 Found 2375 hits with Last Name = 'evans' and Initial = 'c'
Found 2375 hits with Last Name = 'evans' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135249

((S)-1-((2S,4R)-4-(5-fluorobenzo[b]thiophen-2-yl)-2...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2cc(F)ccc2s1 Show InChI InChI=1S/C26H29FN2O2S/c1-16-10-22-23(28-16)4-3-5-24(22)31-15-21(30)14-29-9-8-18(11-17(29)2)26-13-19-12-20(27)6-7-25(19)32-26/h3-7,10,12-13,17-18,21,28,30H,8-9,11,14-15H2,1-2H3/t17-,18+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130163

((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244020
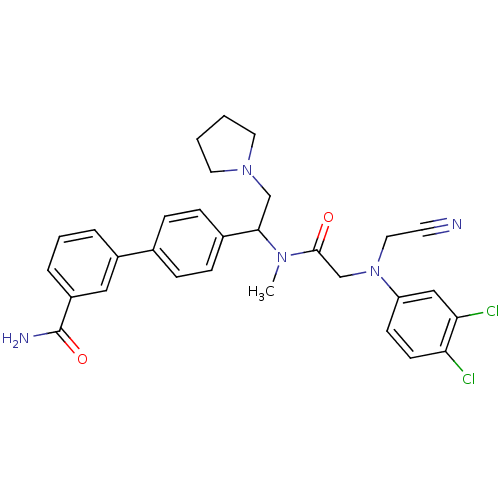
(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130152
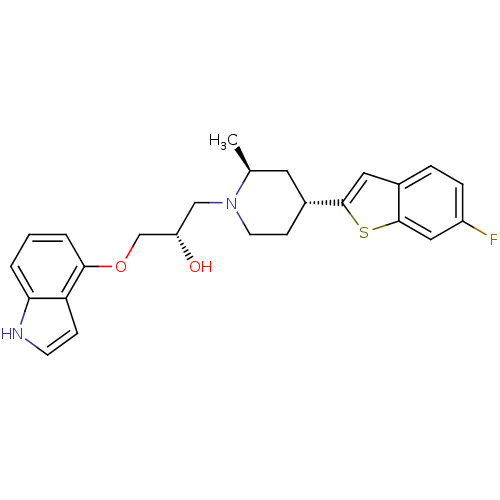
((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(6-fluoroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C25H27FN2O2S/c1-16-11-18(24-12-17-5-6-19(26)13-25(17)31-24)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16,18,20,27,29H,8,10-11,14-15H2,1H3/t16-,18+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377218
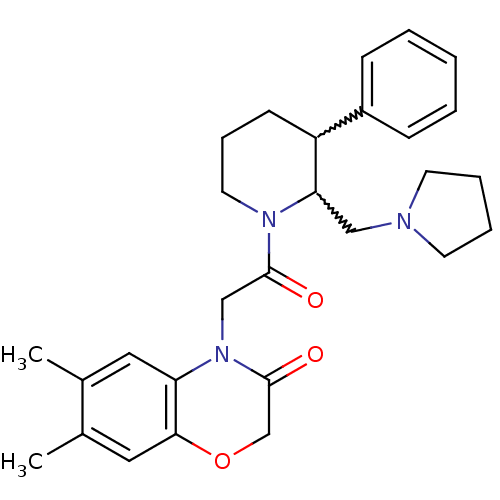
(CHEMBL257171)Show SMILES Cc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H35N3O3/c1-20-15-24-26(16-21(20)2)34-19-28(33)31(24)18-27(32)30-14-8-11-23(22-9-4-3-5-10-22)25(30)17-29-12-6-7-13-29/h3-5,9-10,15-16,23,25H,6-8,11-14,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377220
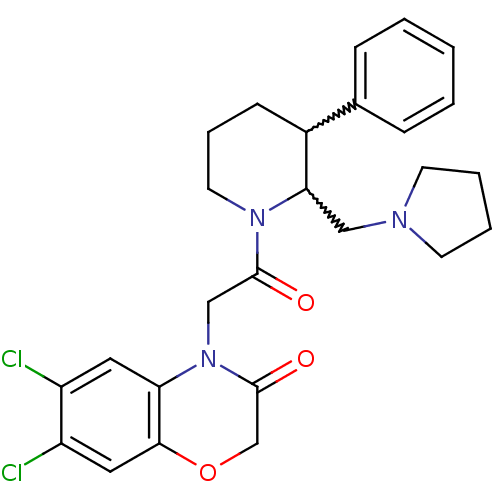
