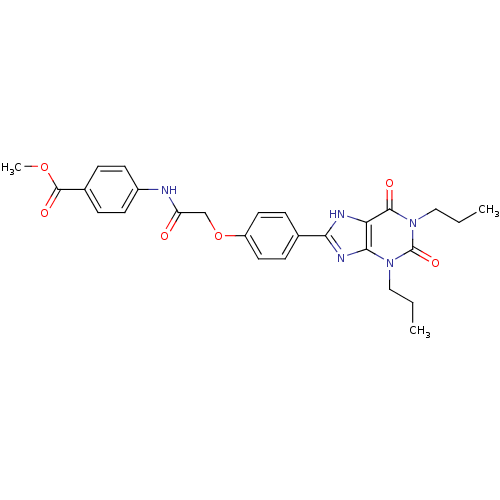 (4-{2-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50086163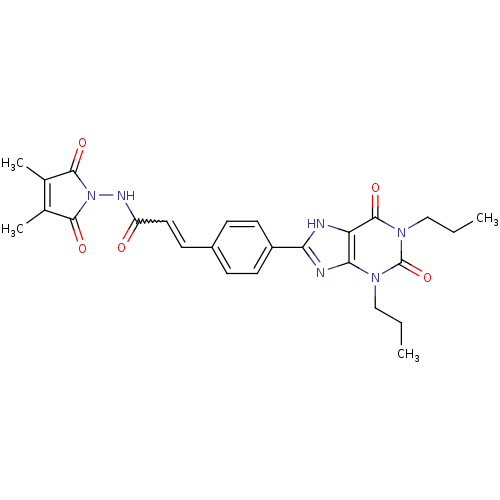 (CHEMBL17294 | N-(3,4-Dimethyl-2,5-dioxo-2,5-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against adenosine A1 receptor in rat brain membrane in presence of [3H]-R-PIA radioligand. | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50086168 (2-(4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against adenosine A1 receptor in rat brain membrane in presence of [3H]-R-PIA radioligand. | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50004566 (9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity for human Adenosine A1 receptor from HEK-293 cells | J Med Chem 39: 4142-8 (1996) Article DOI: 10.1021/jm960482i BindingDB Entry DOI: 10.7270/Q2FQ9VQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086187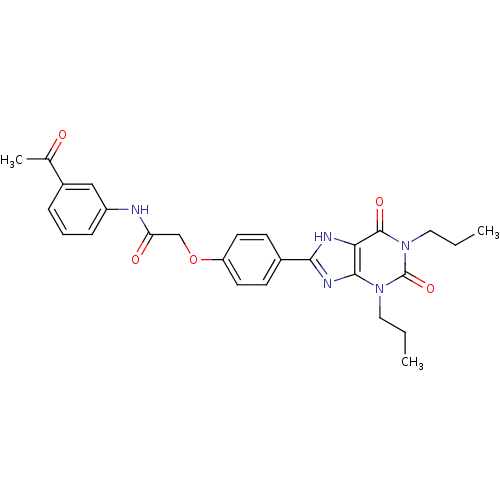 (CHEMBL16534 | N-(3-Acetyl-phenyl)-2-[4-(2,6-dioxo-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50086166 (CHEMBL275605 | N-Benzyl-2-[4-(2,6-dioxo-1,3-diprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against adenosine A1 receptor in rat brain membrane in presence of [3H]-R-PIA radioligand. | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086178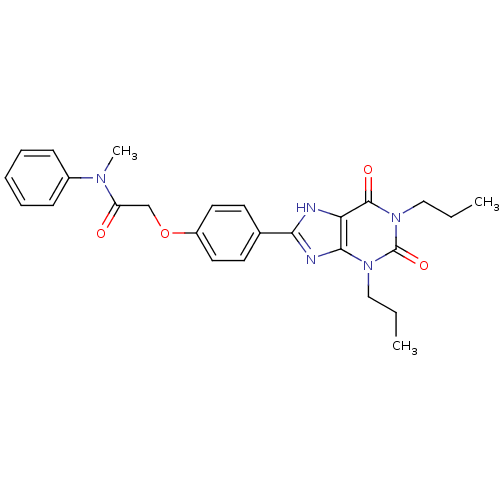 (2-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against human A2B adenosine receptor expressed in HEK-293 cells uisng [3H]-ZM-241,385 or [125I]-IABOPX | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50021085 (2-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against adenosine A1 receptor in rat brain membrane in presence of [3H]-R-PIA radioligand. | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50086164 (CHEMBL275427 | N-(4-Chloro-phenyl)-2-[4-(2,6-dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against adenosine A1 receptor in rat brain membrane in presence of [3H]-R-PIA radioligand. | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50455070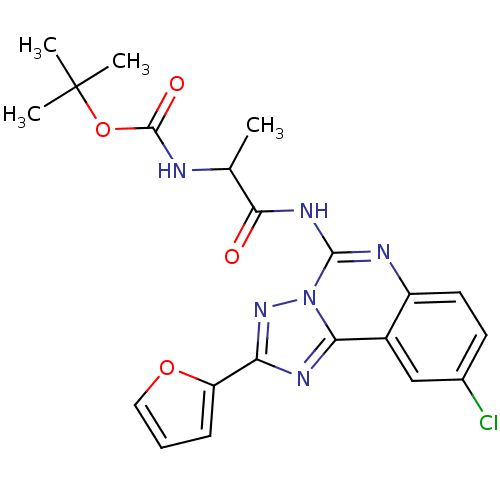 (CHEMBL2114300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat striatal Adenosine A2A receptor vs [3H]-CGS- 21680 | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065755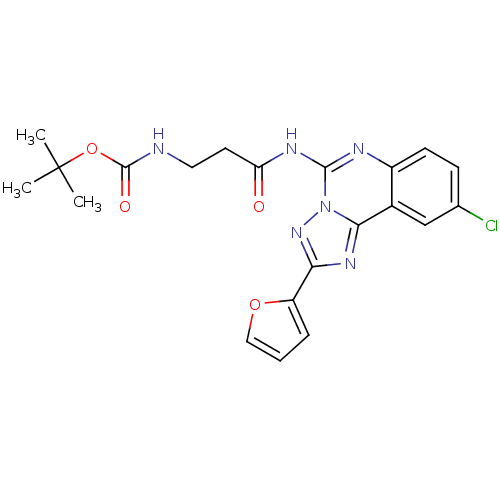 (CHEMBL319539 | [2-(9-Chloro-2-furan-2-yl-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50207816 (CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against recombinant human adenosine A1 receptor expressed in HEK-293 cells in presence of [125I]-IABA radioligand. | J Med Chem 43: 1165-72 (2000) BindingDB Entry DOI: 10.7270/Q2M044NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50065766 (2-(4-Amino-phenyl)-N-(9-chloro-2-furan-2-yl-[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat striatal Adenosine A2A receptor vs [3H]-CGS- 21680 | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50065775 (5-Amino-pentanoic acid (9-chloro-2-furan-2-yl-[1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat brain adenosine A1 receptor vs [3H]-R-PIA | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1523 total ) | Next | Last >> |