

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474150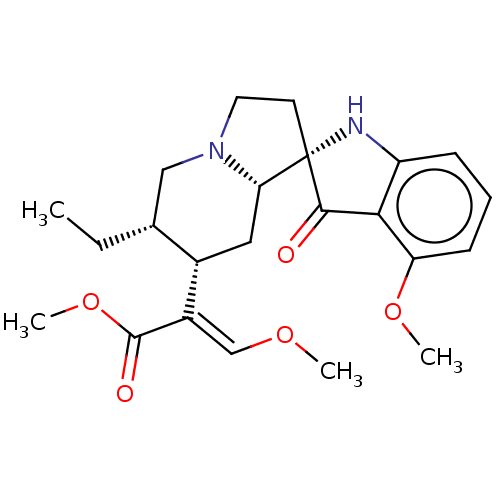 (CHEMBL58362) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor (unknown origin) | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004178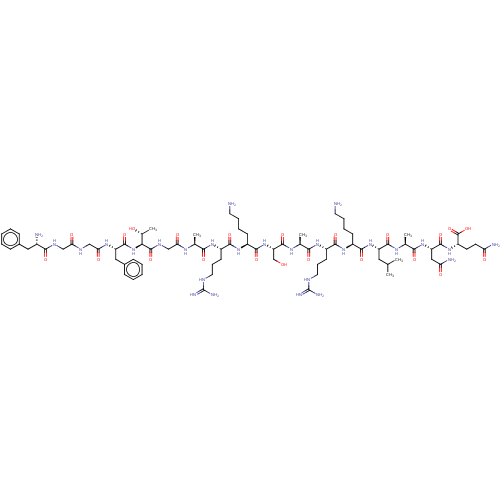 (Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Binding affinity for opioid receptor like type, human Opioid receptor like 1 expressed in membrane homogenates of COS-1 or CHO cells | J Med Chem 45: 5353-7 (2002) BindingDB Entry DOI: 10.7270/Q2VT1ST9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50459408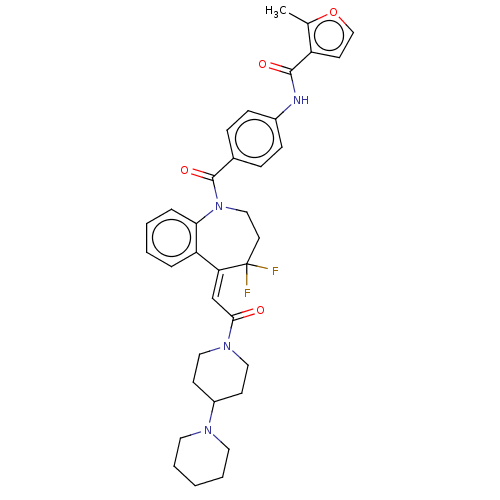 (CHEMBL3307200) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1A receptor expressed in CHO cell membranes | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM85095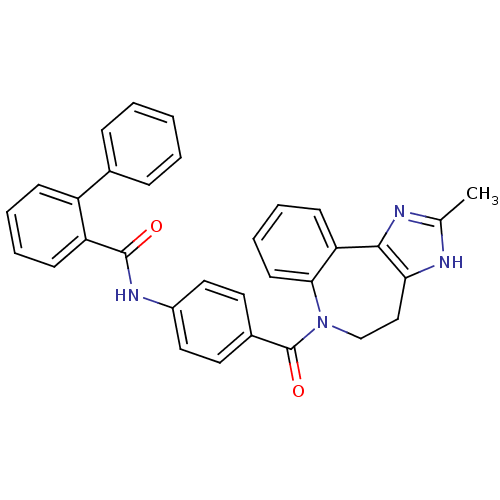 (CAS_151171 | CONIVAPTAN | NSC_151171 | YM087) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]AVP from V1A receptor in Wistar rat liver membranes incubated for 60 mins by microplate scintillation counting method | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50307117 (CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074509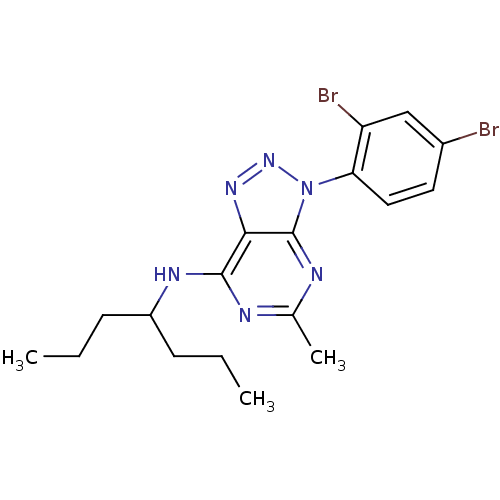 (CHEMBL354886 | [3-(2,4-Dibromo-phenyl)-5-methyl-3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074501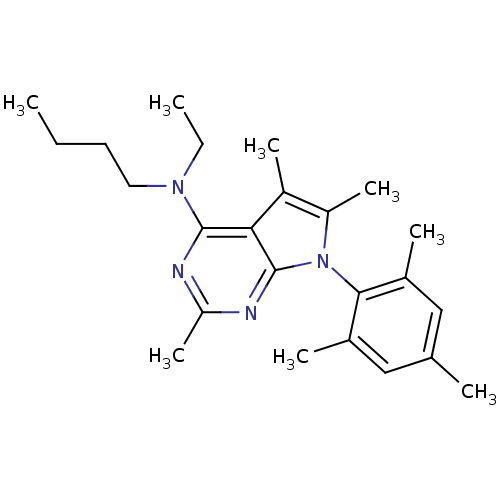 (Butyl-ethyl-[2,5,6-trimethyl-7-(2,4,6-trimethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074492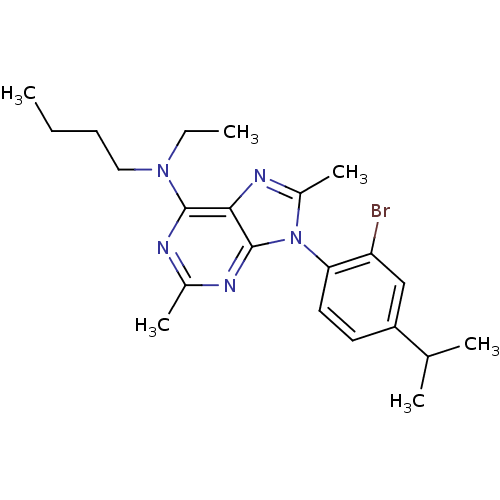 (CHEMBL168554 | [9-(2-Bromo-4-isopropyl-phenyl)-2,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM126004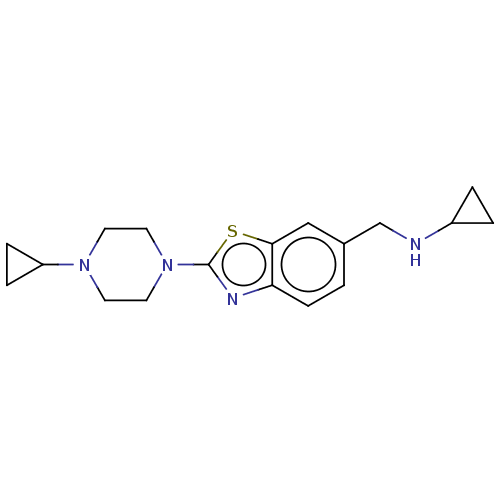 (US8772285, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
High Point Pharmaceuticals, LLC US Patent | Assay Description The ability of the compounds to bind and interact with the human H3 receptor as agonists, inverse agonists and/or antagonists, is determined by a fun... | US Patent US8772285 (2014) BindingDB Entry DOI: 10.7270/Q2P849KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM170440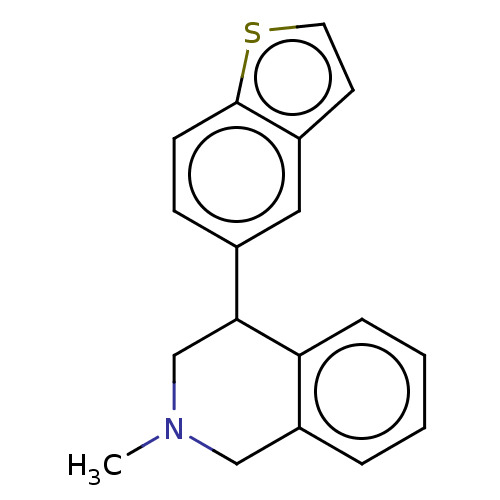 (US9085531, 40) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM170440 (US9085531, 40) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM170452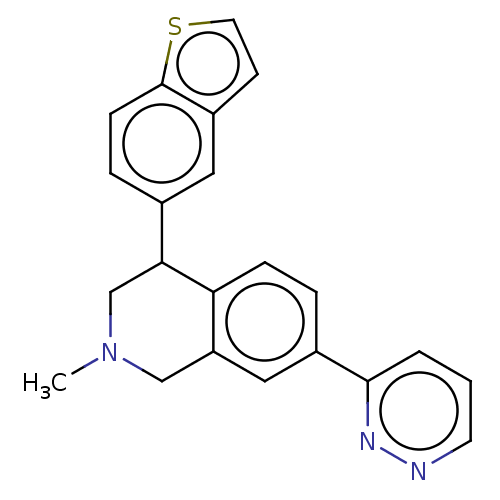 (US9085531, 97) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074465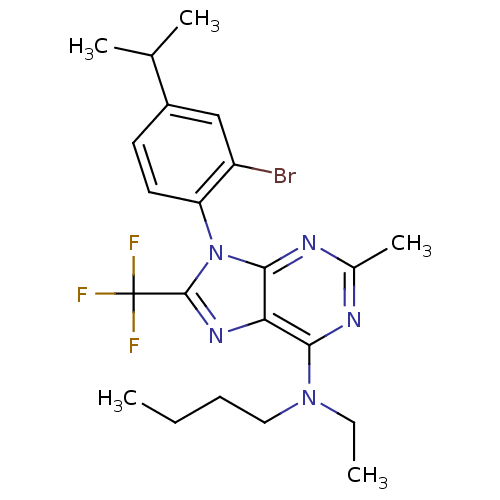 (CHEMBL354982 | [9-(2-Bromo-4-isopropyl-phenyl)-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074500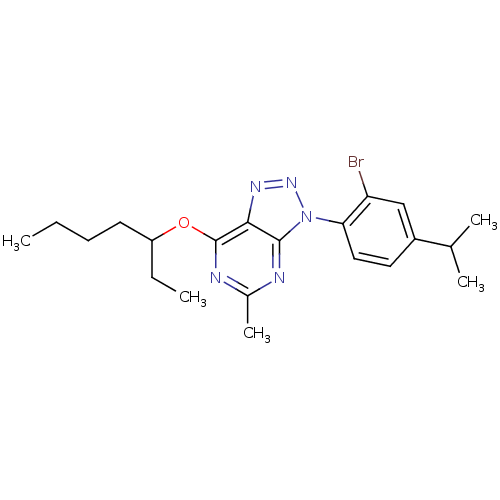 (3-(2-Bromo-4-isopropyl-phenyl)-7-(1-ethyl-pentylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50076613 (CHEMBL367253 | [9-(2-Bromo-4-isopropyl-phenyl)-8-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for transfected human Corticotropin releasing factor receptor expressed in HEK 293E cells using [125I]-TYR-oCRH as the displaced rad... | Bioorg Med Chem Lett 9: 967-72 (1999) BindingDB Entry DOI: 10.7270/Q26H4GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50076602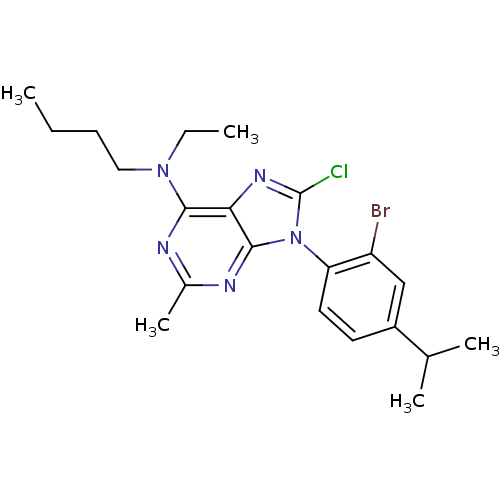 (CHEMBL368772 | [9-(2-Bromo-4-isopropyl-phenyl)-8-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for transfected human Corticotropin releasing factor receptor expressed in HEK 293E cells using [125I]-TYR-oCRH as the displaced rad... | Bioorg Med Chem Lett 9: 967-72 (1999) BindingDB Entry DOI: 10.7270/Q26H4GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50058163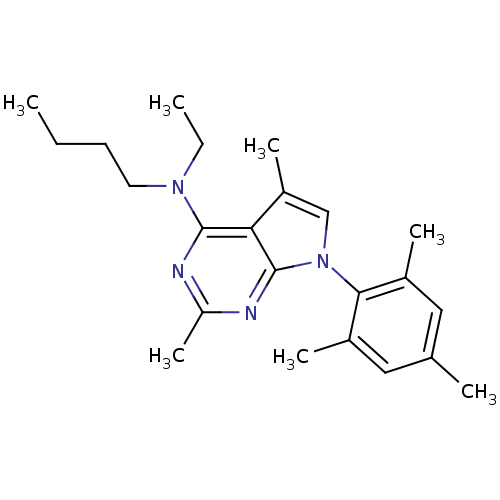 (Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM126003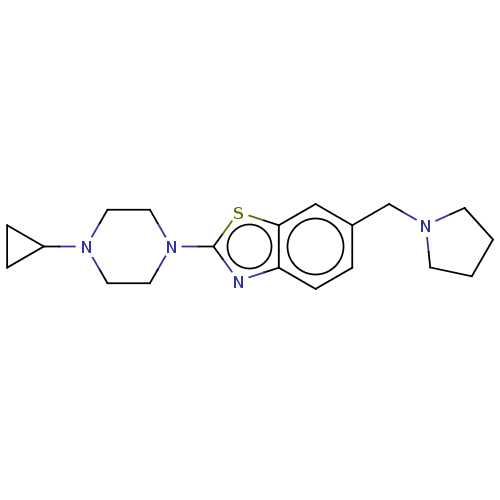 (US8772285, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