(CHEMBL255509)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H29Cl2N3O3/c27-20-13-22-24(14-21(20)28)34-17-26(33)31(22)16-25(32)30-12-6-9-19(18-7-2-1-3-8-18)23(30)15-29-10-4-5-11-29/h1-3,7-8,13-14,19,23H,4-6,9-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130168
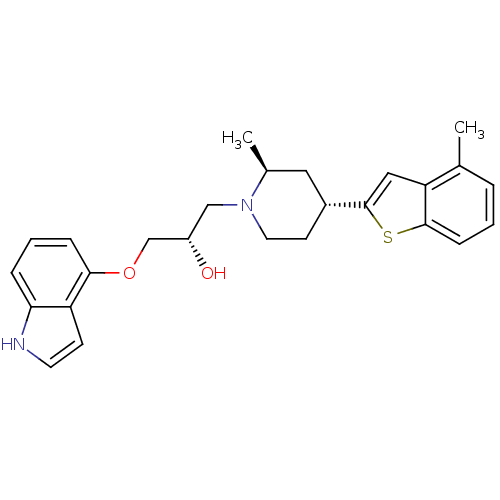
((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-2-methyl-4-(4-...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2c(C)cccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-5-3-8-25-22(17)14-26(31-25)19-10-12-28(18(2)13-19)15-20(29)16-30-24-7-4-6-23-21(24)9-11-27-23/h3-9,11,14,18-20,27,29H,10,12-13,15-16H2,1-2H3/t18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130157
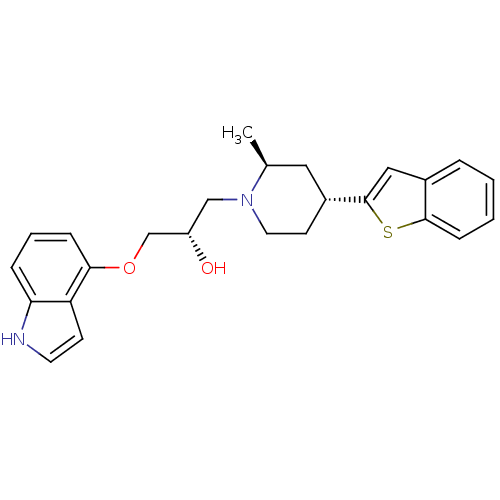
((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccccc2s1 Show InChI InChI=1S/C25H28N2O2S/c1-17-13-19(25-14-18-5-2-3-8-24(18)30-25)10-12-27(17)15-20(28)16-29-23-7-4-6-22-21(23)9-11-26-22/h2-9,11,14,17,19-20,26,28H,10,12-13,15-16H2,1H3/t17-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135246
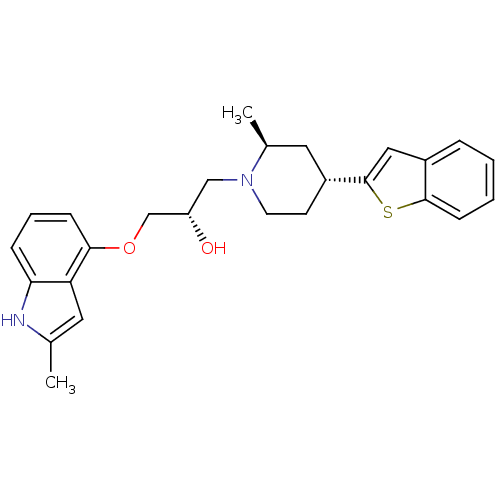
((S)-1-((2S,4R)-4-(benzo[b]thiophen-2-yl)-2-methylp...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]c(C)cc12)c1cc2ccccc2s1 Show InChI InChI=1S/C26H30N2O2S/c1-17-12-22-23(27-17)7-5-8-24(22)30-16-21(29)15-28-11-10-20(13-18(28)2)26-14-19-6-3-4-9-25(19)31-26/h3-9,12,14,18,20-21,27,29H,10-11,13,15-16H2,1-2H3/t18-,20+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130169
