

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13925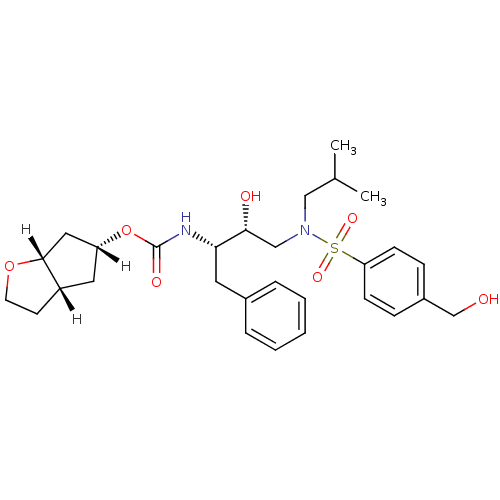 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM4685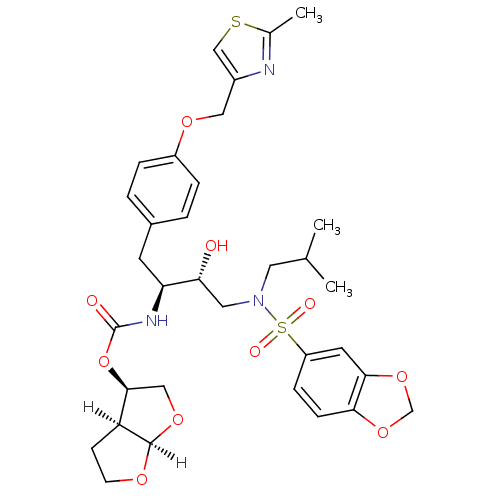 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM9236 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50476647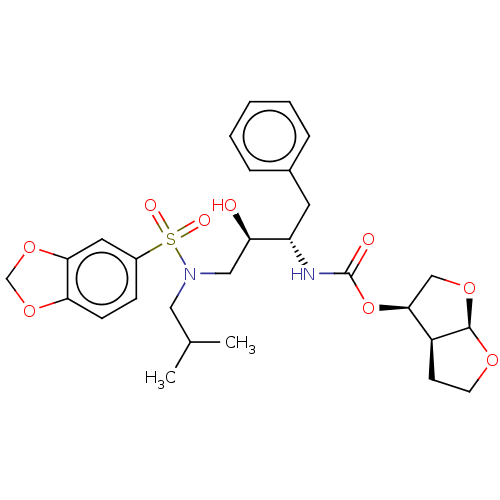 (CHEMBL178593 | GRL-98065) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370083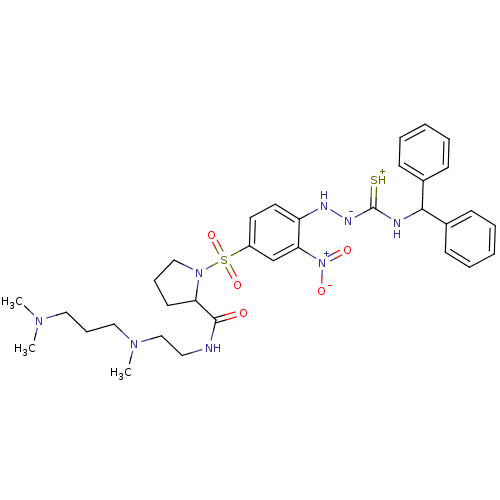 (CHEMBL1907651) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478337 (CHEMBL403306 | GRL-0036A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000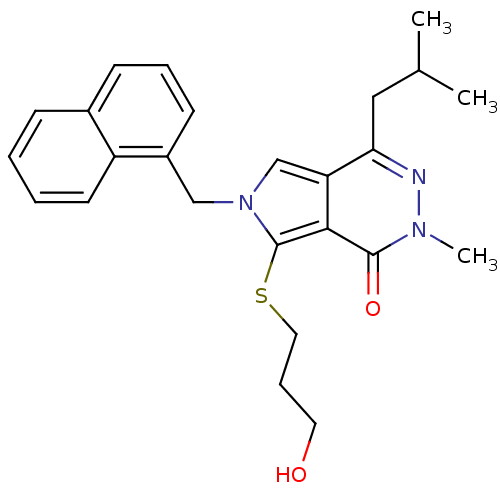 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001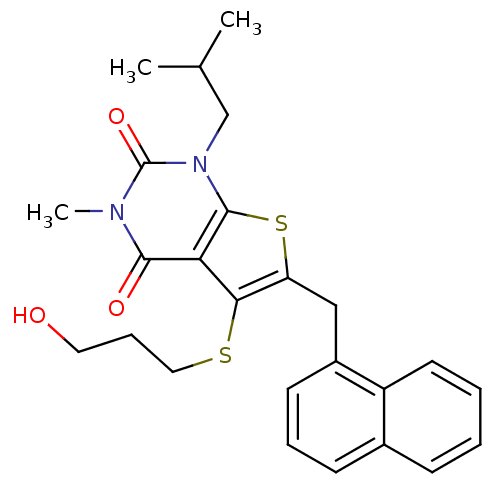 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22009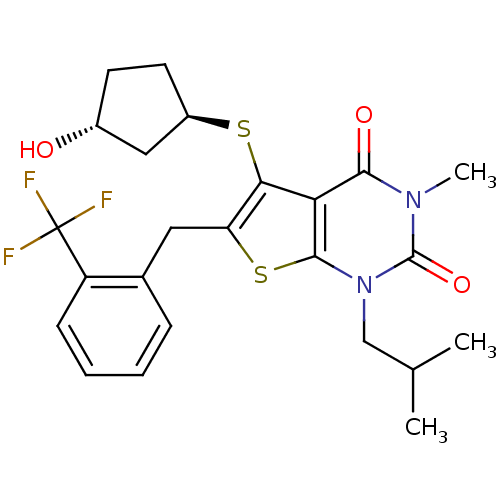 (5-{[(1R,3R)-3-hydroxycyclopentyl]sulfanyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -53.