

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15131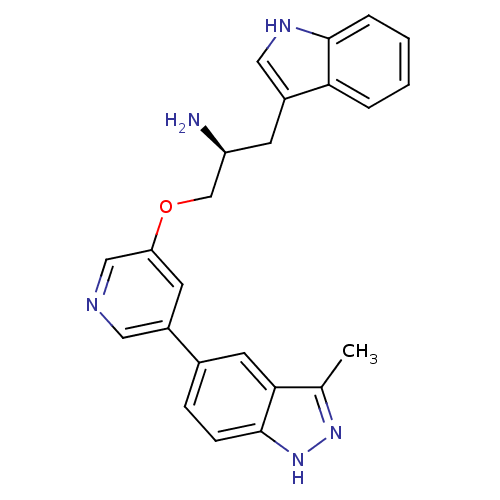 (5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15136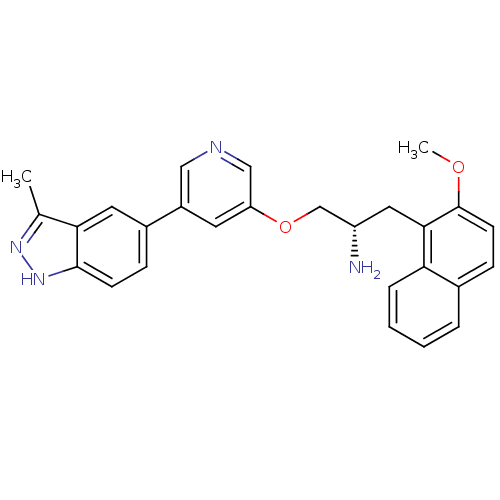 (5-indazolyl pyridine 11e | 5-{5-[(2S)-2-amino-3-(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15067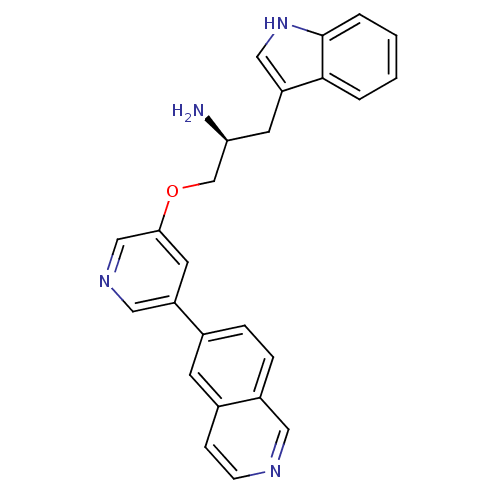 ((2S)-1-(1H-indol-3-yl)-3-(5-isoquinolin-6-ylpyridi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15132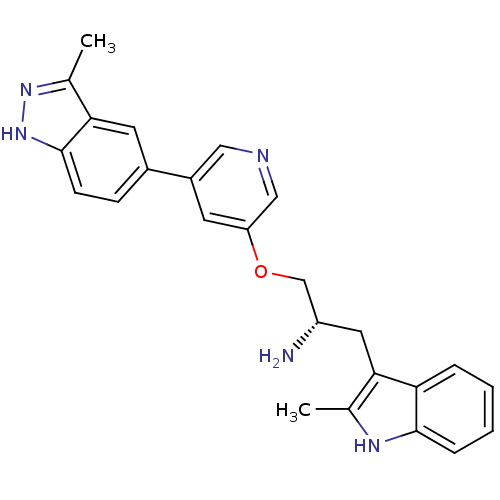 (5-indazolyl pyridine 11a | 5-{5-[(2S)-2-amino-3-(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15134 (5-indazolyl pyridine 11c | 5-{5-[(2S)-2-amino-3-(n...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15125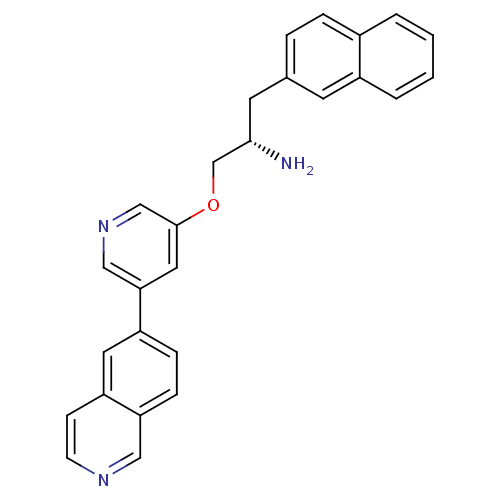 (5-isoquinolinyl pyridine 10d | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15133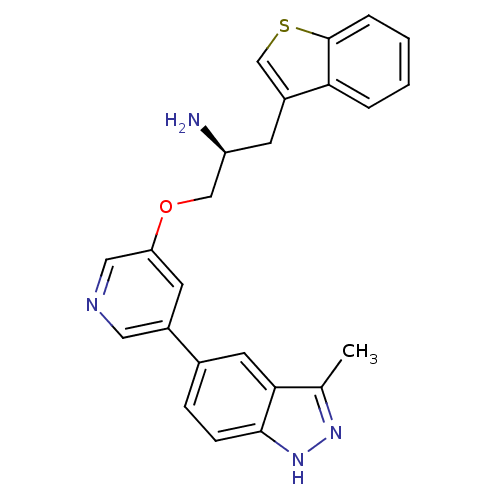 (5-indazolyl pyridine 11b | 5-{5-[(2S)-2-amino-3-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15123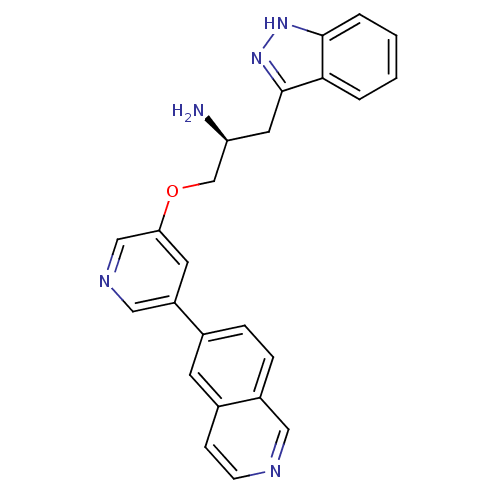 (5-isoquinolinyl pyridine 10b | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15145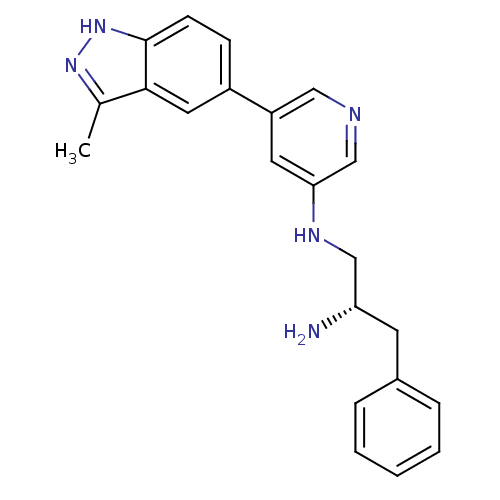 (5-indazolyl pyridine 16 | N-[(2S)-2-amino-3-phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.30 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15135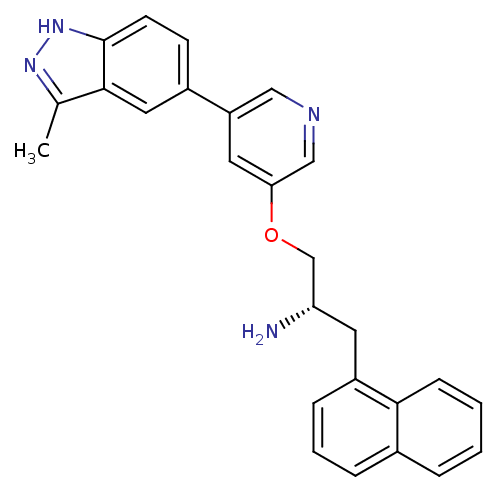 (5-indazolyl pyridine 11d | 5-{5-[(2S)-2-amino-3-(n...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15138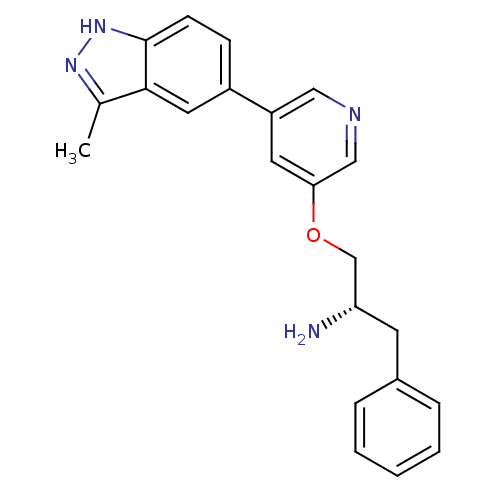 (5-indazolyl pyridine 11g | 5-{5-[(2S)-2-amino-3-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Bos taurus (bovine)) | BDBM15138 (5-indazolyl pyridine 11g | 5-{5-[(2S)-2-amino-3-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses purified recombinant enzyme and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidi... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15122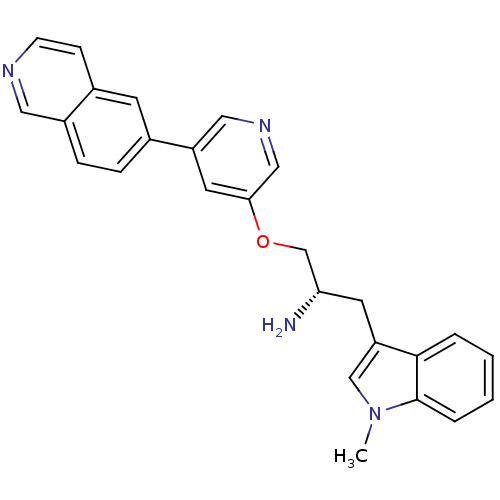 (5-isoquinolinyl pyridine 10a | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54.6 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15124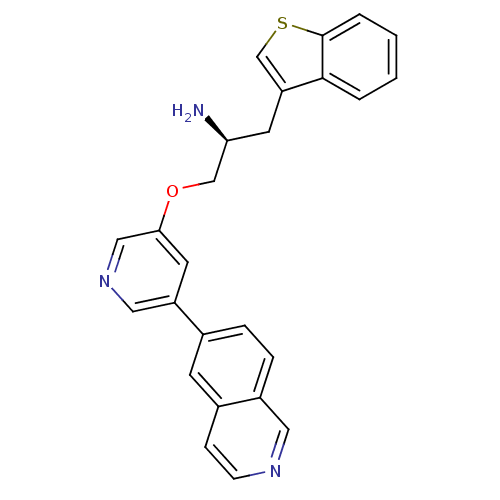 (5-isoquinolinyl pyridine 10c | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 63.7 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15144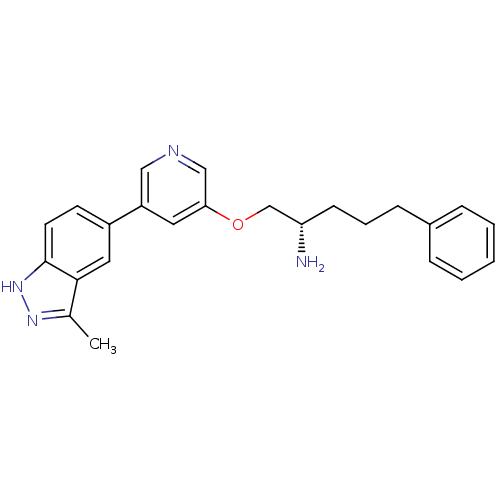 (5-(5-{[(2S)-2-amino-5-phenylpentyl]oxy}pyridin-3-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 