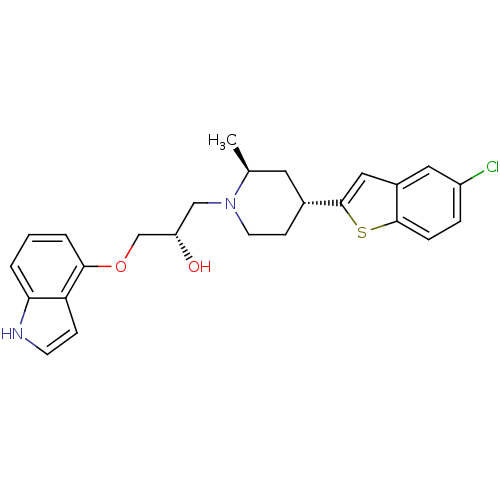
((S)-1-(1H-indol-4-yloxy)-3-((2S,4R)-4-(5-chloroben...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2cc(Cl)ccc2s1 Show InChI InChI=1S/C25H27ClN2O2S/c1-16-11-17(25-13-18-12-19(26)5-6-24(18)31-25)8-10-28(16)14-20(29)15-30-23-4-2-3-22-21(23)7-9-27-22/h2-7,9,12-13,16-17,20,27,29H,8,10-11,14-15H2,1H3/t16-,17+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377217
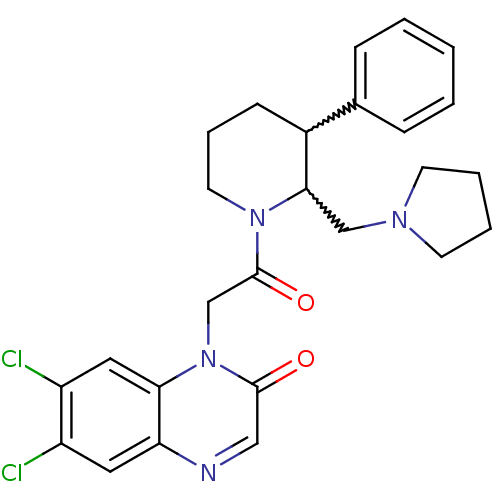
(CHEMBL256989)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H28Cl2N4O2/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-12-6-9-19(18-7-2-1-3-8-18)24(31)16-30-10-4-5-11-30/h1-3,7-8,13-15,19,24H,4-6,9-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50136680
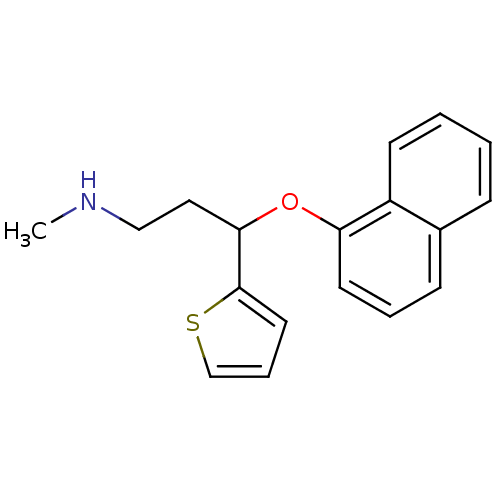
(CHEMBL424660 | N-methyl-3-(1-naphthyloxy)-3-(2-thi...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 45: 935-44 (2003)
Article DOI: 10.1016/s0028-3908(03)00268-5
BindingDB Entry DOI: 10.7270/Q2VQ318R |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50135256
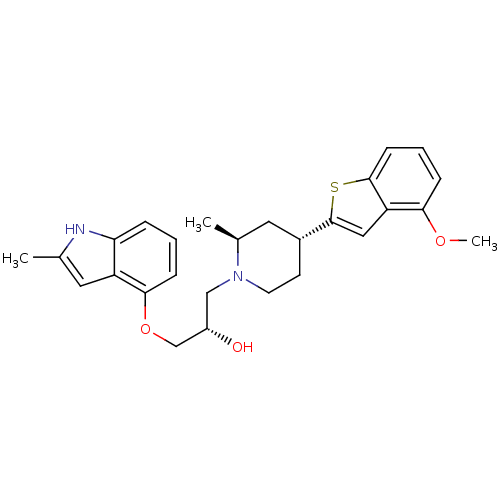
((S)-1-((2S,4R)-4-(4-methoxybenzo[b]thiophen-2-yl)-...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]c(C)cc23)[C@@H](C)C1 Show InChI InChI=1S/C27H32N2O3S/c1-17-12-21-23(28-17)6-4-8-25(21)32-16-20(30)15-29-11-10-19(13-18(29)2)27-14-22-24(31-3)7-5-9-26(22)33-27/h4-9,12,14,18-20,28,30H,10-11,13,15-16H2,1-3H3/t18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM86421
