

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181460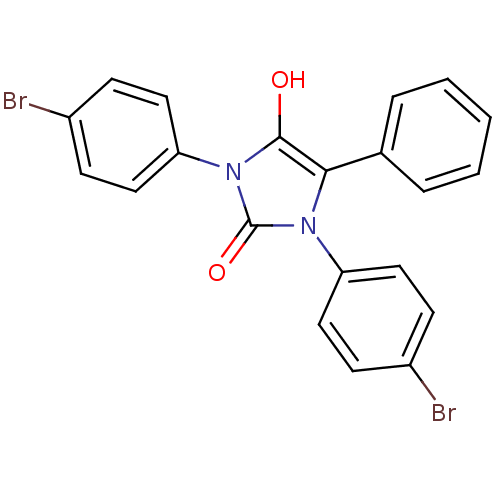 (1,3-bis(4-bromophenyl)-5-phenylimidazolidine-2,4-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50181463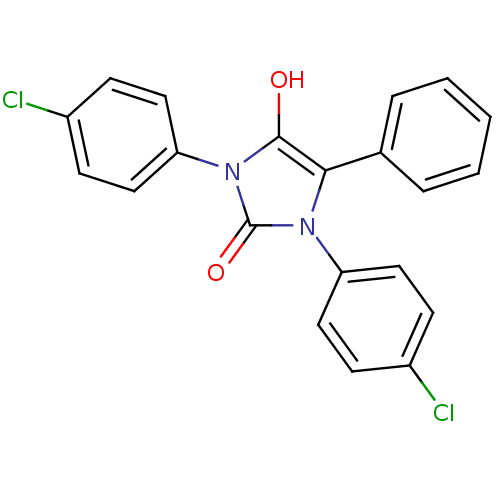 (1,3-bis(4-chlorophenyl)-5-phenylimidazolidine-2,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50181460 (1,3-bis(4-bromophenyl)-5-phenylimidazolidine-2,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181463 (1,3-bis(4-chlorophenyl)-5-phenylimidazolidine-2,4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181462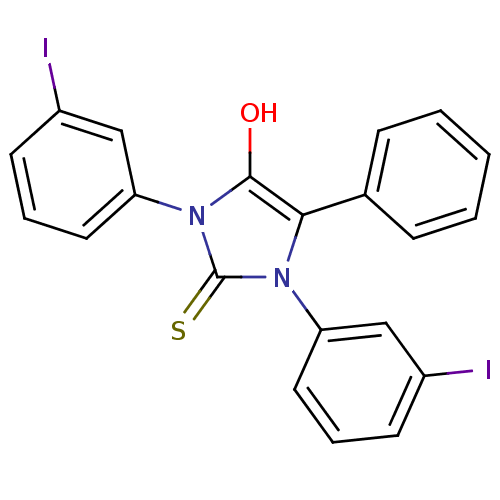 (1,3-bis(3-iodophenyl)-5-phenyl-2-thioxoimidazolidi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181458 (5-(4-bromophenyl)-1,3-bis(4-chlorophenyl)imidazoli...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 905 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181461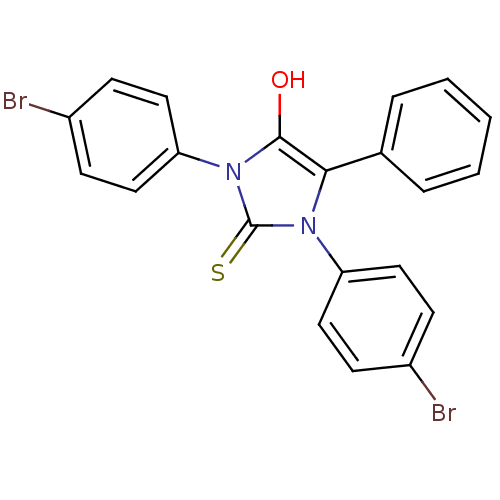 (1,3-bis(4-bromophenyl)-5-phenyl-2-thioxoimidazolid...