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370077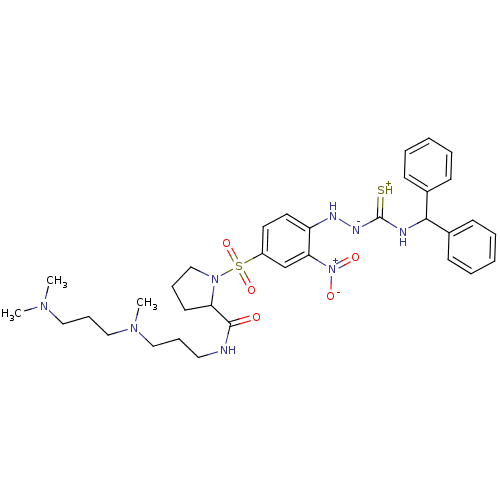 (CHEMBL1907652) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986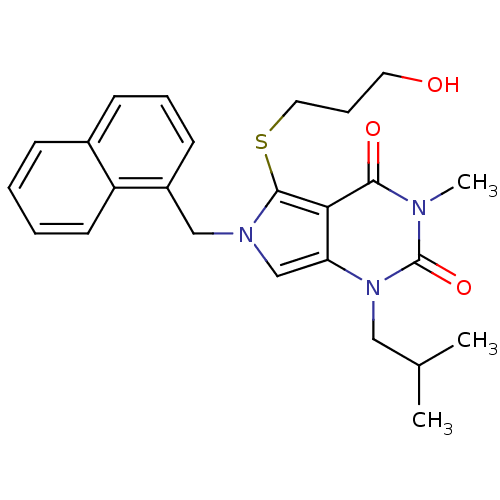 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002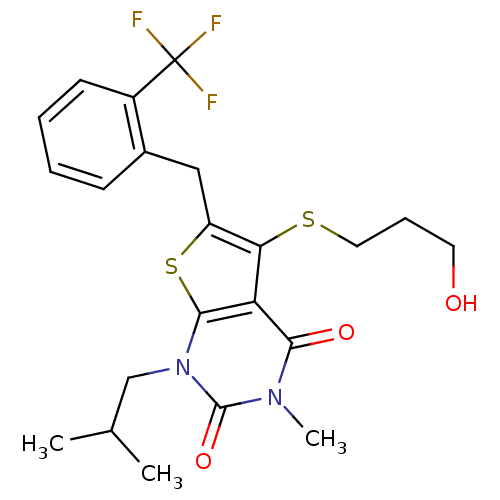 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22010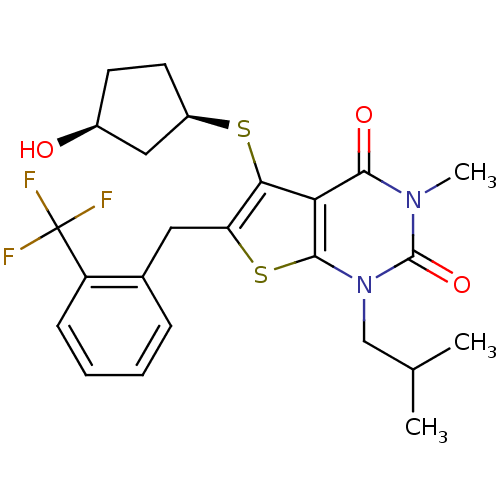 (5-{[(1R,3S)-3-hydroxycyclopentyl]sulfanyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50005463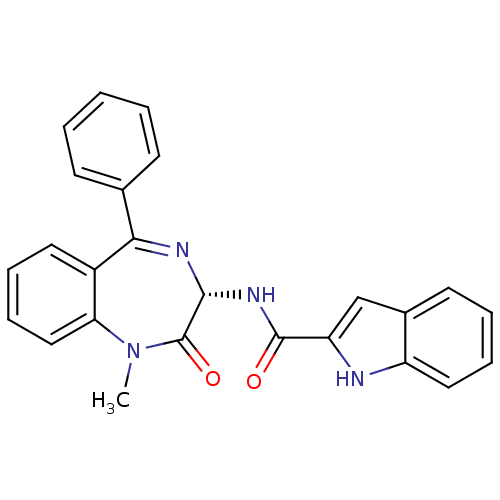 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82403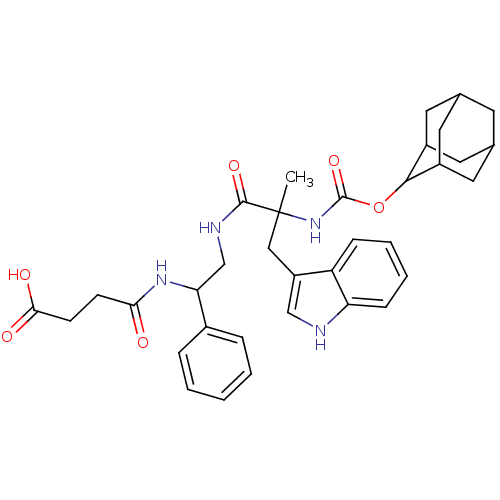 (CAS_108186 | CI-988 | NSC_108186) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82404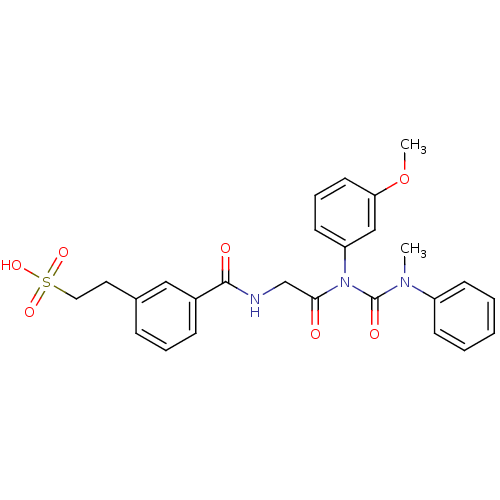 (Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263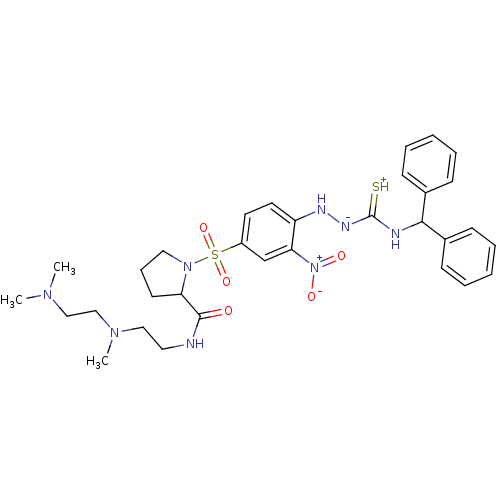 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409120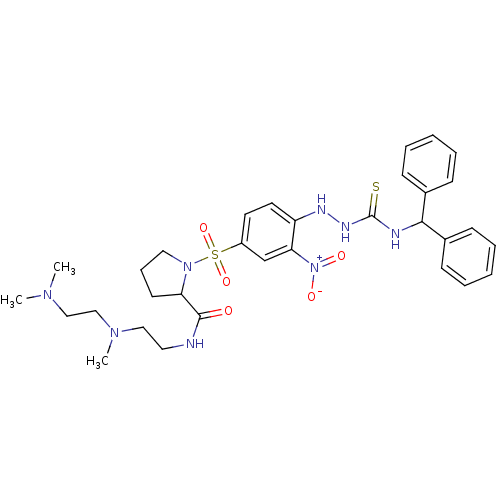 (CHEMBL2112044 | CHEMBL2112937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409120 (CHEMBL2112044 | CHEMBL2112937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22011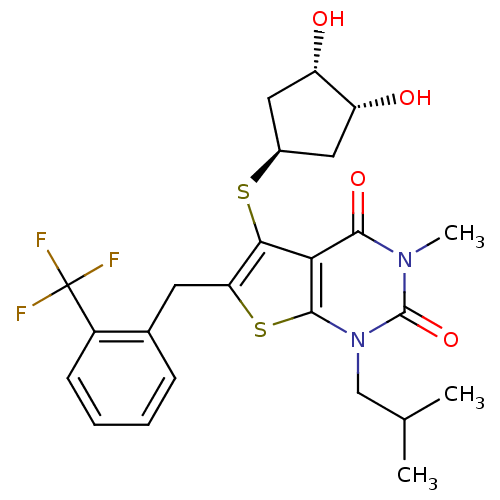 (5-{[(1S,3R,4S)-3,4-dihydroxycyclopentyl]sulfanyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.680 | -51.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347563 (CHEMBL1801740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human Angiotensin receptor 1 | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22025 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.790 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409527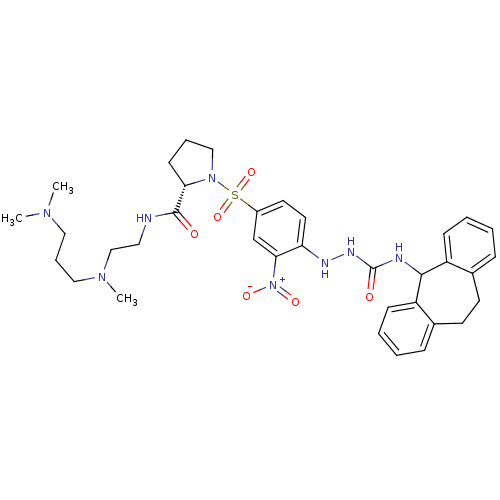 (CHEMBL2112283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82404 (Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50251493 ((2S,4S)-1-(2-(1-(4-cyano-3,5-difluorophenyl)piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma DPP4 | Bioorg Med Chem Lett 18: 4087-91 (2008) Article DOI: 10.1016/j.bmcl.2008.05.101 BindingDB Entry DOI: 10.7270/Q2T153FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22014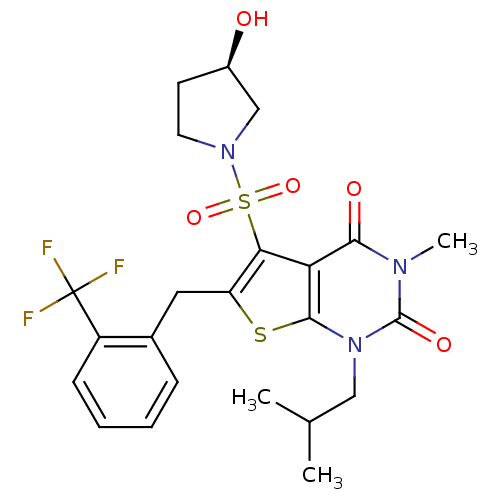 (5-{[(3R)-3-hydroxypyrrolidine-1-]sulfonyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409529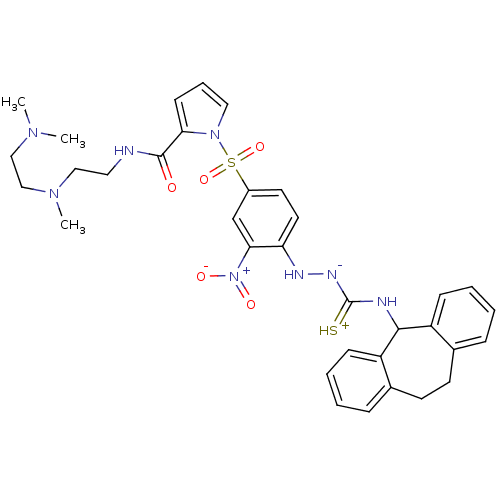 (CHEMBL2112221) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50085685 (4-benzhydrylamino(thioxo)methylhydrazine-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50558239 (CHEMBL4781228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-GR113808 from guinea pig brain 5HT4 receptor in presence of serotonin incubated for 30 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.048 BindingDB Entry DOI: 10.7270/Q2VX0M78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82403 (CAS_108186 | CI-988 | NSC_108186) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22004 (3-methyl-1-(2-methylpropyl)-5-(propan-2-ylsulfanyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22008 (5-{[(1R,2R)-2-hydroxycyclopentyl]sulfanyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409528 (CHEMBL2112220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM28583 (5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in BHK cell membrane measured after 60 mins by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113059 BindingDB Entry DOI: 10.7270/Q2KP85V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22006 (5-(cyclopentylsulfanyl)-3-methyl-1-(2-methylpropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507212 (CHEMBL4585990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM82241 (CAS_5534-95-2 | NSC_444007 | Pentagastrin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22020 (5-[(3-hydroxy-3-methylazetidin-1-yl)carbonyl]-3-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50558245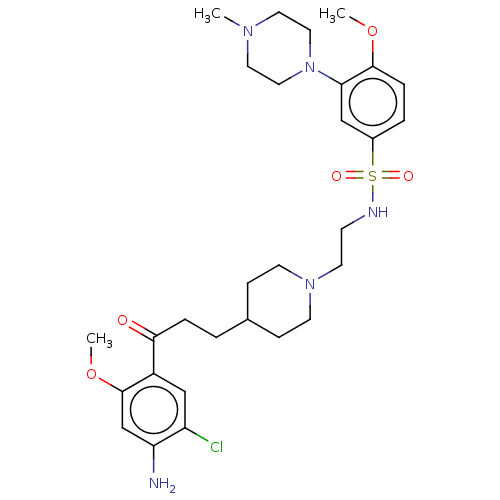 (CHEMBL4789661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-GR113808 from guinea pig brain 5HT4 receptor in presence of serotonin incubated for 30 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2016.05.048 BindingDB Entry DOI: 10.7270/Q2VX0M78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22024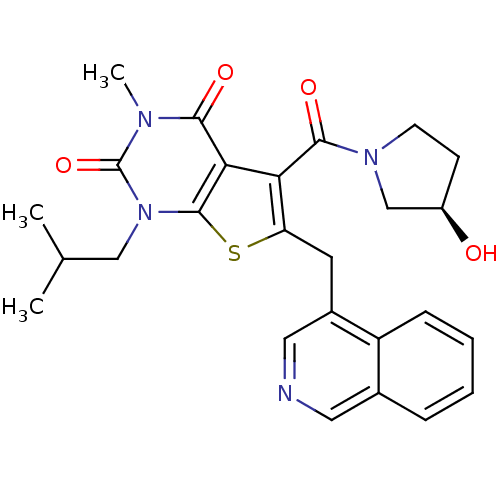 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(iso...