 Found 1348 hits with Last Name = 'cywin' and Initial = 'c'
Found 1348 hits with Last Name = 'cywin' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14028

((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...)Show SMILES C[C@H]1CNCCCN1S(=O)(=O)c1cccc2cncc(C)c12 |r| Show InChI InChI=1S/C16H21N3O2S/c1-12-9-18-11-14-5-3-6-15(16(12)14)22(20,21)19-8-4-7-17-10-13(19)2/h3,5-6,9,11,13,17H,4,7-8,10H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK1 by homogenous luciferase assay |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50027431
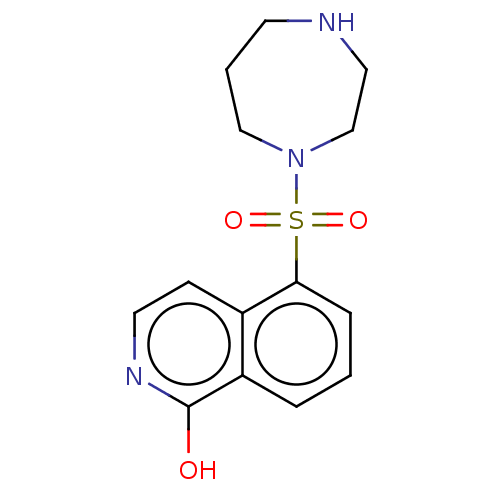
(HYDROXYFASUDIL | Hydroxy-Fasudil)Show InChI InChI=1S/C14H17N3O3S/c18-14-12-3-1-4-13(11(12)5-7-16-14)21(19,20)17-9-2-6-15-8-10-17/h1,3-5,7,15H,2,6,8-10H2,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK1 by homogenous luciferase assay |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14029
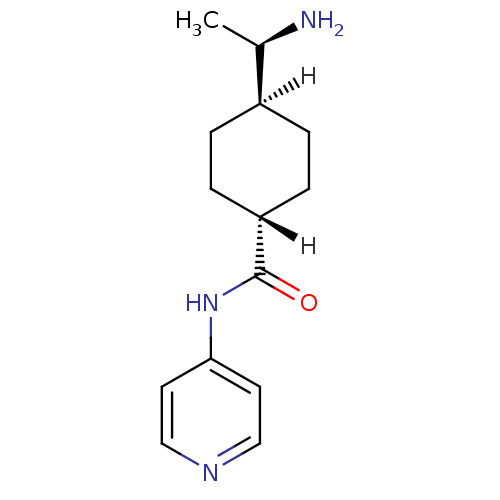
((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK1 by homogenous luciferase assay |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14029

((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK2 by homogenous luciferase assay |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026260
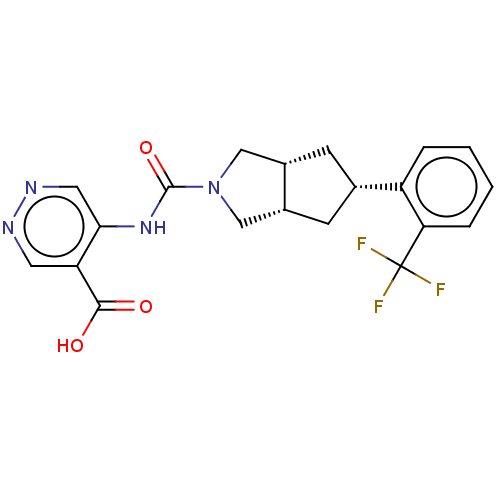
(CHEMBL3359024)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)Nc1cnncc1C(O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C20H19F3N4O3/c21-20(22,23)16-4-2-1-3-14(16)11-5-12-9-27(10-13(12)6-11)19(30)26-17-8-25-24-7-15(17)18(28)29/h1-4,7-8,11-13H,5-6,9-10H2,(H,28,29)(H,24,26,30)/t11-,12-,13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.121 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50019040
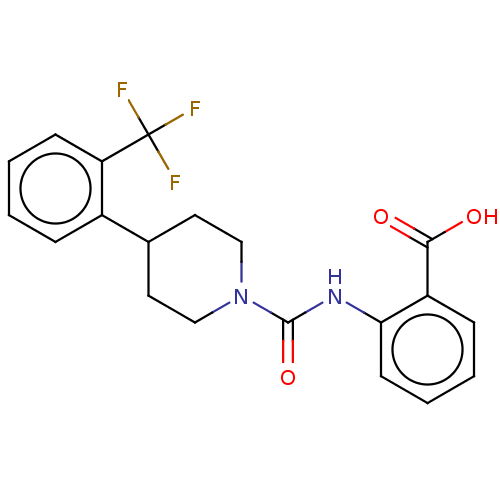
(CHEMBL1230001)Show SMILES OC(=O)c1ccccc1NC(=O)N1CCC(CC1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C20H19F3N2O3/c21-20(22,23)16-7-3-1-5-14(16)13-9-11-25(12-10-13)19(28)24-17-8-4-2-6-15(17)18(26)27/h1-8,13H,9-12H2,(H,24,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026258
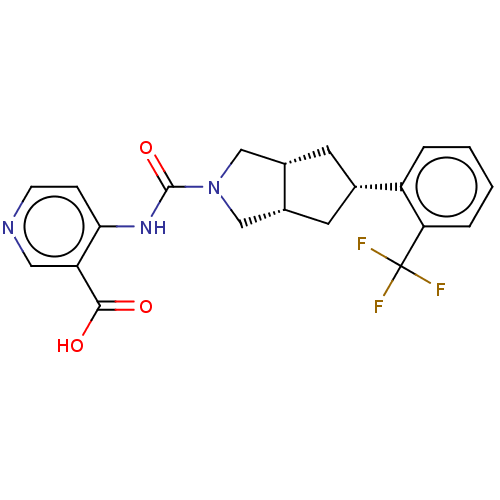
(CHEMBL3359022)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)Nc1ccncc1C(O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C21H20F3N3O3/c22-21(23,24)17-4-2-1-3-15(17)12-7-13-10-27(11-14(13)8-12)20(30)26-18-5-6-25-9-16(18)19(28)29/h1-6,9,12-14H,7-8,10-11H2,(H,28,29)(H,25,26,30)/t12-,13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.179 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026259
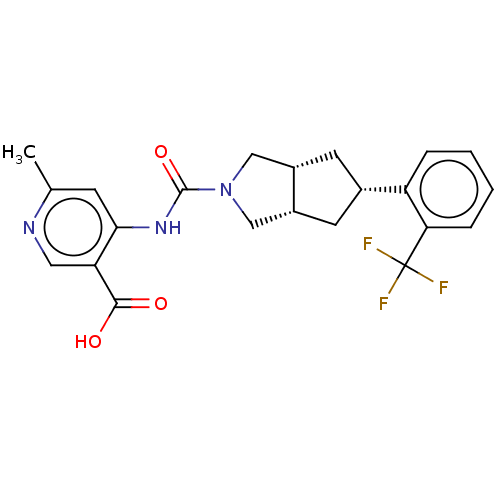
(CHEMBL3359023)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)Nc1cc(C)ncc1C(O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C22H22F3N3O3/c1-12-6-19(17(9-26-12)20(29)30)27-21(31)28-10-14-7-13(8-15(14)11-28)16-4-2-3-5-18(16)22(23,24)25/h2-6,9,13-15H,7-8,10-11H2,1H3,(H,29,30)(H,26,27,31)/t13-,14-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.235 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026255