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50181458 (5-(4-bromophenyl)-1,3-bis(4-chlorophenyl)imidazoli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181459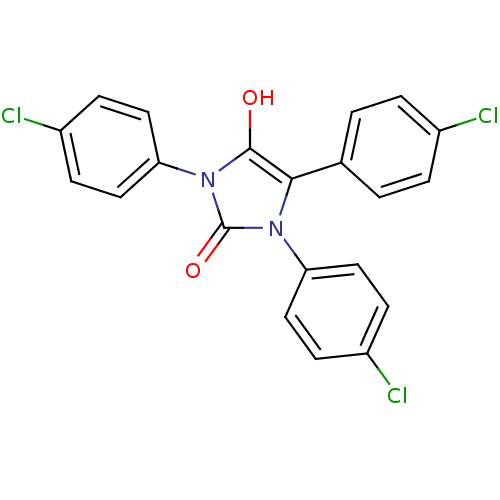 (1,3,5-tris(4-chlorophenyl)imidazolidine-2,4-dione ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181466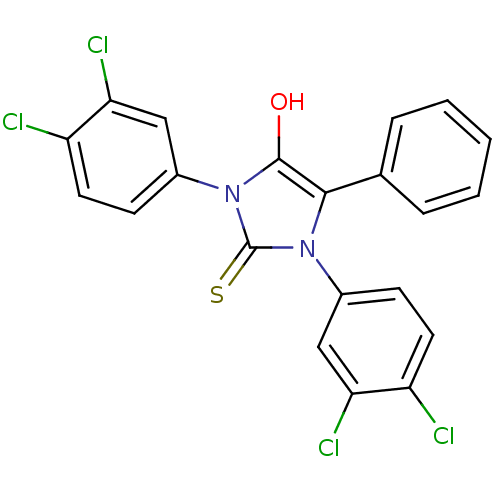 (1,3-bis(4,3-dichlorophenyl)-5-phenyl-2-thioxoimida...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181469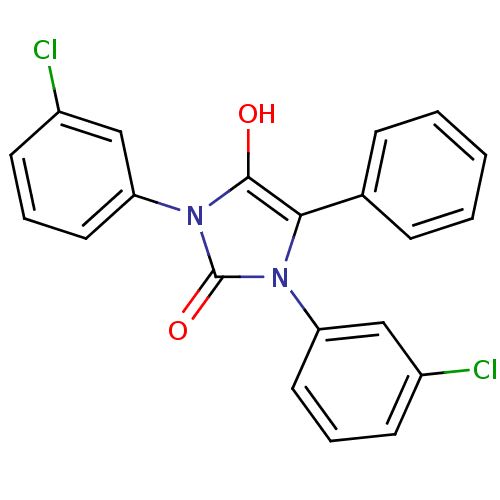 (1,3-bis(3-chlorophenyl)-5-phenylimidazolidine-2,4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181456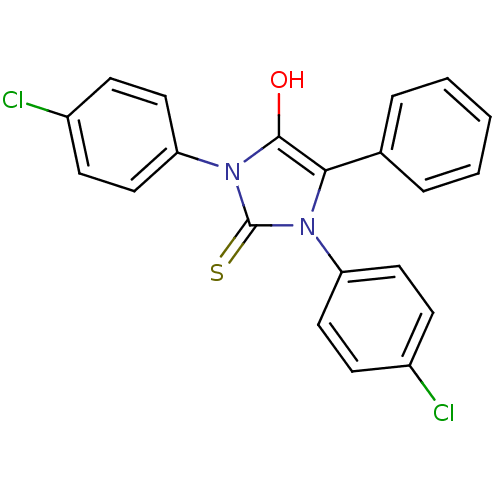 (1,3-bis(4-chlorophenyl)-5-phenyl-2-thioxoimidazoli...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50181459 (1,3,5-tris(4-chlorophenyl)imidazolidine-2,4-dione ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181455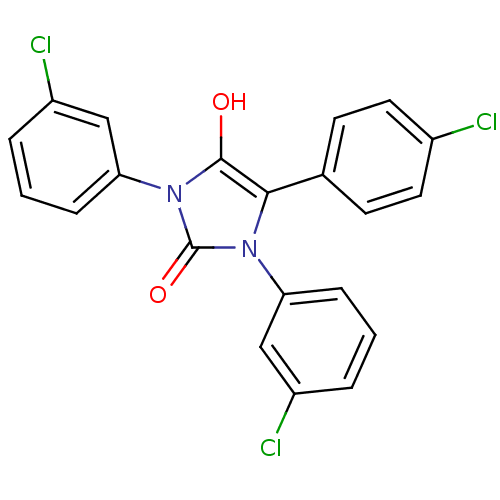 (1,3-bis(3-chlorophenyl)-5-(4-chlorophenyl)imidazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181472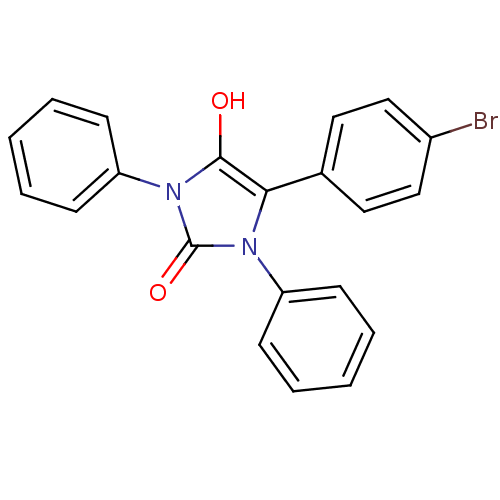 (5-(4-bromophenyl)-1,3-diphenylimidazolidine-2,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181470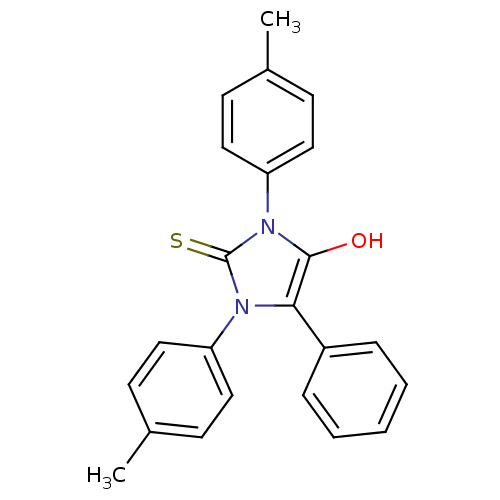 (1,3-bis(4-methylphenyl)-5-phenyl-2-thioxoimidazoli...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181471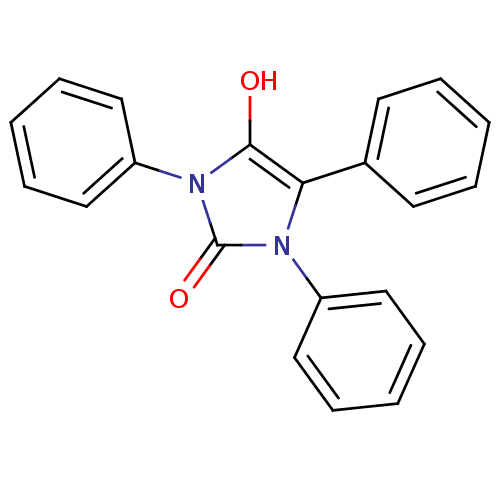 (1,3,5-triphenylimidazolidine-2,4-dione | CHEMBL381...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181467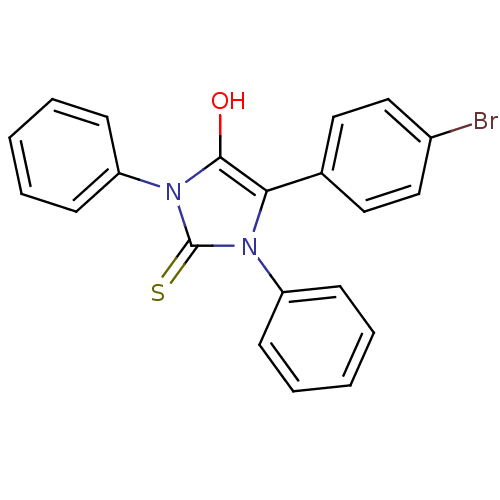 (5-(4-bromophenyl)-1,3-diphenyl-2-thioxoimidazolidi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181468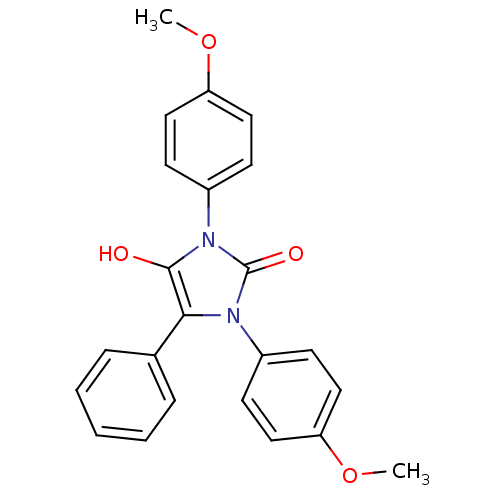 (1,3-bis(4-methoxyphenyl)-5-phenylimidazolidine-2,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181465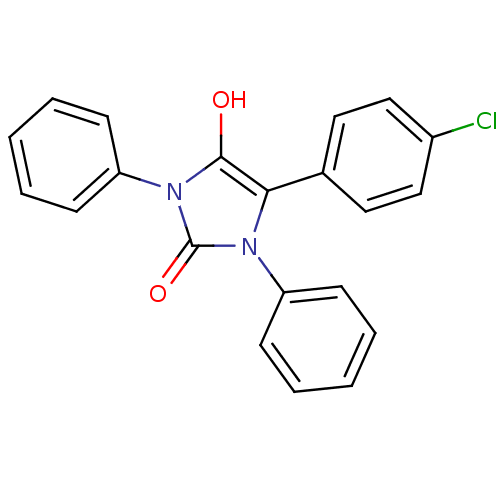 (5-(4-chlorophenyl)-1,3-diphenylimidazolidine-2,4-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181457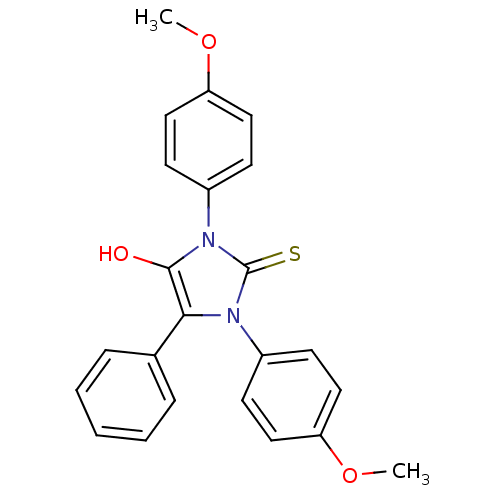 (1,3-bis(4-methoxyphenyl)-5-phenyl-2-thioxoimidazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50181464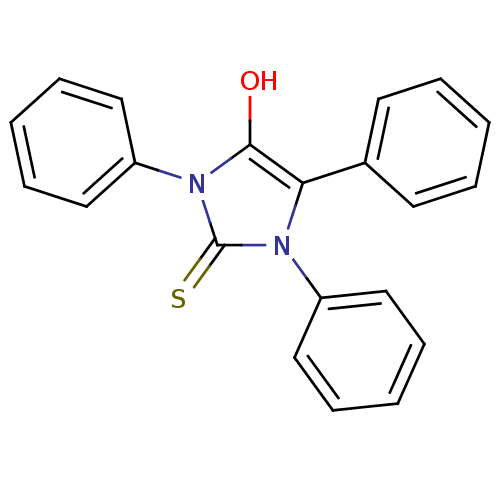 (1,3,5-triphenyl-2-thioxoimidazolidin-4-one | CHEMB...