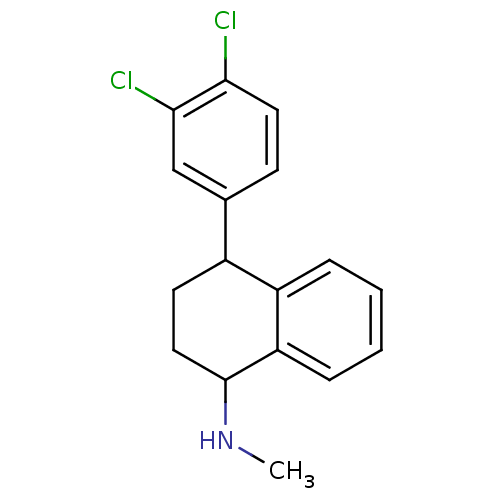
(CAS_79617-96-2 | NSC_68617 | SERTRALINE)Show InChI InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 45: 935-44 (2003)
Article DOI: 10.1016/s0028-3908(03)00268-5
BindingDB Entry DOI: 10.7270/Q2VQ318R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377215
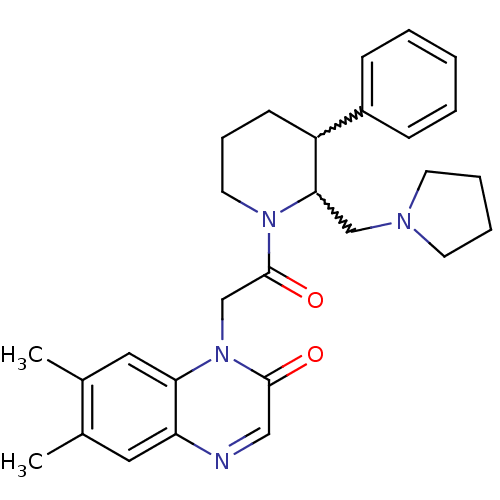
(CHEMBL257415)Show SMILES Cc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H34N4O2/c1-20-15-24-25(16-21(20)2)32(27(33)17-29-24)19-28(34)31-14-8-11-23(22-9-4-3-5-10-22)26(31)18-30-12-6-7-13-30/h3-5,9-10,15-17,23,26H,6-8,11-14,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082406

(1-(2-fluorophenoxy)-3-spiro[1,3-dihydrobenzo[e]iso...)Show SMILES O[C@H](COc1ccccc1F)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C25H26FNO3/c26-23-7-3-4-8-24(23)29-16-19(28)15-27-13-11-25(12-14-27)22-10-9-18-5-1-2-6-20(18)21(22)17-30-25/h1-10,19,28H,11-17H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130167
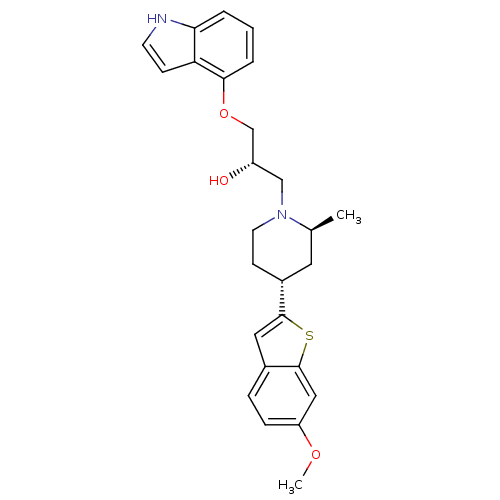
((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(6-methoxy-b...)Show SMILES COc1ccc2cc(sc2c1)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-12-19(25-13-18-6-7-21(30-2)14-26(18)32-25)9-11-28(17)15-20(29)16-31-24-5-3-4-23-22(24)8-10-27-23/h3-8,10,13-14,17,19-20,27,29H,9,11-12,15-16H2,1-2H3/t17-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082405