(CHEMBL3359017)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)Nc1ccc(F)cc1C(O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C22H20F4N2O3/c23-15-5-6-19(17(9-15)20(29)30)27-21(31)28-10-13-7-12(8-14(13)11-28)16-3-1-2-4-18(16)22(24,25)26/h1-6,9,12-14H,7-8,10-11H2,(H,27,31)(H,29,30)/t12-,13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.285 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026253
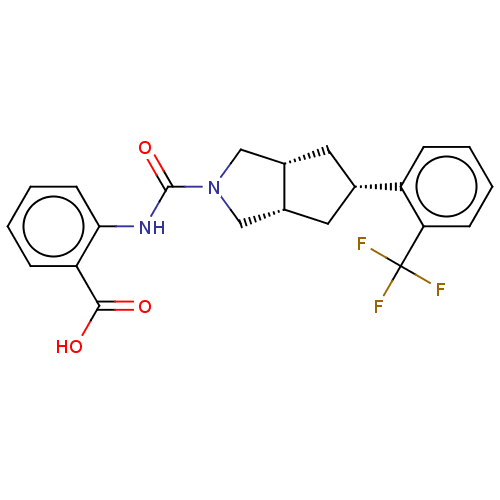
(CHEMBL3359014)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)Nc1ccccc1C(O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N2O3/c23-22(24,25)18-7-3-1-5-16(18)13-9-14-11-27(12-15(14)10-13)21(30)26-19-8-4-2-6-17(19)20(28)29/h1-8,13-15H,9-12H2,(H,26,30)(H,28,29)/t13-,14-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.294 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026256
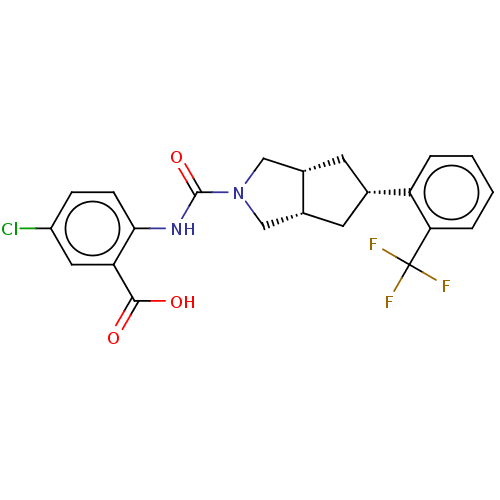
(CHEMBL3359019)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)Nc1ccc(Cl)cc1C(O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C22H20ClF3N2O3/c23-15-5-6-19(17(9-15)20(29)30)27-21(31)28-10-13-7-12(8-14(13)11-28)16-3-1-2-4-18(16)22(24,25)26/h1-6,9,12-14H,7-8,10-11H2,(H,27,31)(H,29,30)/t12-,13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026254
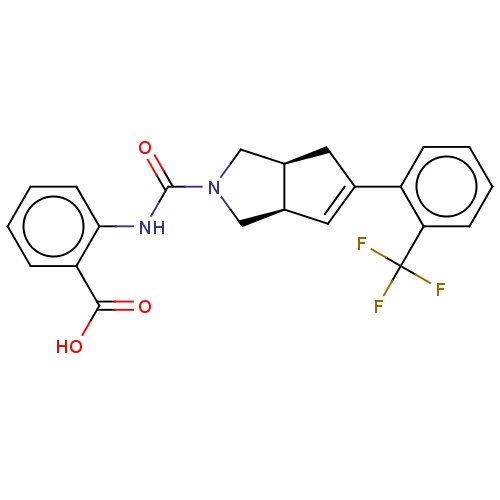
(CHEMBL3359015)Show SMILES [H][C@@]12CN(C[C@]1([H])C=C(C2)c1ccccc1C(F)(F)F)C(=O)Nc1ccccc1C(O)=O |r,c:8| Show InChI InChI=1S/C22H19F3N2O3/c23-22(24,25)18-7-3-1-5-16(18)13-9-14-11-27(12-15(14)10-13)21(30)26-19-8-4-2-6-17(19)20(28)29/h1-9,14-15H,10-12H2,(H,26,30)(H,28,29)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.481 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026251
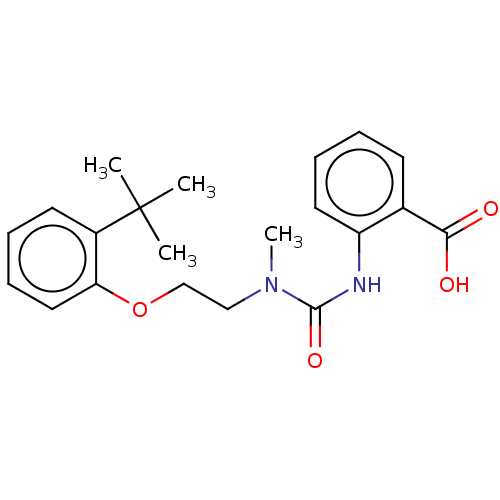
(CHEMBL3359005)Show SMILES CN(CCOc1ccccc1C(C)(C)C)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C21H26N2O4/c1-21(2,3)16-10-6-8-12-18(16)27-14-13-23(4)20(26)22-17-11-7-5-9-15(17)19(24)25/h5-12H,13-14H2,1-4H3,(H,22,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.554 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026244
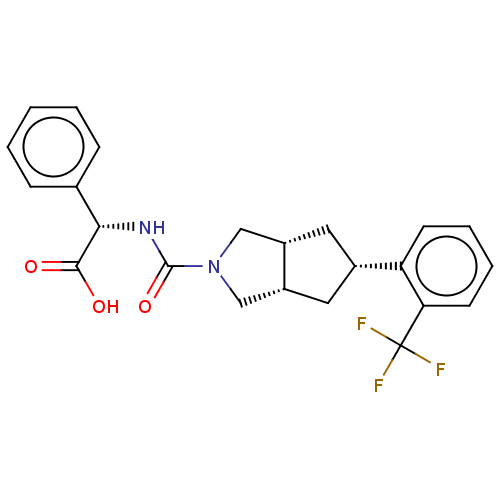
(CHEMBL3359028)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)N[C@H](C(O)=O)c1ccccc1)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C23H23F3N2O3/c24-23(25,26)19-9-5-4-8-18(19)15-10-16-12-28(13-17(16)11-15)22(31)27-20(21(29)30)14-6-2-1-3-7-14/h1-9,15-17,20H,10-13H2,(H,27,31)(H,29,30)/t15-,16-,17+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.829 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50319985
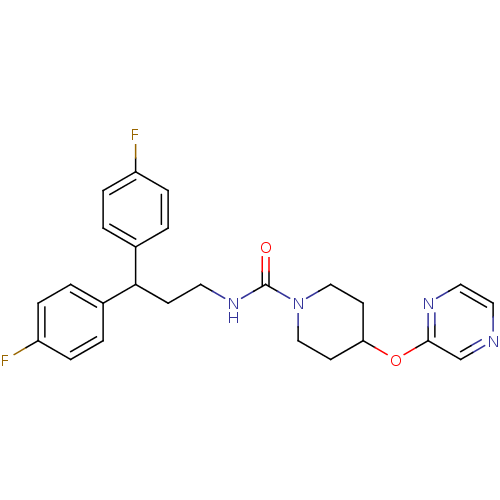
(CHEMBL1083622 | N-(3,3-bis(4-fluorophenyl)propyl)-...)Show SMILES Fc1ccc(cc1)C(CCNC(=O)N1CCC(CC1)Oc1cnccn1)c1ccc(F)cc1 Show InChI InChI=1S/C25H26F2N4O2/c26-20-5-1-18(2-6-20)23(19-3-7-21(27)8-4-19)9-12-30-25(32)31-15-10-22(11-16-31)33-24-17-28-13-14-29-24/h1-8,13-14,17,22-23H,9-12,15-16H2,(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 20: 3703-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.078
BindingDB Entry DOI: 10.7270/Q2BV7GTJ |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026257
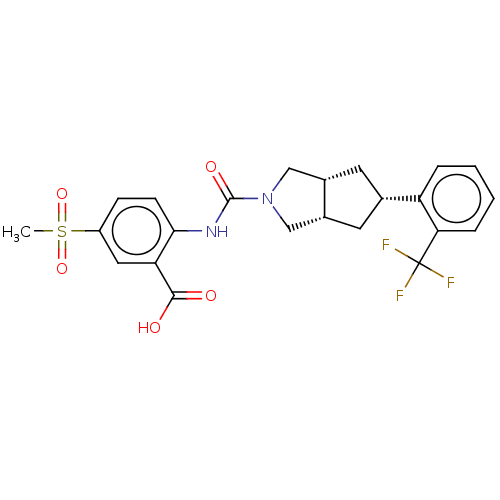