High Point Pharmaceuticals, LLC US Patent | Assay Description The ability of the compounds to bind and interact with the human H3 receptor as agonists, inverse agonists and/or antagonists, is determined by a fun... | US Patent US8772285 (2014) BindingDB Entry DOI: 10.7270/Q2P849KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074482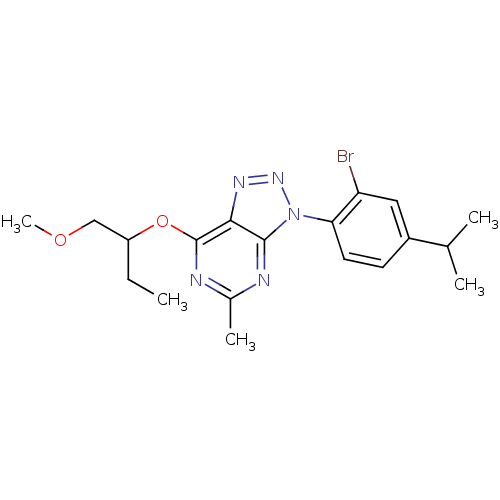 (3-(2-Bromo-4-isopropyl-phenyl)-7-(1-methoxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074470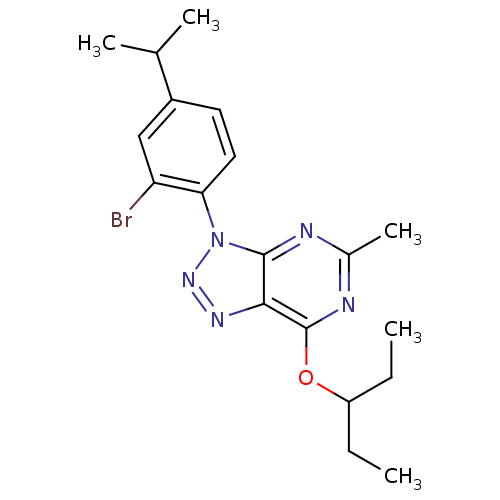 (3-(2-Bromo-4-isopropyl-phenyl)-7-(1-ethyl-propoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM170442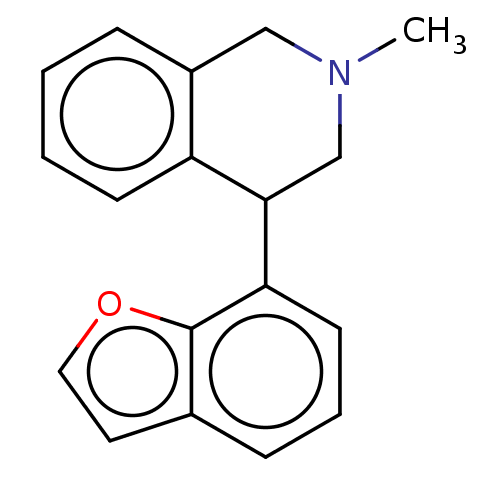 (US9085531, 19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50057073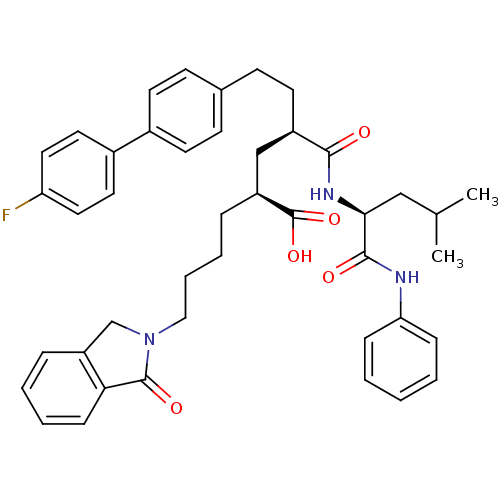 ((2S,4R)-6-(4'-Fluoro-biphenyl-4-yl)-4-((S)-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) | J Med Chem 40: 1026-40 (1997) Article DOI: 10.1021/jm960465t BindingDB Entry DOI: 10.7270/Q2H70DXR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074349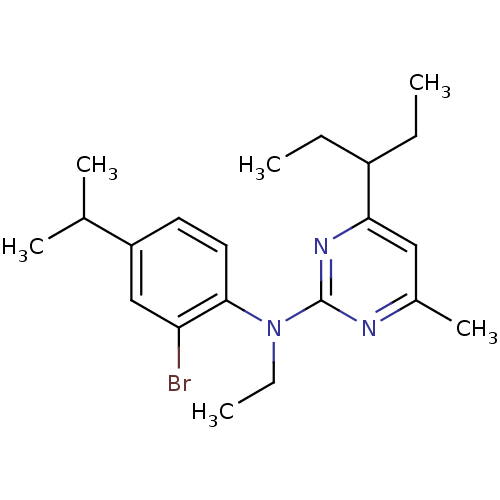 ((2-Bromo-4-isopropyl-phenyl)-ethyl-[4-(1-ethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for human recombinant corticotropin releasing factor receptor 1 | J Med Chem 42: 805-18 (1999) Article DOI: 10.1021/jm980222w BindingDB Entry DOI: 