70.6 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15126 (5-isoquinolinyl pyridine 10e | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 85 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15127 (5-isoquinolinyl pyridine 10f | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 89.6 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15137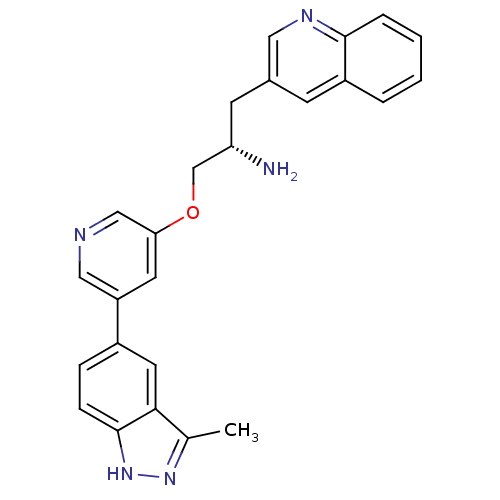 (3-[(2S)-2-amino-3-{[5-(3-methyl-1H-indazol-5-yl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 185 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15143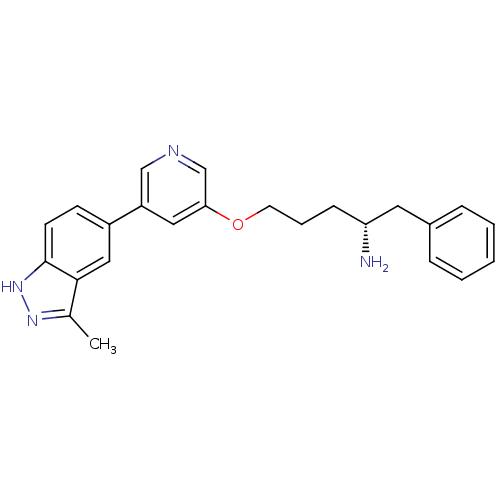 (5-(5-{[(4R)-4-amino-5-phenylpentyl]oxy}pyridin-3-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 197 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15141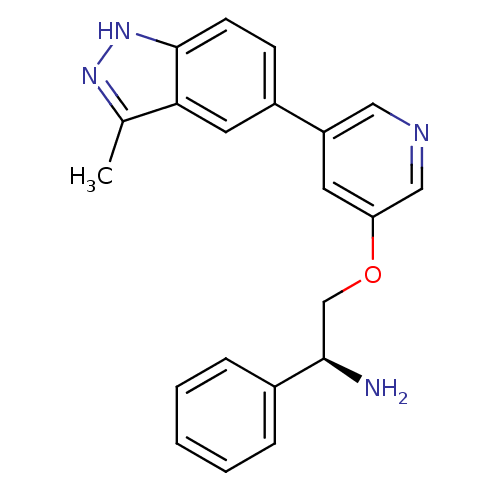 (5-indazolyl pyridine 11j | 5-{5-[(2S)-2-amino-2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 233 | -37.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15146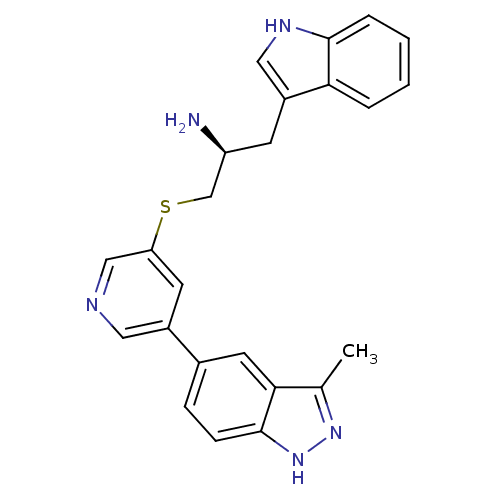 (5-(5-{[(2S)-2-amino-3-(1H-indol-3-yl)propyl]sulfan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 244 | -37.