(CHEMBL3359020)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)Nc1ccc(cc1C(O)=O)S(C)(=O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C23H23F3N2O5S/c1-34(32,33)16-6-7-20(18(10-16)21(29)30)27-22(31)28-11-14-8-13(9-15(14)12-28)17-4-2-3-5-19(17)23(24,25)26/h2-7,10,13-15H,8-9,11-12H2,1H3,(H,27,31)(H,29,30)/t13-,14-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026250
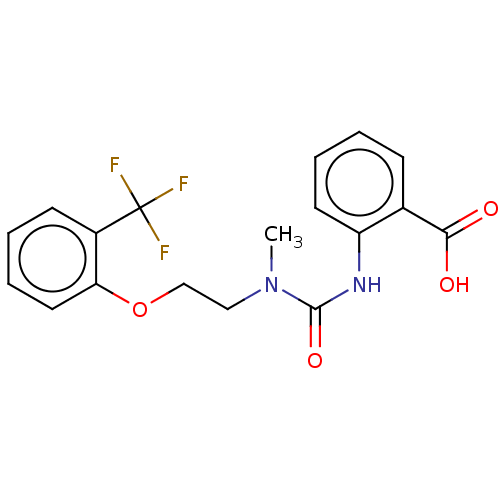
(CHEMBL3358470)Show SMILES CN(CCOc1ccccc1C(F)(F)F)C(=O)Nc1ccccc1C(O)=O Show InChI InChI=1S/C18H17F3N2O4/c1-23(17(26)22-14-8-4-2-6-12(14)16(24)25)10-11-27-15-9-5-3-7-13(15)18(19,20)21/h2-9H,10-11H2,1H3,(H,22,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196941
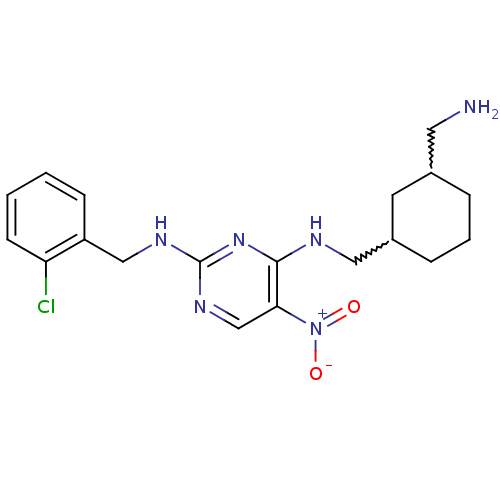
(CHEMBL246365 | N2-(2-chlorobenzyl)-N4-((3-(aminome...)Show SMILES NCC1CCCC(CNc2nc(NCc3ccccc3Cl)ncc2[N+]([O-])=O)C1 |w:6.6,2.1| Show InChI InChI=1S/C19H25ClN6O2/c20-16-7-2-1-6-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-5-3-4-13(8-14)9-21/h1-2,6-7,12-14H,3-5,8-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50275692
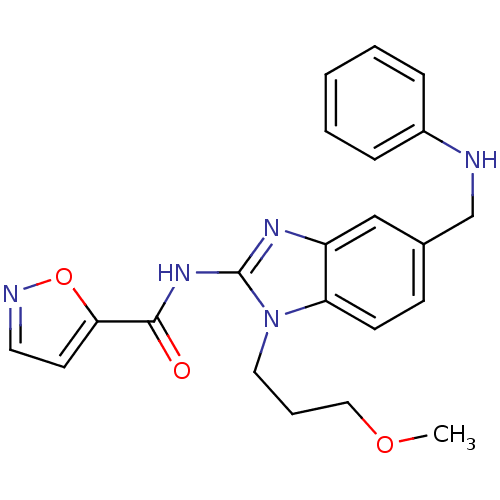
(CHEMBL528989 | N-(1-(3-methoxypropyl)-5-((phenylam...)Show SMILES COCCCn1c(NC(=O)c2ccno2)nc2cc(CNc3ccccc3)ccc12 Show InChI InChI=1S/C22H23N5O3/c1-29-13-5-12-27-19-9-8-16(15-23-17-6-3-2-4-7-17)14-18(19)25-22(27)26-21(28)20-10-11-24-30-20/h2-4,6-11,14,23H,5,12-13,15H2,1H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) by DELPHIA assay |
Bioorg Med Chem Lett 18: 5541-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.016
BindingDB Entry DOI: 10.7270/Q2ZC82PX |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50319986
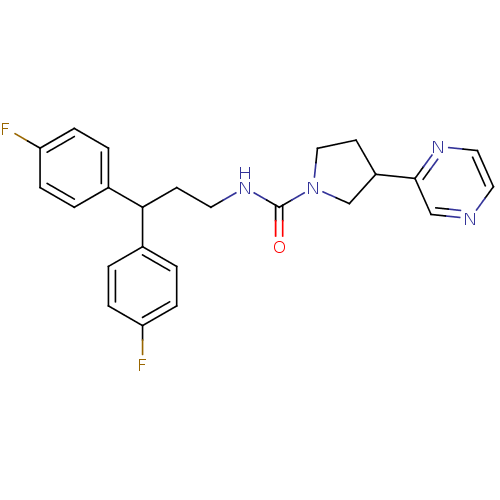
(CHEMBL1085743 | N-(3,3-bis(4-fluorophenyl)propyl)-...)Show SMILES Fc1ccc(cc1)C(CCNC(=O)N1CCC(C1)c1cnccn1)c1ccc(F)cc1 Show InChI InChI=1S/C24H24F2N4O/c25-20-5-1-17(2-6-20)22(18-3-7-21(26)8-4-18)9-11-29-24(31)30-14-10-19(16-30)23-15-27-12-13-28-23/h1-8,12-13,15,19,22H,9-11,14,16H2,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 20: 3703-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.078
BindingDB Entry DOI: 10.7270/Q2BV7GTJ |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026249

(CHEMBL3358466)Show InChI InChI=1S/C14H14F3NO3/c15-14(16,17)11-4-2-1-3-10(11)9-5-7-18(8-6-9)12(19)13(20)21/h1-4,9H,5-8H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50104147
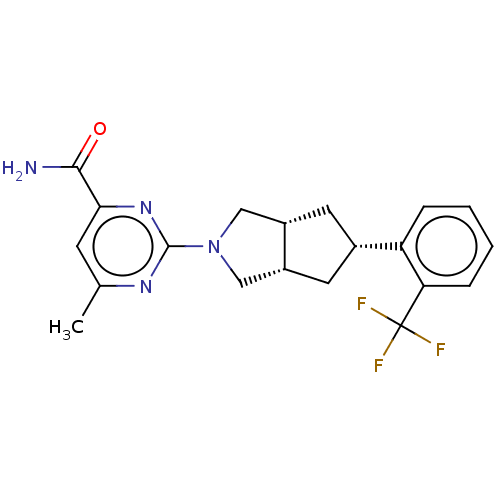
(CHEMBL3593524)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)c1nc(C)cc(n1)C(N)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C20H21F3N4O/c1-11-6-17(18(24)28)26-19(25-11)27-9-13-7-12(8-14(13)10-27)15-4-2-3-5-16(15)20(21,22)23/h2-6,12-14H,7-10H2,1H3,(H2,24,28)/t12-,13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-retinol from RBP4 (unknown origin) by scintillation proximity assay |
J Med Chem 58: 5863-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00423
BindingDB Entry DOI: 10.7270/Q20Z751F |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50104159
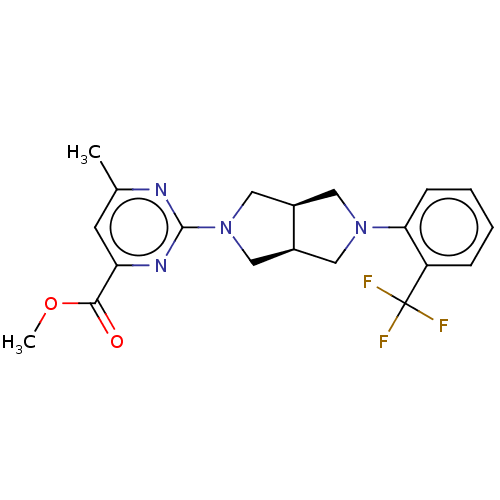