10.7270/Q29S1Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50057050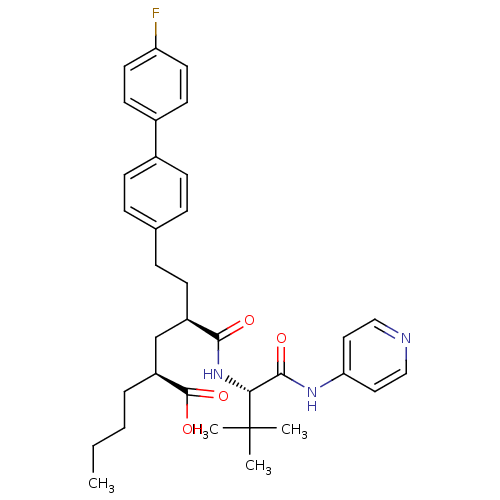 ((2S,4R)-2-Butyl-4-[(S)-2,2-dimethyl-1-(pyridin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) | J Med Chem 40: 1026-40 (1997) Article DOI: 10.1021/jm960465t BindingDB Entry DOI: 10.7270/Q2H70DXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM170444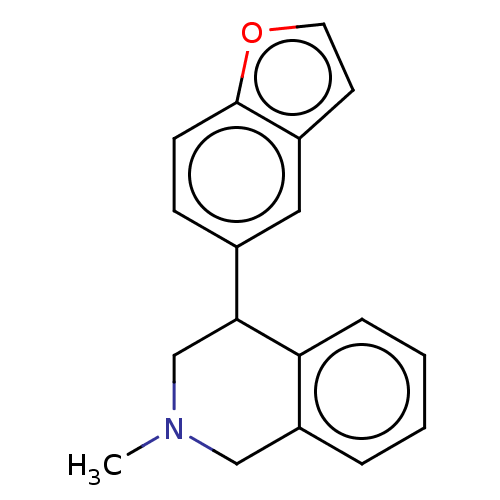 (US9085531, 14) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM170447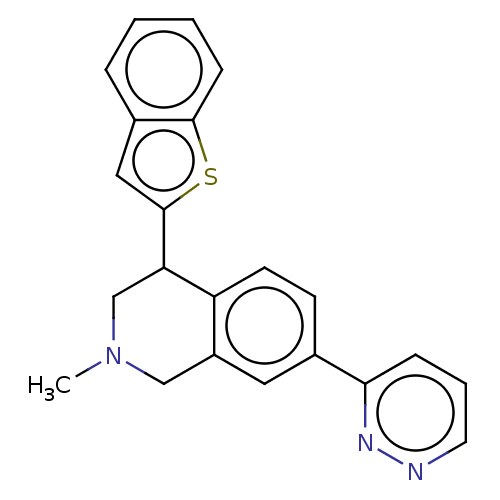 (US9085531, 70) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM170452 (US9085531, 97) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074468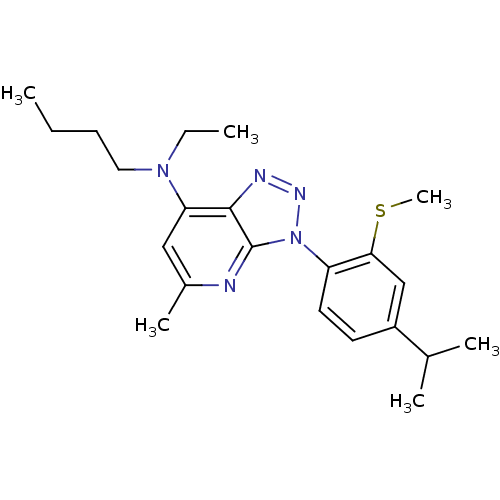 (Butyl-ethyl-[3-(4-isopropyl-2-methylsulfanyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50459401 (CHEMBL4210854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1A receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50411239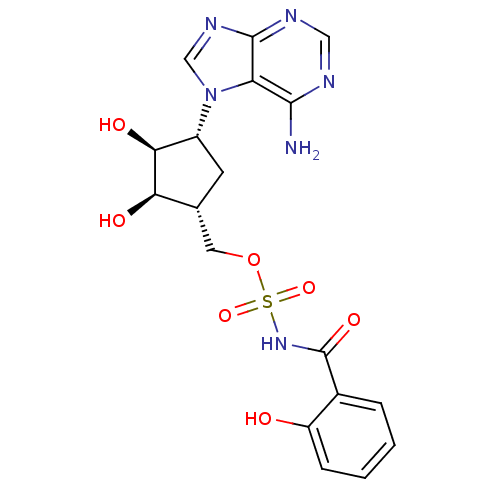 (CHEMBL218023) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA by [32P]PPi-ATP exchange assay | J Med Chem 49: 7623-35 (2006) Article DOI: 10.1021/jm061068d BindingDB Entry DOI: 10.7270/Q20Z74H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM170455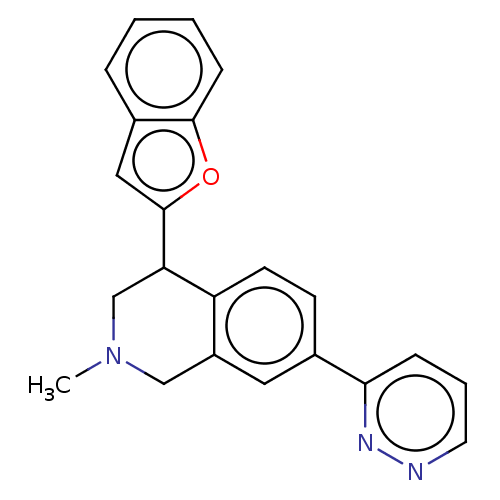 (US9085531, 117) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM60509 (US9085531, 125) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50307117 (CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V1A receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074474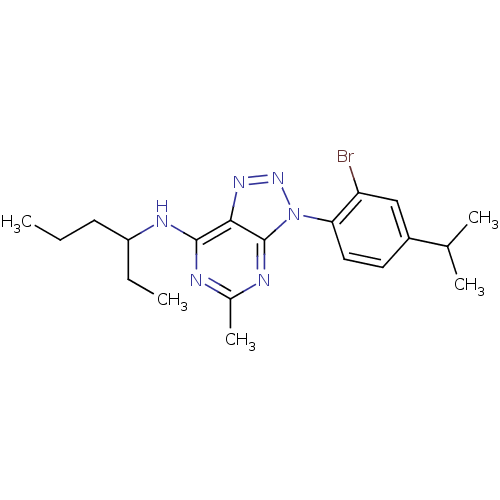 (CHEMBL166669 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074459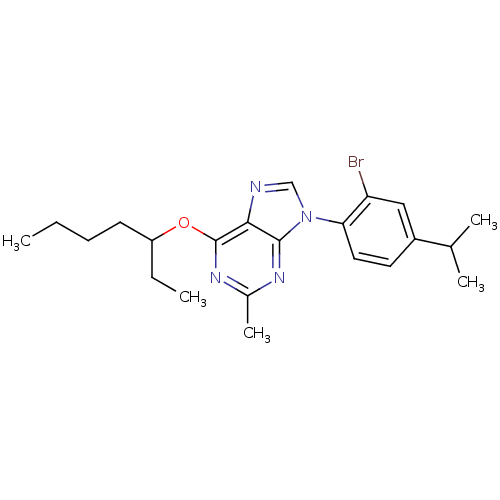 (9-(2-Bromo-4-isopropyl-phenyl)-6-(1-ethyl-pentylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM60513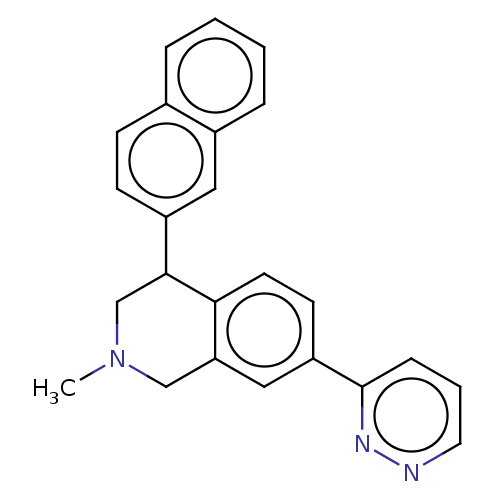 (US9085531, 137) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM170454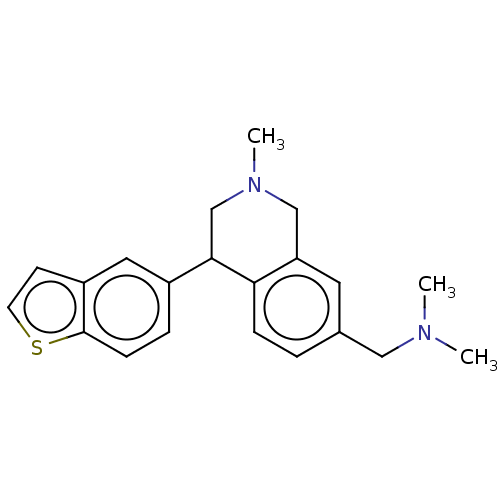 (US9085531, 102) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM85095 (CAS_151171 | CONIVAPTAN | NSC_151171 | YM087) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]AVP from V2 receptor in Wistar rat kidney membranes incubated for 60 mins by microplate scintillation counting method | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50474150 (CHEMBL58362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor (unknown origin) | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074458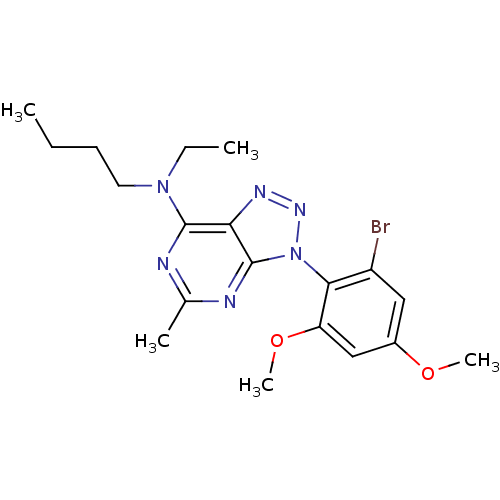 (CHEMBL9633 | [3-(2-Bromo-4,6-dimethoxy-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074354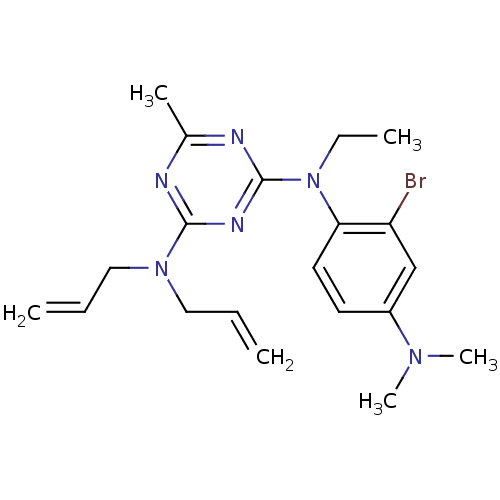 (CHEMBL168131 | N,N-Diallyl-N'-(2-bromo-4-dimethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for human recombinant corticotropin releasing factor receptor 1 | J Med Chem 42: 805-18 (1999) Article DOI: 10.1021/jm980222w BindingDB Entry DOI: 10.7270/Q29S1Q63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50076608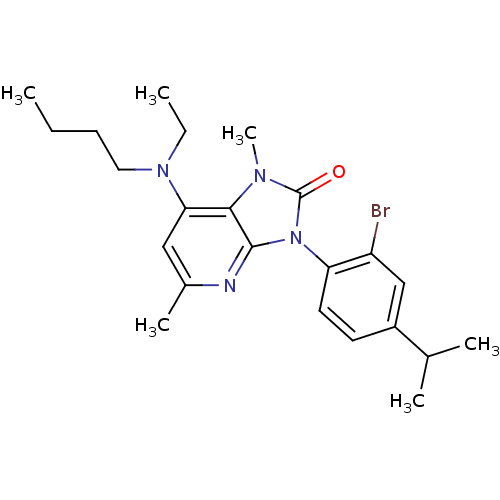 (7-(Butyl-ethyl-amino)-1,5-dimethyl-3-phenyl-1,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity for transfected human Corticotropin releasing factor receptor expressed in HEK 293E cells using [125I]-TYR-oCRH as the displaced rad... | Bioorg Med Chem Lett 9: 967-72 (1999) BindingDB Entry DOI: 10.7270/Q26H4GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074452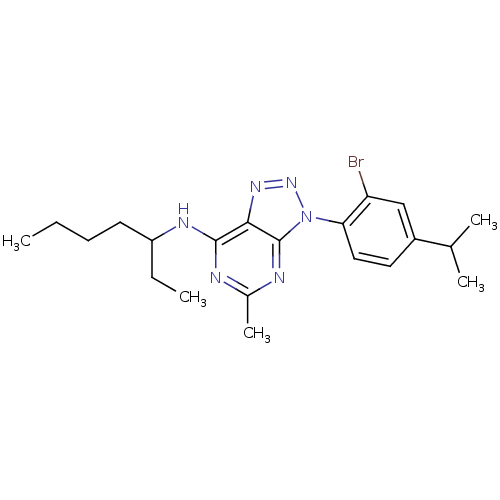 (CHEMBL169952 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074453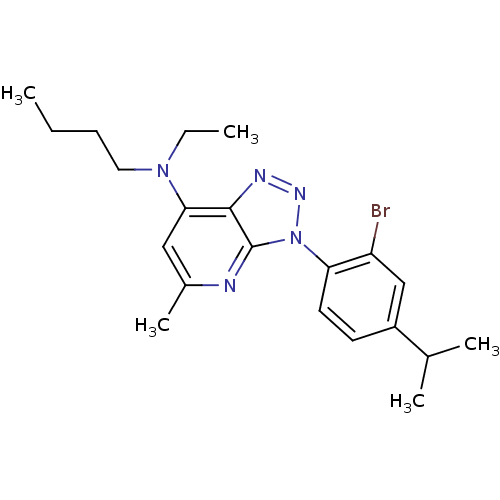 (CHEMBL171202 | [3-(2-Bromo-4-isopropyl-phenyl)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50411241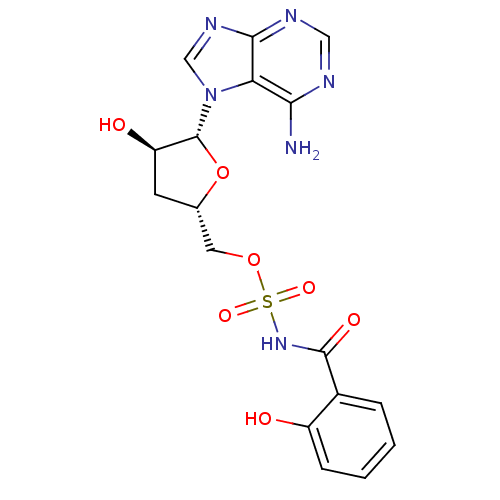 (CHEMBL387162) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA by [32P]PPi-ATP exchange assay | J Med Chem 49: 7623-35 (2006) Article DOI: 10.1021/jm061068d BindingDB Entry DOI: 10.7270/Q20Z74H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM170449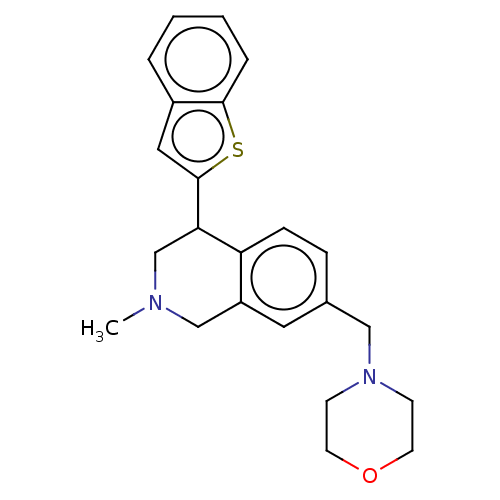 (US9085531, 78) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM60503 (US9085531, 56) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM170454 (US9085531, 102) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Albany Molecular Research, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description In order to evaluate the relative affinity of the various compounds at the NE, DA and 5HT transporters, HEK293E cell lines were developed to express ... | US Patent US9085531 (2015) BindingDB Entry DOI: 10.7270/Q24X56JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50074522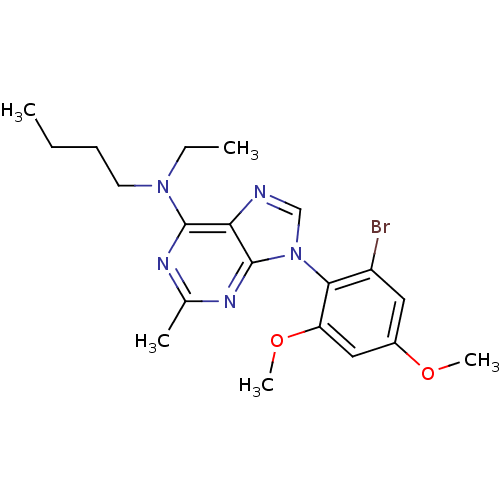 (CHEMBL354940 | [9-(2-Bromo-4,6-dimethoxy-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity to recombinant human Corticotropin releasing factor receptor 1 (hCRF1) expressed in 293EBNA cells | J Med Chem 42: 833-48 (1999) Article DOI: 10.1021/jm980224g BindingDB Entry DOI: 10.7270/Q2610ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2803 total ) | Next | Last >> |