(1-phenoxy-3-spiro[1,3-dihydrobenzo[e]isobenzofuran...)Show SMILES O[C@H](COc1ccccc1)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C25H27NO3/c27-20(17-28-21-7-2-1-3-8-21)16-26-14-12-25(13-15-26)24-11-10-19-6-4-5-9-22(19)23(24)18-29-25/h1-11,20,27H,12-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50130165
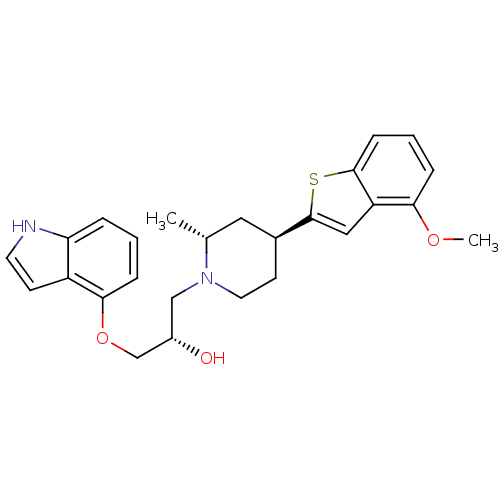
((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6S)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377227

(CHEMBL255462)Show SMILES Clc1ccc2sc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2c1 |w:16.25,17.18| Show InChI InChI=1S/C25H28ClN3O2S/c26-19-10-11-23-21(15-19)29(25(31)32-23)17-24(30)28-14-6-9-20(18-7-2-1-3-8-18)22(28)16-27-12-4-5-13-27/h1-3,7-8,10-11,15,20,22H,4-6,9,12-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082413
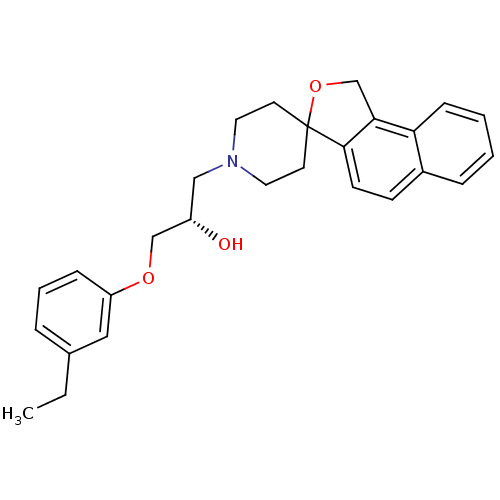
(1-(3-ethylphenoxy)-3-spiro[1,3-dihydrobenzo[e]isob...)Show SMILES CCc1cccc(OC[C@@H](O)CN2CCC3(CC2)OCc2c3ccc3ccccc23)c1 Show InChI InChI=1S/C27H31NO3/c1-2-20-6-5-8-23(16-20)30-18-22(29)17-28-14-12-27(13-15-28)26-11-10-21-7-3-4-9-24(21)25(26)19-31-27/h3-11,16,22,29H,2,12-15,17-19H2,1H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50128368
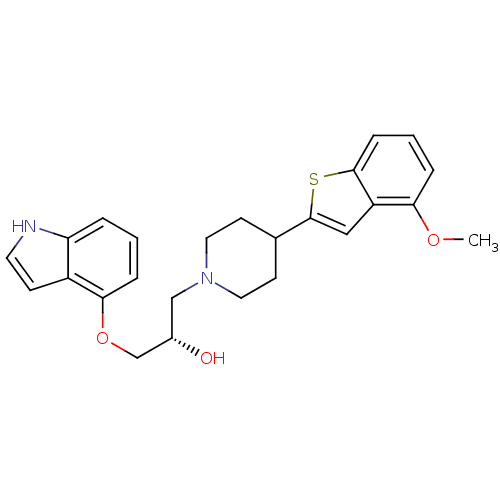
((S)-1-(1H-Indol-4-yloxy)-3-[4-(4-methoxy-benzo[b]t...)Show SMILES COc1cccc2sc(cc12)C1CCN(C[C@H](O)COc2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C25H28N2O3S/c1-29-22-5-3-7-24-20(22)14-25(31-24)17-9-12-27(13-10-17)15-18(28)16-30-23-6-2-4-21-19(23)8-11-26-21/h2-8,11,14,17-18,26,28H,9-10,12-13,15-16H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50128367