(CHEMBL3594229 | US10787453, Compound 66)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1ccccc1C(F)(F)F)c1nc(C)cc(n1)C(=O)OC |r| Show InChI InChI=1S/C20H21F3N4O2/c1-12-7-16(18(28)29-2)25-19(24-12)27-10-13-8-26(9-14(13)11-27)17-6-4-3-5-15(17)20(21,22)23/h3-7,13-14H,8-11H2,1-2H3/t13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-retinol from RBP4 (unknown origin) by scintillation proximity assay |
J Med Chem 58: 5863-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00423
BindingDB Entry DOI: 10.7270/Q20Z751F |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026248

(CHEMBL3358465)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)Nc1ccccc1C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C26H24F3N3O4S/c27-26(28,29)22-12-6-4-10-20(22)18-14-16-32(17-15-18)25(34)30-23-13-7-5-11-21(23)24(33)31-37(35,36)19-8-2-1-3-9-19/h1-13,18H,14-17H2,(H,30,34)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50319986

(CHEMBL1085743 | N-(3,3-bis(4-fluorophenyl)propyl)-...)Show SMILES Fc1ccc(cc1)C(CCNC(=O)N1CCC(C1)c1cnccn1)c1ccc(F)cc1 Show InChI InChI=1S/C24H24F2N4O/c25-20-5-1-17(2-6-20)22(18-3-7-21(26)8-4-18)9-11-29-24(31)30-14-10-19(16-30)23-15-27-12-13-28-23/h1-8,12-13,15,19,22H,9-11,14,16H2,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assay |
Bioorg Med Chem Lett 20: 3703-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.078
BindingDB Entry DOI: 10.7270/Q2BV7GTJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50308898

(3-[3-(4-Amido-phenyl)-ureidomethyl]-N-(1,2,3,4-tet...)Show SMILES NC(=O)c1ccc(NC(=O)NCc2cccc(c2)C(=O)Nc2ccc3CCNCc3c2)cc1 Show InChI InChI=1S/C25H25N5O3/c26-23(31)18-5-7-21(8-6-18)30-25(33)28-14-16-2-1-3-19(12-16)24(32)29-22-9-4-17-10-11-27-15-20(17)13-22/h1-9,12-13,27H,10-11,14-15H2,(H2,26,31)(H,29,32)(H2,28,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ROCK2 by IMAP assay |
J Med Chem 53: 759-77 (2010)
Article DOI: 10.1021/jm9014263
BindingDB Entry DOI: 10.7270/Q2V125RD |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50319984
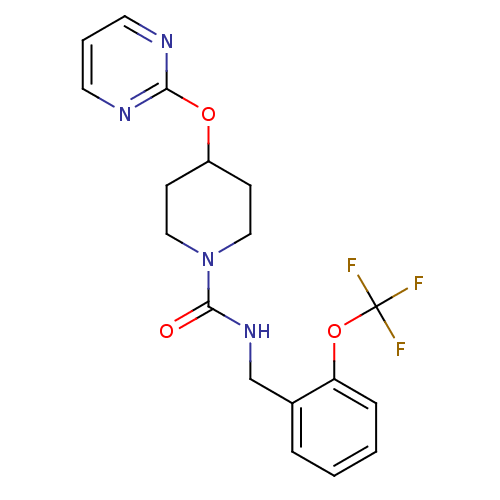
(4-(pyrimidin-2-yloxy)-N-(2-(trifluoromethoxy)benzy...)Show SMILES FC(F)(F)Oc1ccccc1CNC(=O)N1CCC(CC1)Oc1ncccn1 Show InChI InChI=1S/C18H19F3N4O3/c19-18(20,21)28-15-5-2-1-4-13(15)12-24-17(26)25-10-6-14(7-11-25)27-16-22-8-3-9-23-16/h1-5,8-9,14H,6-7,10-12H2,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 20: 3703-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.078
BindingDB Entry DOI: 10.7270/Q2BV7GTJ |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50104179
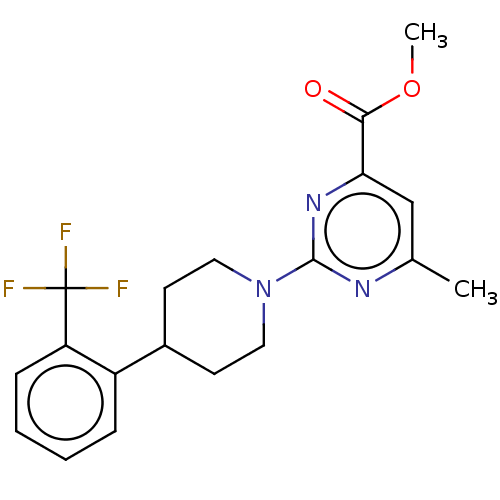
(CHEMBL3594225)Show SMILES COC(=O)c1cc(C)nc(n1)N1CCC(CC1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C19H20F3N3O2/c1-12-11-16(17(26)27-2)24-18(23-12)25-9-7-13(8-10-25)14-5-3-4-6-15(14)19(20,21)22/h3-6,11,13H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-retinol from RBP4 (unknown origin) by scintillation proximity assay |
J Med Chem 58: 5863-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00423
BindingDB Entry DOI: 10.7270/Q20Z751F |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196957