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156581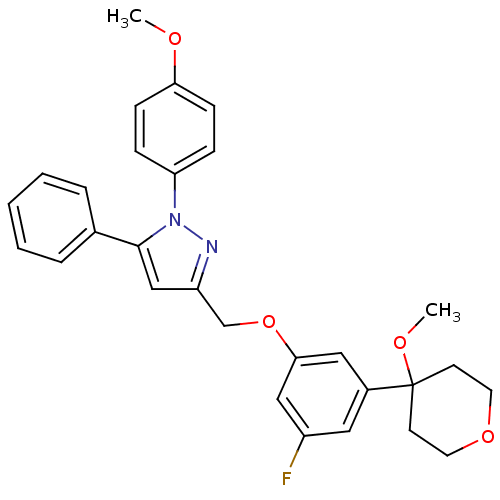 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cells | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000829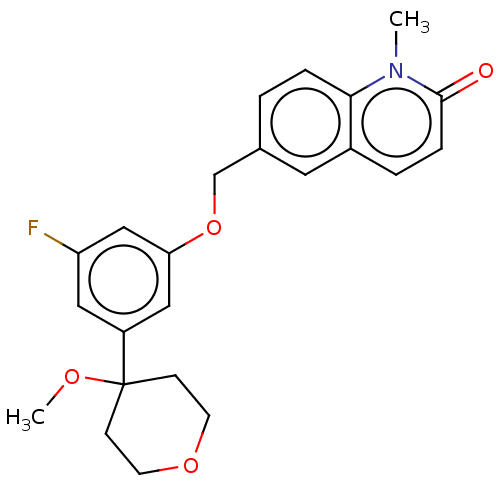 (6-((3-fluoro-5-(4-methoxytetrahydro-2H-pyran-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156577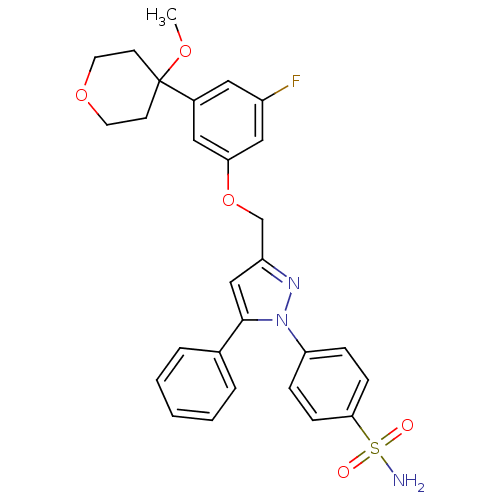 (1-(4-aminosulfonylphenyl)-3-[3-fluoro-5-(4-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110485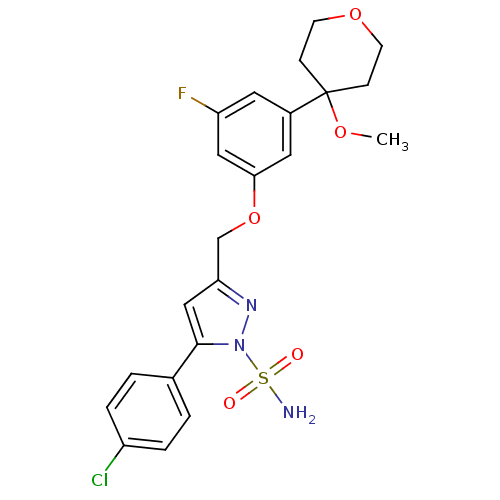 (5-(4-Chloro-phenyl)-3-[3-fluoro-5-(4-methoxy-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110483 (2-[3-Fluoro-5-(4-methoxy-tetrahydro-pyran-4-yl)-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50177863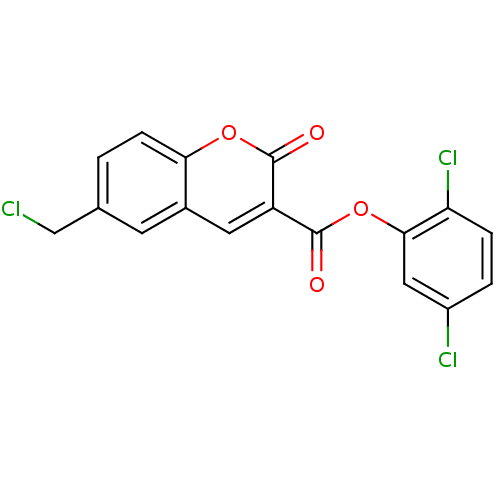 (2,5-dichlorophenyl 6-(chloromethyl)-2-oxo-2H-chrom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | J Med Chem 48: 7592-603 (2005) Article DOI: 10.1021/jm050448g BindingDB Entry DOI: 10.7270/Q2V987MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110485 (5-(4-Chloro-phenyl)-3-[3-fluoro-5-(4-methoxy-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase activity of compound evaluated as determined by the inhibition of calcium ionophore-induced leukotriene B4 production in... | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156584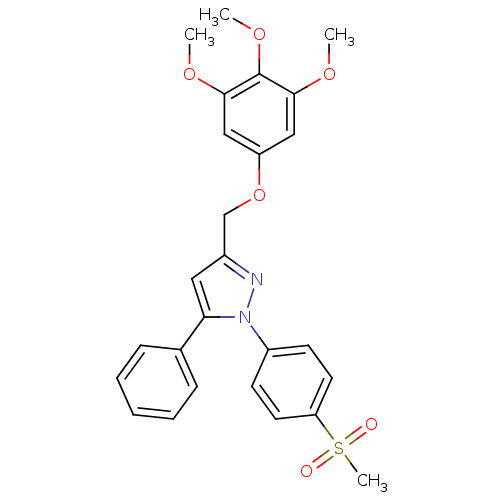 (1-(4-methanesulfonylphenyl)-3-[(3,4,5-trimethoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110483 (2-[3-Fluoro-5-(4-methoxy-tetrahydro-pyran-4-yl)-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera Curated by ChEMBL | Assay Description The 5-lipoxygenase activity of the compound was determined by the inhibition of calcium ionophore-induced leukotriene B4 production in human blood. | Bioorg Med Chem Lett 12: 779-82 (2002) BindingDB Entry DOI: 10.7270/Q20864MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156581 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156594 (3-[(3-fluoro-5-methoxy)phenoxymethyl]-1-(4-methane...