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15142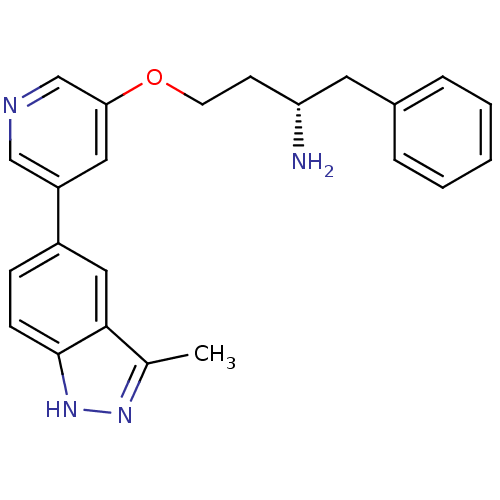 (5-indazolyl pyridine 11k | 5-{5-[(3S)-3-amino-4-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 289 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15140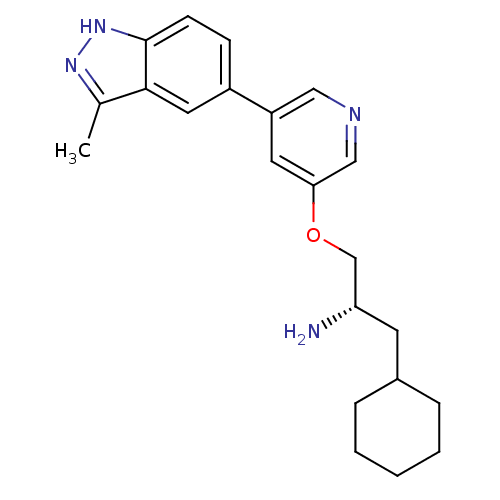 (5-indazolyl pyridine 11i | 5-{5-[(2S)-2-amino-3-cy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 368 | -36.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15130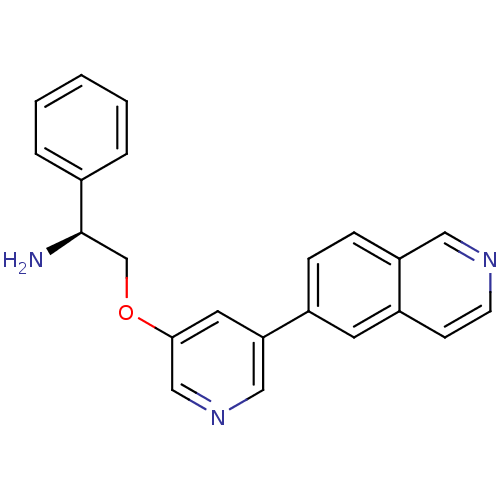 (5-isoquinolinyl pyridine 10i | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 465 | -35.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15147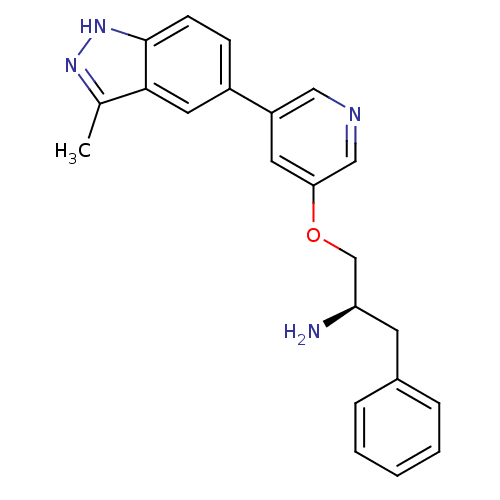 (5-indazolyl pyridine 24 | 5-{5-[(2R)-2-amino-3-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 626 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15129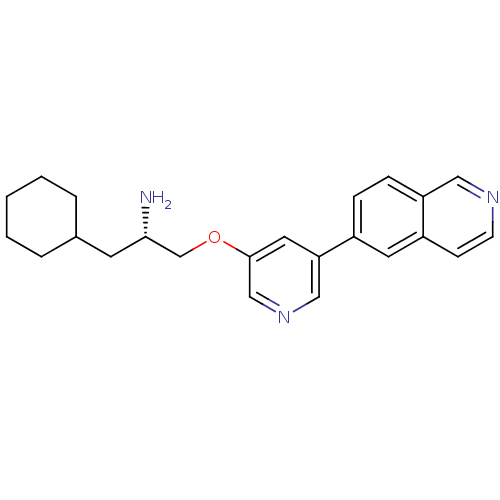 (5-isoquinolinyl pyridine 10h | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 672 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15128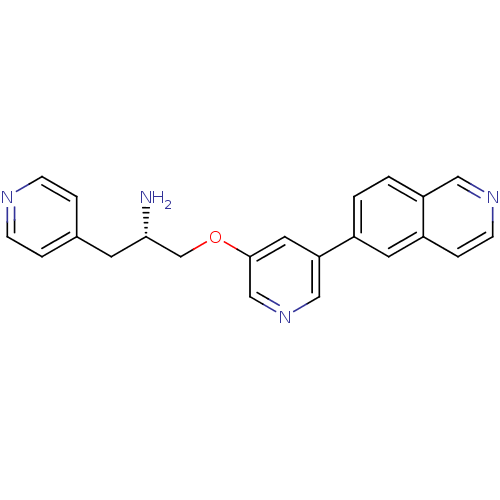 (5-isoquinolinyl pyridine 10g | 6-{5-[(2S)-2-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.53E+3 | -32.