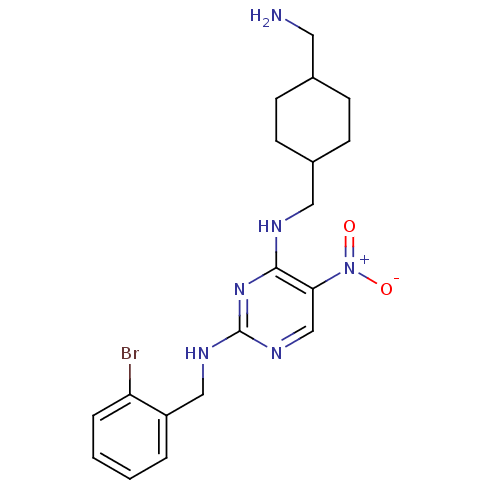
(CHEMBL246175 | N2-(2-bromobenzyl)-N4-((4-(aminomet...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3Br)ncc2[N+]([O-])=O)CC1 |(5.93,-24.27,;5.93,-22.73,;4.6,-21.95,;3.26,-22.72,;1.93,-21.95,;1.94,-20.42,;.61,-19.65,;.6,-18.11,;-.73,-17.34,;-2.07,-18.11,;-3.4,-17.34,;-4.74,-18.11,;-6.07,-17.34,;-7.41,-18.11,;-8.74,-17.33,;-10.07,-18.1,;-10.07,-19.64,;-8.73,-20.41,;-7.4,-19.64,;-6.07,-20.41,;-3.4,-15.8,;-2.07,-15.03,;-.74,-15.79,;.6,-15.01,;1.93,-15.78,;.59,-13.47,;3.26,-19.63,;4.6,-20.41,)| Show InChI InChI=1S/C19H25BrN6O2/c20-16-4-2-1-3-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-7-5-13(9-21)6-8-14/h1-4,12-14H,5-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50121562
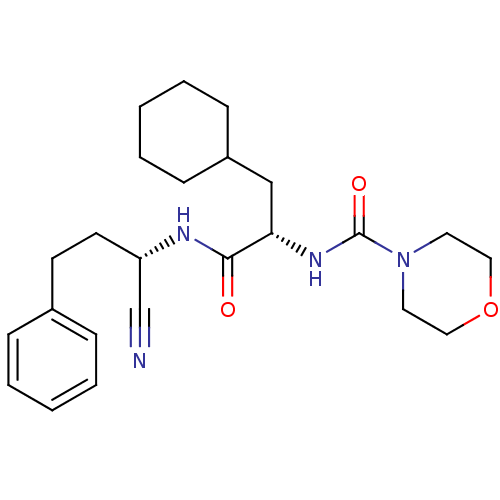
(CHEMBL153239 | Morpholine-4-carboxylic acid [1-(1-...)Show SMILES O=C(N[C@@H](CCc1ccccc1)C#N)[C@H](CC1CCCCC1)NC(=O)N1CCOCC1 Show InChI InChI=1S/C24H34N4O3/c25-18-21(12-11-19-7-3-1-4-8-19)26-23(29)22(17-20-9-5-2-6-10-20)27-24(30)28-13-15-31-16-14-28/h1,3-4,7-8,20-22H,2,5-6,9-17H2,(H,26,29)(H,27,30)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus |
J Med Chem 45: 5471-82 (2002)
BindingDB Entry DOI: 10.7270/Q2P26XG7 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50121542
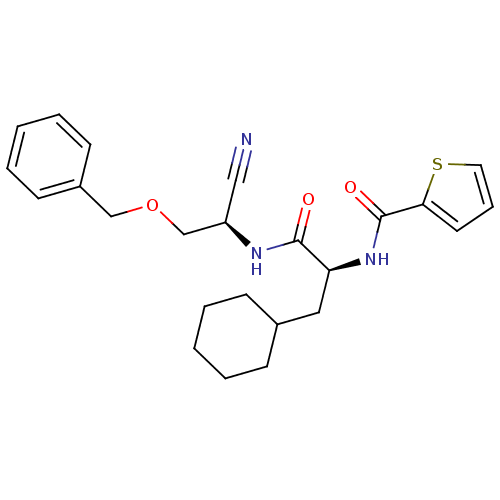
(CHEMBL155560 | Thiophene-2-carboxylic acid {1-[(be...)Show SMILES O=C(N[C@@H](COCc1ccccc1)C#N)[C@H](CC1CCCCC1)NC(=O)c1cccs1 Show InChI InChI=1S/C24H29N3O3S/c25-15-20(17-30-16-19-10-5-2-6-11-19)26-23(28)21(14-18-8-3-1-4-9-18)27-24(29)22-12-7-13-31-22/h2,5-7,10-13,18,20-21H,1,3-4,8-9,14,16-17H2,(H,26,28)(H,27,29)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus |
J Med Chem 45: 5471-82 (2002)
BindingDB Entry DOI: 10.7270/Q2P26XG7 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196932

(CHEMBL392642 | N2-(2-(methylthio)benzyl)-N4-((4-(a...)Show SMILES CSc1ccccc1CNc1ncc(c(NCC2CCC(CN)CC2)n1)[N+]([O-])=O |(25.65,-13.62,;25.64,-12.08,;24.31,-11.32,;22.98,-12.09,;21.64,-11.32,;21.64,-9.78,;22.97,-9.01,;24.3,-9.79,;25.64,-9.02,;26.97,-9.79,;28.31,-9.02,;28.31,-7.47,;29.64,-6.7,;30.97,-7.47,;30.98,-9.02,;32.31,-9.79,;32.32,-11.33,;33.65,-12.09,;33.64,-13.63,;34.97,-14.4,;36.31,-13.63,;37.64,-14.4,;37.64,-15.94,;36.31,-12.09,;34.97,-11.31,;29.64,-9.79,;32.31,-6.69,;33.64,-7.45,;32.3,-5.15,)| Show InChI InChI=1S/C20H28N6O2S/c1-29-18-5-3-2-4-16(18)12-23-20-24-13-17(26(27)28)19(25-20)22-11-15-8-6-14(10-21)7-9-15/h2-5,13-15H,6-12,21H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026247
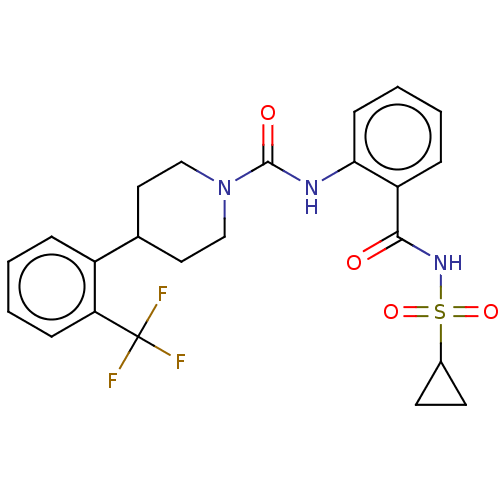
(CHEMBL3358464)Show SMILES FC(F)(F)c1ccccc1C1CCN(CC1)C(=O)Nc1ccccc1C(=O)NS(=O)(=O)C1CC1 Show InChI InChI=1S/C23H24F3N3O4S/c24-23(25,26)19-7-3-1-5-17(19)15-11-13-29(14-12-15)22(31)27-20-8-4-2-6-18(20)21(30)28-34(32,33)16-9-10-16/h1-8,15-16H,9-14H2,(H,27,31)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026242
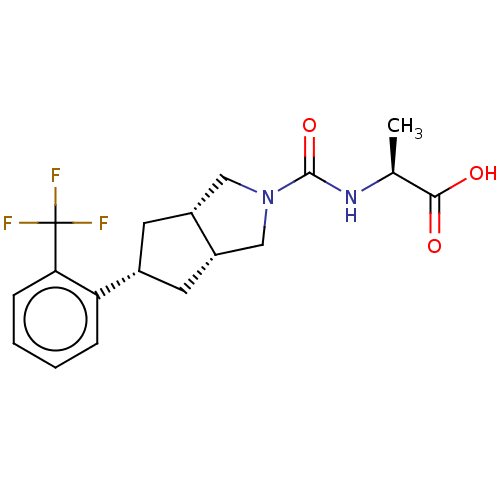