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038001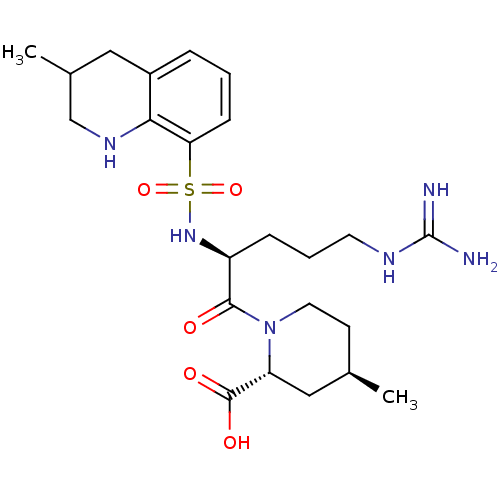 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | J Med Chem 48: 7592-603 (2005) Article DOI: 10.1021/jm050448g BindingDB Entry DOI: 10.7270/Q2V987MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50120981 (1,1,1-trifluoro-N-(3-phenoxypyridin-4-yl)methanesu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège Curated by ChEMBL | Assay Description The inhibitory activity against cyclooxygenase Prostaglandin G/H synthase 2 (COX-2) measured as PGE-2 production after blood coagulation using human ... | J Med Chem 45: 5182-5 (2002) BindingDB Entry DOI: 10.7270/Q2WS8SM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156580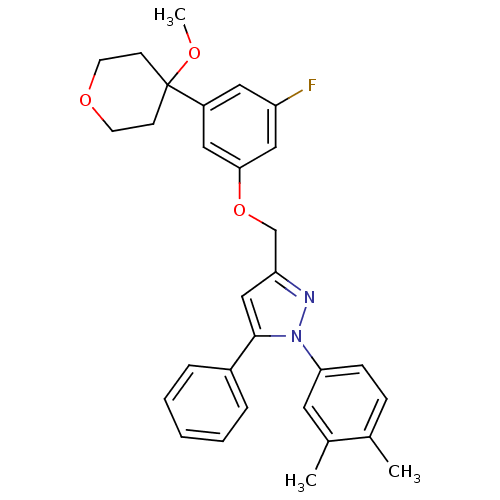 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156590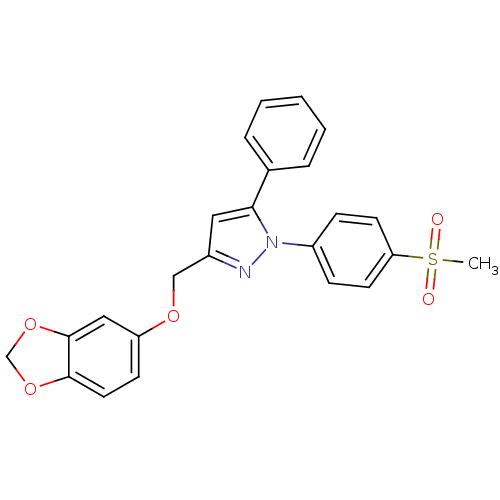 (1-(4-methanesulfonylphenyl)-3-[(3,4-methylenedioxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156585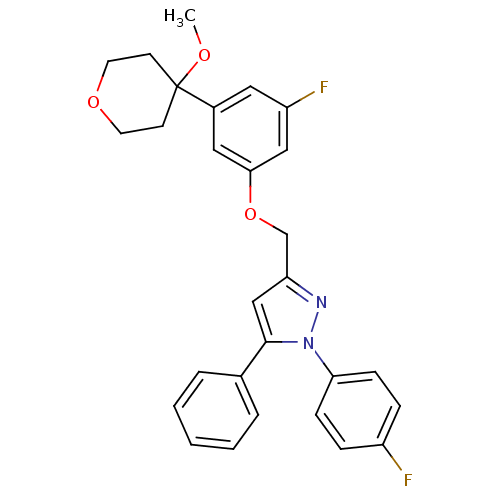 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 148 total ) | Next | Last >> |