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15139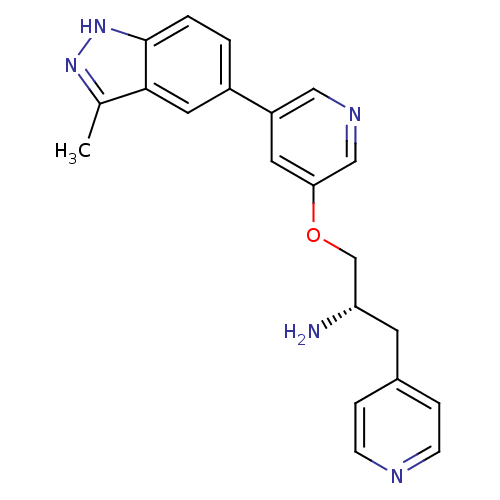 (5-indazolyl pyridine 11h | 5-{5-[(2S)-2-amino-3-(p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.28E+3 | -31.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15131 (5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | J Med Chem 50: 2990-3003 (2007) Article DOI: 10.1021/jm0701019 BindingDB Entry DOI: 10.7270/Q24X562F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM16531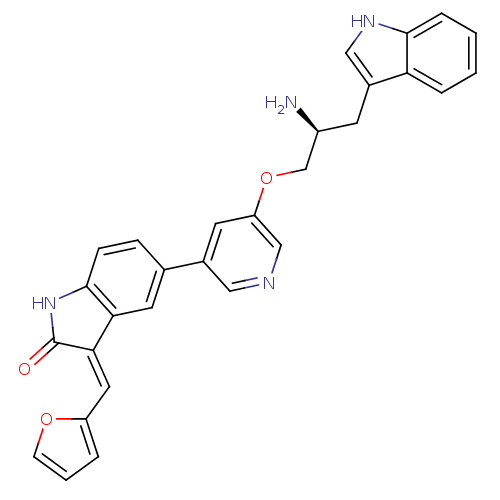 ((3Z)-5-(5-{[(2S)-2-amino-3-(1H-indol-3-yl)propyl]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | J Med Chem 50: 2990-3003 (2007) Article DOI: 10.1021/jm0701019 BindingDB Entry DOI: 10.7270/Q24X562F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM156397 (US9018381, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 [1-204] (Homo sapiens (Human)) | BDBM204937 (US9248140, 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 [1-204] (Homo sapiens (Human)) | BDBM204936 (US9248140, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM156398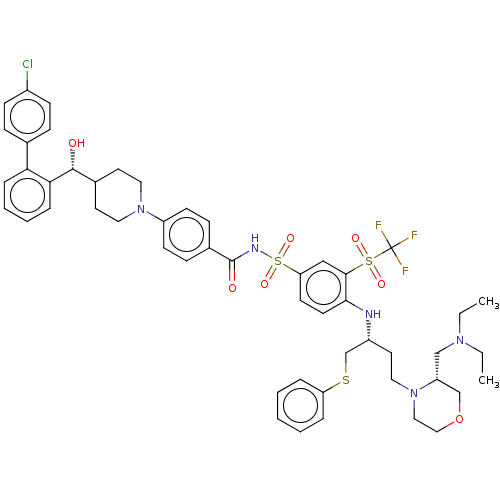 (US9018381, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM156378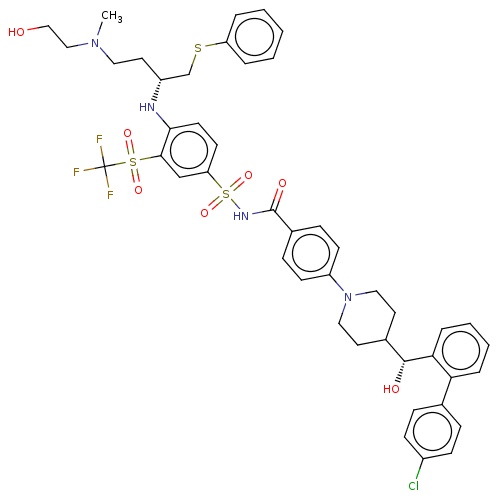 (US9018381, 3 | US9248140, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM156377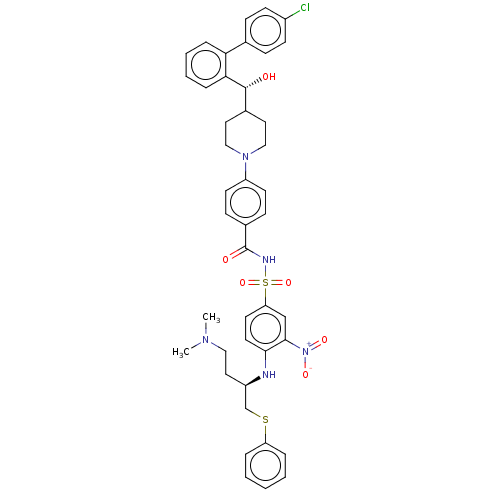 (US9018381, 2 | US9248140, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 [1-204] (Homo sapiens (Human)) | BDBM156377 (US9018381, 2 | US9248140, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 [1-204] (Homo sapiens (Human)) | BDBM156378 (US9018381, 3 | US9248140, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM16532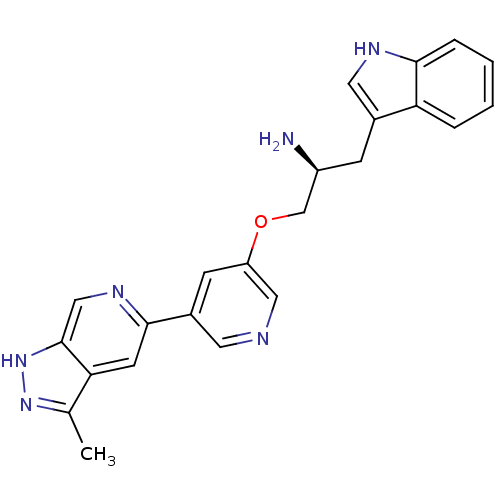 ((2S)-1-(1H-indol-3-yl)-3-{[5-(3-methyl-1H-pyrazolo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | J Med Chem 50: 2990-3003 (2007) Article DOI: 10.1021/jm0701019 BindingDB Entry DOI: 10.7270/Q24X562F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 [1-204] (Homo sapiens (Human)) | BDBM156402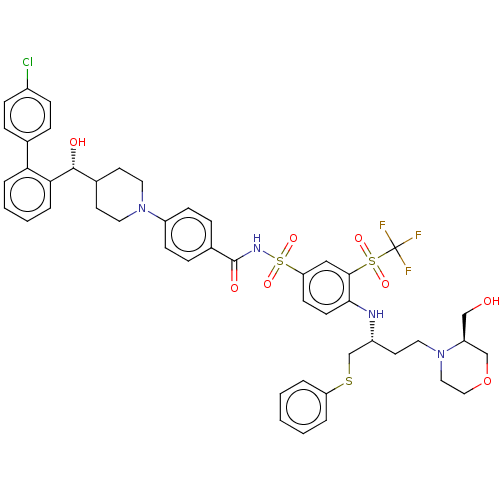 (US9018381, 25 | US9248140, 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 [1-204] (Homo sapiens (Human)) | BDBM156401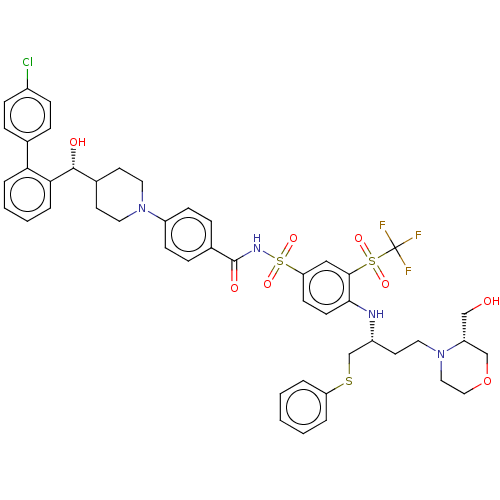 (US9018381, 24 | US9248140, 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM156401 (US9018381, 24 | US9248140, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 [1-204] (Homo sapiens (Human)) | BDBM156403 (US9018381, 26 | US9248140, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM156403 (US9018381, 26 | US9248140, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM156402 (US9018381, 25 | US9248140, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM156403 (US9018381, 26 | US9248140, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM156398 (US9018381, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-e) may be determined using a variety of kno... | US Patent US9018381 (2015) BindingDB Entry DOI: 10.7270/Q2BC3X8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 [1-204] (Homo sapiens (Human)) | BDBM156380 (US9018381, 4 | US9248140, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 [1-209] (Homo sapiens (Human)) | BDBM156403 (US9018381, 26 | US9248140, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 [1-209] (Homo sapiens (Human)) | BDBM204937 (US9248140, 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB US Patent | Assay Description Bcl-xL and Bcl-2 FP binding affinity of compound(s) of Formulae (I), (I-a), (I-b), (I-c), (I-d) and/or (I-c) may be determined using a variety of kno... | US Patent US9248140 (2016) BindingDB Entry DOI: 10.7270/Q21G0K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1392 total ) | Next | Last >> |