(CHEMBL3359026)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)N[C@@H](C)C(O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C18H21F3N2O3/c1-10(16(24)25)22-17(26)23-8-12-6-11(7-13(12)9-23)14-4-2-3-5-15(14)18(19,20)21/h2-5,10-13H,6-9H2,1H3,(H,22,26)(H,24,25)/t10-,11-,12-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50121554
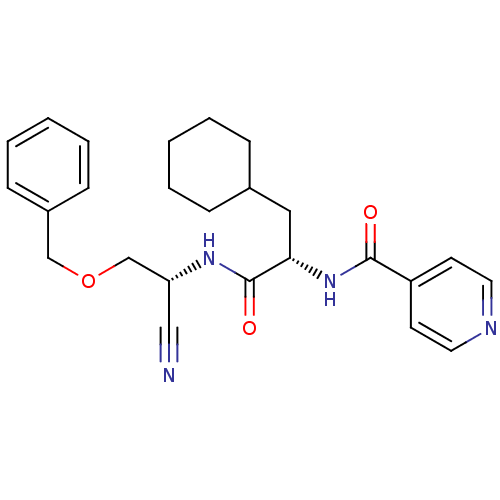
(CHEMBL356155 | N-{1-[(Benzyloxymethyl-cyano-methyl...)Show SMILES O=C(N[C@@H](COCc1ccccc1)C#N)[C@H](CC1CCCCC1)NC(=O)c1ccncc1 Show InChI InChI=1S/C25H30N4O3/c26-16-22(18-32-17-20-9-5-2-6-10-20)28-25(31)23(15-19-7-3-1-4-8-19)29-24(30)21-11-13-27-14-12-21/h2,5-6,9-14,19,22-23H,1,3-4,7-8,15,17-18H2,(H,28,31)(H,29,30)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus |
J Med Chem 45: 5471-82 (2002)
BindingDB Entry DOI: 10.7270/Q2P26XG7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50275651
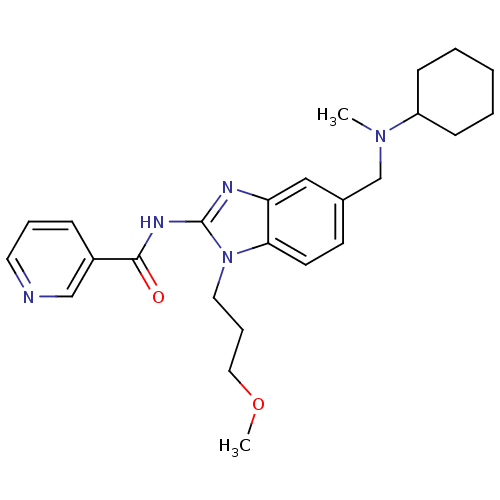
(CHEMBL487357 | N-(5-((cyclohexyl(methyl)amino)meth...)Show SMILES COCCCn1c(NC(=O)c2cccnc2)nc2cc(CN(C)C3CCCCC3)ccc12 Show InChI InChI=1S/C25H33N5O2/c1-29(21-9-4-3-5-10-21)18-19-11-12-23-22(16-19)27-25(30(23)14-7-15-32-2)28-24(31)20-8-6-13-26-17-20/h6,8,11-13,16-17,21H,3-5,7,9-10,14-15,18H2,1-2H3,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) by DELPHIA assay |
Bioorg Med Chem Lett 18: 5541-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.016
BindingDB Entry DOI: 10.7270/Q2ZC82PX |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196961
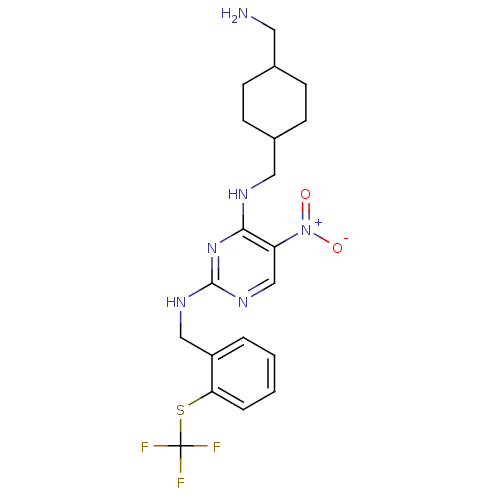
(CHEMBL246174 | N2-(2-(trifluoromethylthio)benzyl)-...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3SC(F)(F)F)ncc2[N+]([O-])=O)CC1 |(21.67,-14.36,;21.67,-12.82,;20.34,-12.05,;19,-12.82,;17.68,-12.05,;17.68,-10.51,;16.35,-9.75,;16.35,-8.21,;15.01,-7.44,;13.67,-8.21,;12.34,-7.44,;11,-8.21,;9.67,-7.44,;8.34,-8.21,;7.01,-7.43,;5.67,-8.2,;5.67,-9.74,;7.01,-10.51,;8.34,-9.74,;9.68,-10.51,;9.68,-12.05,;9.67,-13.58,;11.22,-12.05,;8.14,-12.05,;12.34,-5.9,;13.67,-5.12,;15,-5.89,;16.34,-5.11,;17.68,-5.88,;16.33,-3.57,;19.01,-9.73,;20.34,-10.51,)| Show InChI InChI=1S/C20H25F3N6O2S/c21-20(22,23)32-17-4-2-1-3-15(17)11-26-19-27-12-16(29(30)31)18(28-19)25-10-14-7-5-13(9-24)6-8-14/h1-4,12-14H,5-11,24H2,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50121549
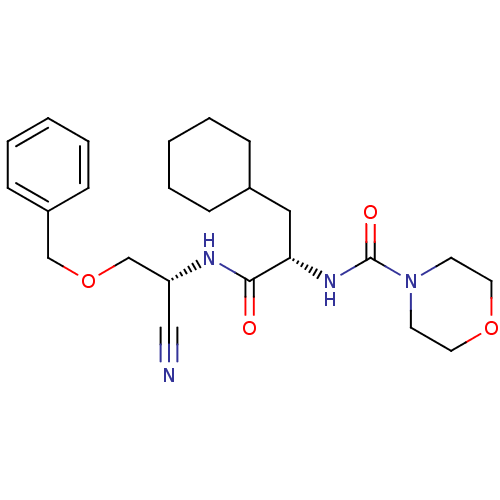
(CHEMBL347111 | Morpholine-4-carboxylic acid {1-[(b...)Show SMILES O=C(N[C@@H](COCc1ccccc1)C#N)[C@H](CC1CCCCC1)NC(=O)N1CCOCC1 Show InChI InChI=1S/C24H34N4O4/c25-16-21(18-32-17-20-9-5-2-6-10-20)26-23(29)22(15-19-7-3-1-4-8-19)27-24(30)28-11-13-31-14-12-28/h2,5-6,9-10,19,21-22H,1,3-4,7-8,11-15,17-18H2,(H,26,29)(H,27,30)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus |
J Med Chem 45: 5471-82 (2002)
BindingDB Entry DOI: 10.7270/Q2P26XG7 |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50104157
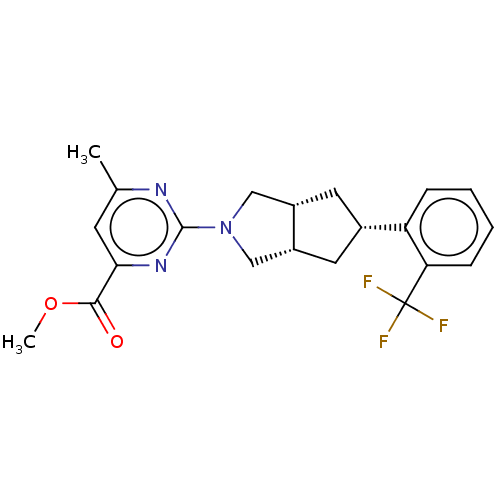
(CHEMBL3594231)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)c1nc(C)cc(n1)C(=O)OC)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N3O2/c1-12-7-18(19(28)29-2)26-20(25-12)27-10-14-8-13(9-15(14)11-27)16-5-3-4-6-17(16)21(22,23)24/h3-7,13-15H,8-11H2,1-2H3/t13-,14-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-retinol from RBP4 (unknown origin) by scintillation proximity assay |