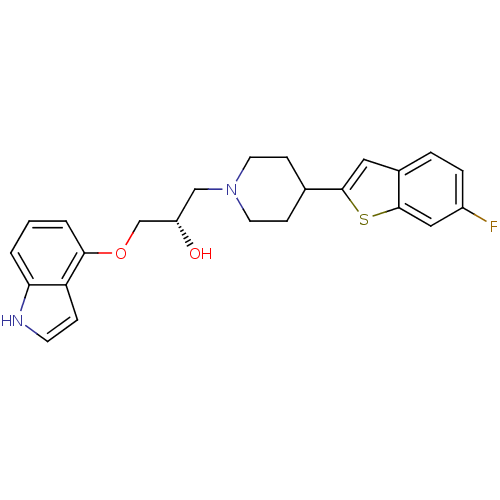
((S)-1-(1H-indol-4-yloxy)-3-(4-(6-fluorobenzo[b]thi...)Show SMILES O[C@H](COc1cccc2[nH]ccc12)CN1CCC(CC1)c1cc2ccc(F)cc2s1 Show InChI InChI=1S/C24H25FN2O2S/c25-18-5-4-17-12-23(30-24(17)13-18)16-7-10-27(11-8-16)14-19(28)15-29-22-3-1-2-21-20(22)6-9-26-21/h1-6,9,12-13,16,19,26,28H,7-8,10-11,14-15H2/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377224
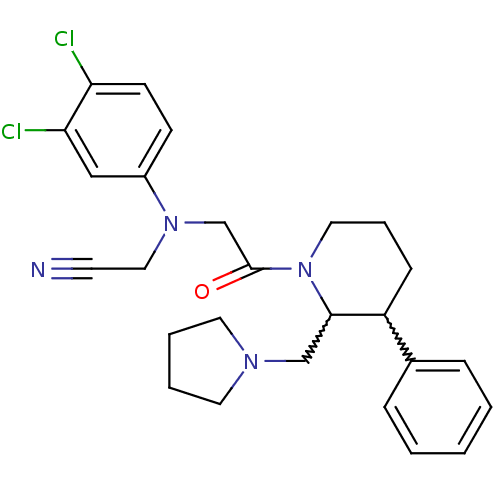
(CHEMBL257767)Show SMILES Clc1ccc(cc1Cl)N(CC#N)CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1 |w:19.29,20.22| Show InChI InChI=1S/C26H30Cl2N4O/c27-23-11-10-21(17-24(23)28)31(16-12-29)19-26(33)32-15-6-9-22(20-7-2-1-3-8-20)25(32)18-30-13-4-5-14-30/h1-3,7-8,10-11,17,22,25H,4-6,9,13-16,18-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359016
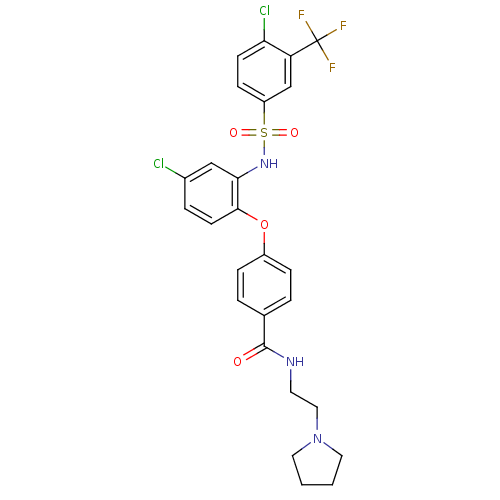
(CHEMBL1924017)Show SMILES FC(F)(F)c1cc(ccc1Cl)S(=O)(=O)Nc1cc(Cl)ccc1Oc1ccc(cc1)C(=O)NCCN1CCCC1 Show InChI InChI=1S/C26H24Cl2F3N3O4S/c27-18-5-10-24(23(15-18)33-39(36,37)20-8-9-22(28)21(16-20)26(29,30)31)38-19-6-3-17(4-7-19)25(35)32-11-14-34-12-1-2-13-34/h3-10,15-16,33H,1-2,11-14H2,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377219
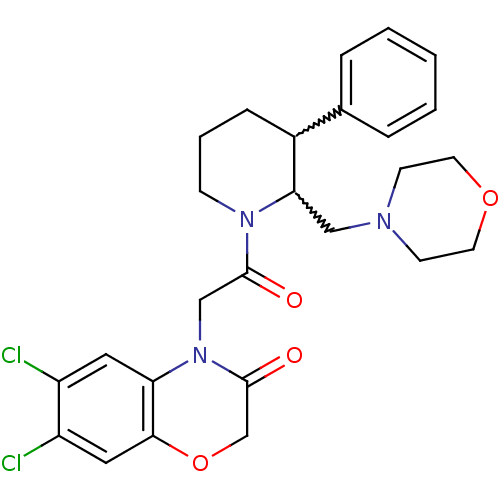