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50085684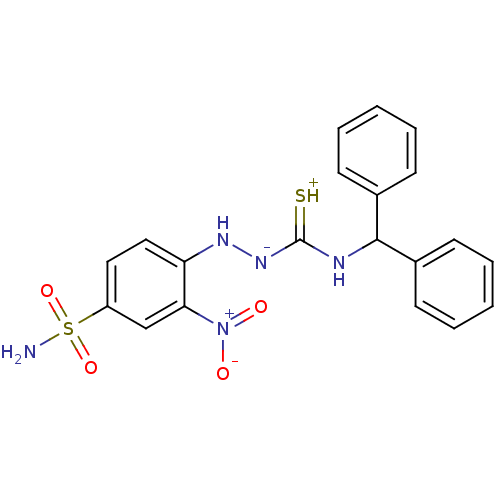 (4-benzhydrylamino(thioxo)methylhydrazine-3-nitro-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562651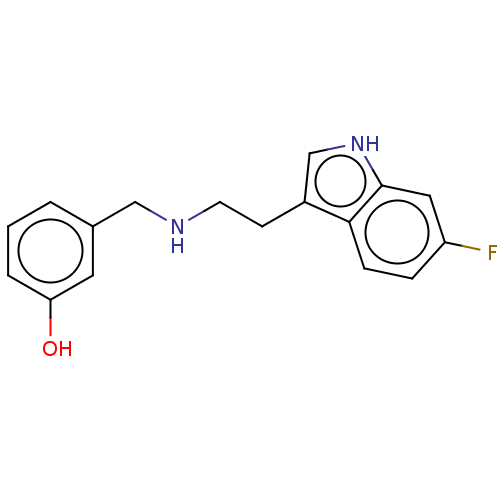 (CHEMBL4788250) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membrane measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113059 BindingDB Entry DOI: 10.7270/Q2KP85V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50370080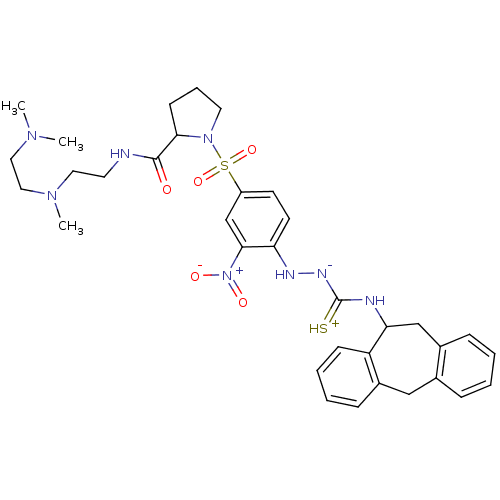 (CHEMBL1907656) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22026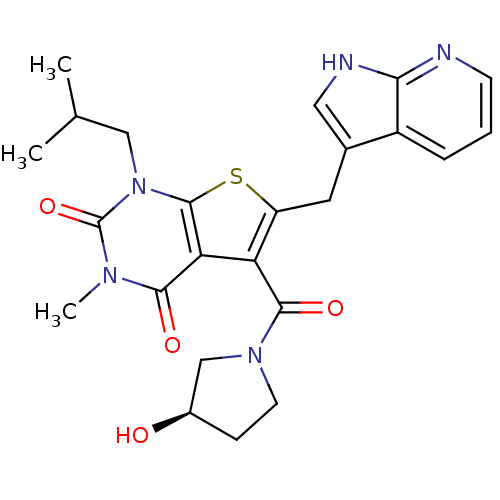 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (GUINEA PIG) | BDBM50061220 (1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 273: 1015-22 (1995) BindingDB Entry DOI: 10.7270/Q2QV3K1C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1953 total ) | Next | Last >> |