J Med Chem 58: 5863-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00423
BindingDB Entry DOI: 10.7270/Q20Z751F |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026246
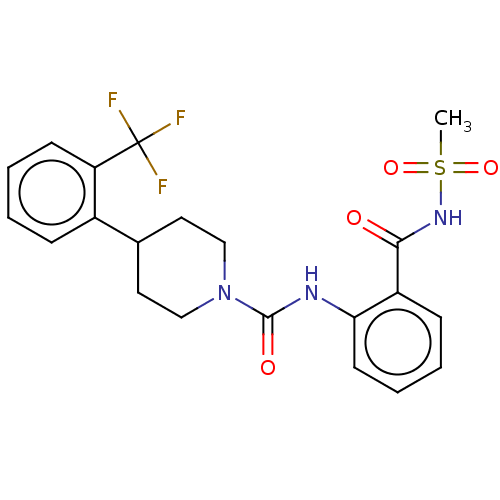
(CHEMBL3358463)Show SMILES CS(=O)(=O)NC(=O)c1ccccc1NC(=O)N1CCC(CC1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C21H22F3N3O4S/c1-32(30,31)26-19(28)16-7-3-5-9-18(16)25-20(29)27-12-10-14(11-13-27)15-6-2-4-8-17(15)21(22,23)24/h2-9,14H,10-13H2,1H3,(H,25,29)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50026241
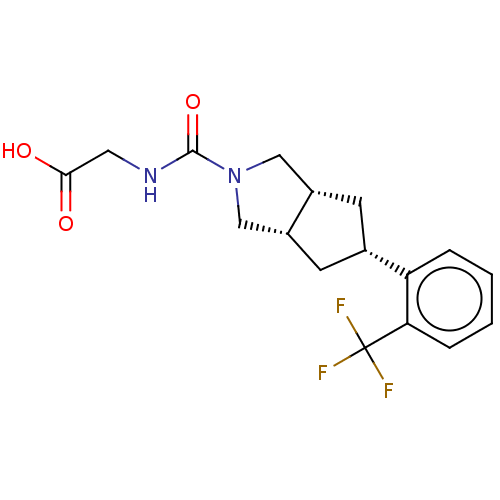
(CHEMBL3359025)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)C(=O)NCC(O)=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C17H19F3N2O3/c18-17(19,20)14-4-2-1-3-13(14)10-5-11-8-22(9-12(11)6-10)16(25)21-7-15(23)24/h1-4,10-12H,5-9H2,(H,21,25)(H,23,24)/t10-,11-,12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Antagonist activity at maltose binding protein-tagged RBP4 (unknown origin) expressed in Escherichia coli assessed as inhibition of retinol-induced p... |
J Med Chem 57: 7731-57 (2014)
Article DOI: 10.1021/jm5010013
BindingDB Entry DOI: 10.7270/Q2FB54JT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057000
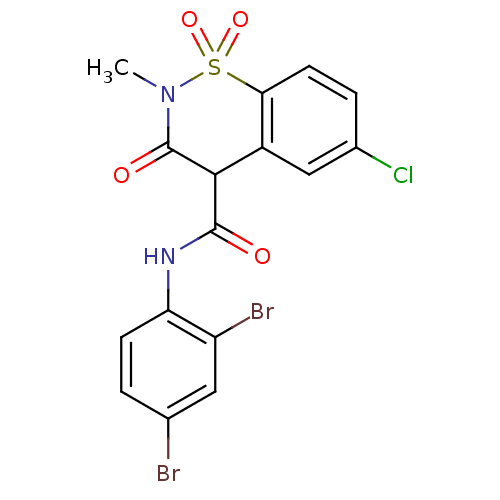
(6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...)Show SMILES CN1C(=O)C(C(=O)Nc2ccc(Br)cc2Br)c2cc(Cl)ccc2S1(=O)=O Show InChI InChI=1S/C16H11Br2ClN2O4S/c1-21-16(23)14(10-7-9(19)3-5-13(10)26(21,24)25)15(22)20-12-4-2-8(17)6-11(12)18/h2-7,14H,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... |
J Med Chem 40: 980-9 (1997)
Article DOI: 10.1021/jm9607010
BindingDB Entry DOI: 10.7270/Q2WH2P37 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196936
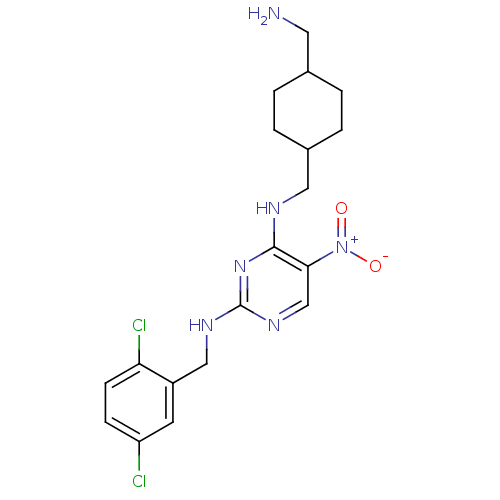
(CHEMBL246386 | N2-(2,5-dichlorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3cc(Cl)ccc3Cl)ncc2[N+]([O-])=O)CC1 |(6.39,-4.5,;6.4,-2.96,;5.07,-2.19,;3.73,-2.95,;2.4,-2.19,;2.41,-.65,;1.07,.12,;1.07,1.66,;-.27,2.42,;-1.6,1.65,;-2.94,2.42,;-4.27,1.65,;-5.6,2.42,;-6.94,1.66,;-8.27,2.43,;-9.6,1.66,;-10.94,2.43,;-9.61,.12,;-8.26,-.65,;-6.94,.12,;-5.6,-.64,;-2.93,3.97,;-1.61,4.74,;-.27,3.97,;1.06,4.75,;2.4,3.99,;1.06,6.29,;3.73,.13,;5.07,-.65,)| Show InChI InChI=1S/C19H24Cl2N6O2/c20-15-5-6-16(21)14(7-15)10-24-19-25-11-17(27(28)29)18(26-19)23-9-13-3-1-12(8-22)2-4-13/h5-7,11-13H,1-4,8-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50121575
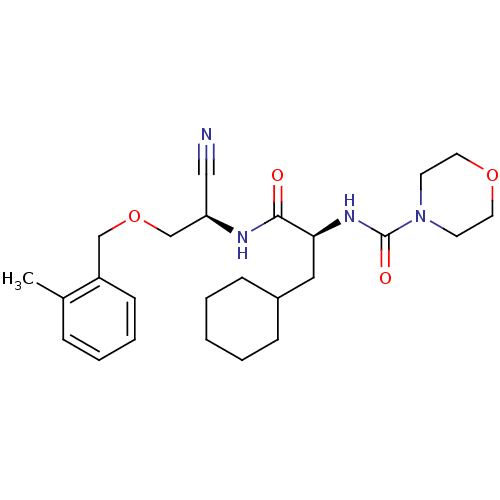
(CHEMBL356442 | Morpholine-4-carboxylic acid (1-{[c...)Show SMILES Cc1ccccc1COC[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)N1CCOCC1)C#N Show InChI InChI=1S/C25H36N4O4/c1-19-7-5-6-10-21(19)17-33-18-22(16-26)27-24(30)23(15-20-8-3-2-4-9-20)28-25(31)29-11-13-32-14-12-29/h5-7,10,20,22-23H,2-4,8-9,11-15,17-18H2,1H3,(H,27,30)(H,28,31)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus |
J Med Chem 45: 5471-82 (2002)
BindingDB Entry DOI: 10.7270/Q2P26XG7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50319983
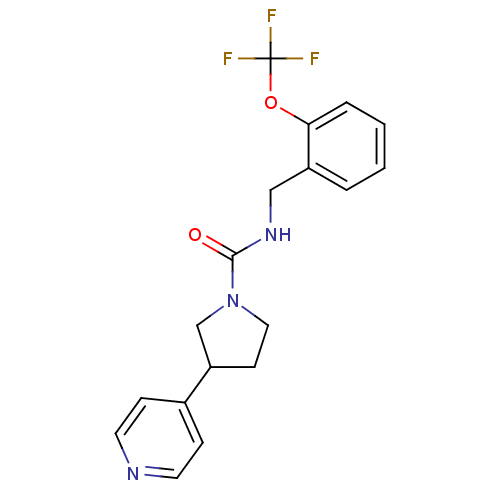
(3-(pyridin-4-yl)-N-(2-(trifluoromethoxy)benzyl)pyr...)Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)26-16-4-2-1-3-14(16)11-23-17(25)24-10-7-15(12-24)13-5-8-22-9-6-13/h1-6,8-9,15H,7,10-12H2,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 20: 3703-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.078
BindingDB Entry DOI: 10.7270/Q2BV7GTJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50325446
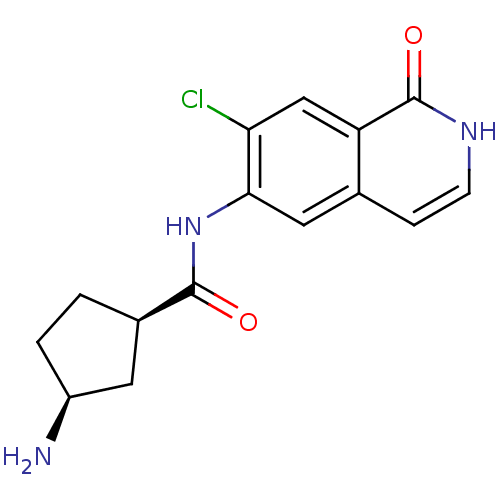
(CHEMBL1222573 | cis-3-amino-N-(7-chloro-1-oxo-1,2-...)Show SMILES N[C@H]1CC[C@H](C1)C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl |r| Show InChI InChI=1S/C15H16ClN3O2/c16-12-7-11-8(3-4-18-15(11)21)6-13(12)19-14(20)9-1-2-10(17)5-9/h3-4,6-7,9-10H,1-2,5,17H2,(H,18,21)(H,19,20)/t9-,10+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 5153-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.014
BindingDB Entry DOI: 10.7270/Q2RR1ZDC |
More data for this
Ligand-Target Pair | |
Retinol-binding protein 4
(Homo sapiens (Human)) | BDBM50104146
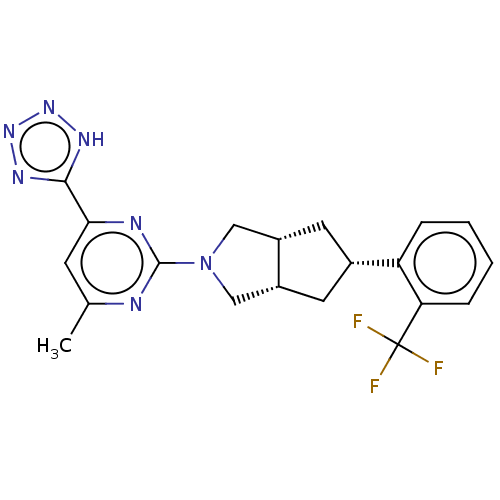
(CHEMBL3593525)Show SMILES [H][C@@]12C[C@@H](C[C@]1([H])CN(C2)c1nc(C)cc(n1)-c1nnn[nH]1)c1ccccc1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-retinol from RBP4 (unknown origin) by scintillation proximity assay |
J Med Chem 58: 5863-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00423
BindingDB Entry DOI: 10.7270/Q20Z751F |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50121569

(CHEMBL153783 | Morpholine-4-carboxylic acid {1-[(b...)Show SMILES CC(C)(C)C[C@H](NC(=O)N1CCOCC1)C(=O)N[C@@H](COCc1ccccc1)C#N Show InChI InChI=1S/C22H32N4O4/c1-22(2,3)13-19(25-21(28)26-9-11-29-12-10-26)20(27)24-18(14-23)16-30-15-17-7-5-4-6-8-17/h4-8,18-19H,9-13,15-16H2,1-3H3,(H,24,27)(H,25,28)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus |
J Med Chem 45: 5471-82 (2002)
BindingDB Entry DOI: 10.7270/Q2P26XG7 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50121581

(CHEMBL150253 | Morpholine-4-carboxylic acid (1-{[(...)Show SMILES Clc1ccccc1COC[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)N1CCOCC1)C#N Show InChI InChI=1S/C24H33ClN4O4/c25-21-9-5-4-8-19(21)16-33-17-20(15-26)27-23(30)22(14-18-6-2-1-3-7-18)28-24(31)29-10-12-32-13-11-29/h4-5,8-9,18,20,22H,1-3,6-7,10-14,16-17H2,(H,27,30)(H,28,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus |
J Med Chem 45: 5471-82 (2002)
BindingDB Entry DOI: 10.7270/Q2P26XG7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057004
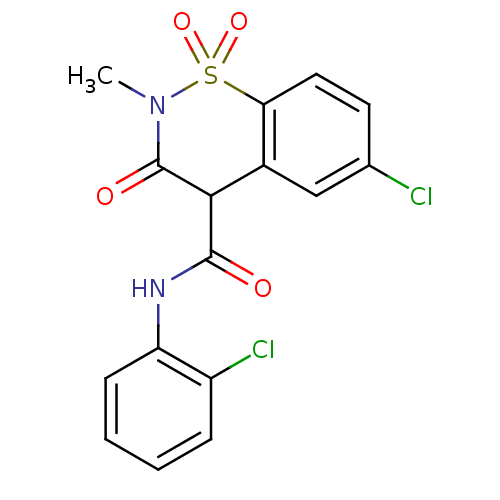
(6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...)Show SMILES CN1C(=O)C(C(=O)Nc2ccccc2Cl)c2cc(Cl)ccc2S1(=O)=O Show InChI InChI=1S/C16H12Cl2N2O4S/c1-20-16(22)14(15(21)19-12-5-3-2-4-11(12)18)10-8-9(17)6-7-13(10)25(20,23)24/h2-8,14H,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... |
J Med Chem 40: 980-9 (1997)
Article DOI: 10.1021/jm9607010
BindingDB Entry DOI: 10.7270/Q2WH2P37 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data