(CHEMBL402520)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1Cl |w:16.26,17.18| Show InChI InChI=1S/C26H29Cl2N3O4/c27-20-13-22-24(14-21(20)28)35-17-26(33)31(22)16-25(32)30-8-4-7-19(18-5-2-1-3-6-18)23(30)15-29-9-11-34-12-10-29/h1-3,5-6,13-14,19,23H,4,7-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082408
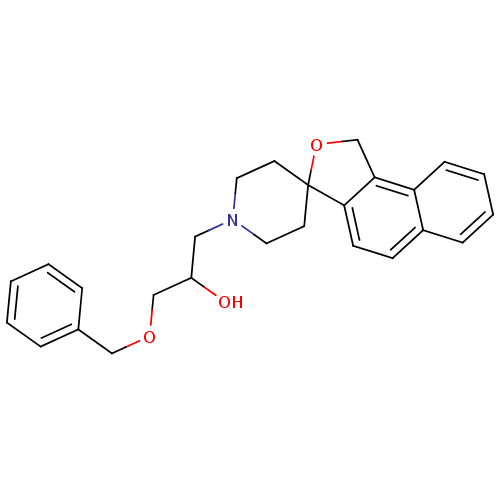
(1-benzyloxy-3-spiro[1,3-dihydrobenzo[e]isobenzofur...)Show SMILES OC(COCc1ccccc1)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C26H29NO3/c28-22(18-29-17-20-6-2-1-3-7-20)16-27-14-12-26(13-15-27)25-11-10-21-8-4-5-9-23(21)24(25)19-30-26/h1-11,22,28H,12-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082410
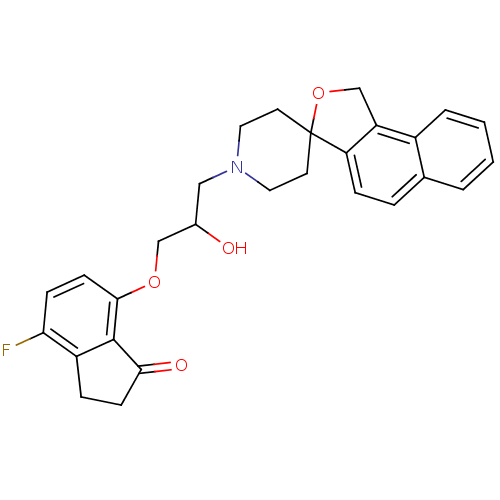
(4-fluoro-7-[2-hydroxy-3-spiro[1,3-dihydrobenzo[e]i...)Show SMILES OC(COc1ccc(F)c2CCC(=O)c12)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C28H28FNO4/c29-24-8-10-26(27-21(24)6-9-25(27)32)33-16-19(31)15-30-13-11-28(12-14-30)23-7-5-18-3-1-2-4-20(18)22(23)17-34-28/h1-5,7-8,10,19,31H,6,9,11-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130161

((S)-1-(1H-Indol-4-yloxy)-3-[(4R,6R)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082411
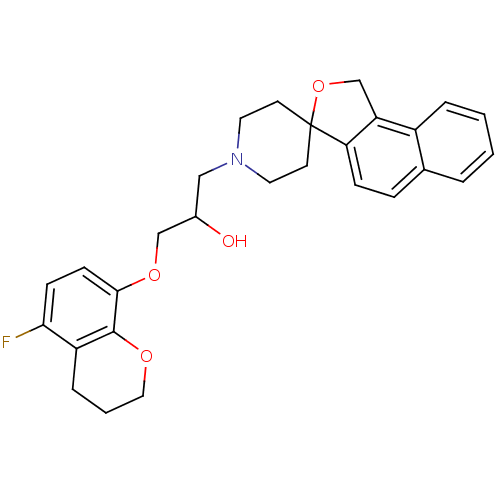
(1-(5-fluoro-3,4-dihydro-2H-8-chromenyloxy)-3-spiro...)Show SMILES OC(COc1ccc(F)c2CCCOc12)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C28H30FNO4/c29-25-9-10-26(27-22(25)6-3-15-32-27)33-17-20(31)16-30-13-11-28(12-14-30)24-8-7-19-4-1-2-5-21(19)23(24)18-34-28/h1-2,4-5,7-10,20,31H,3,6,11-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244019
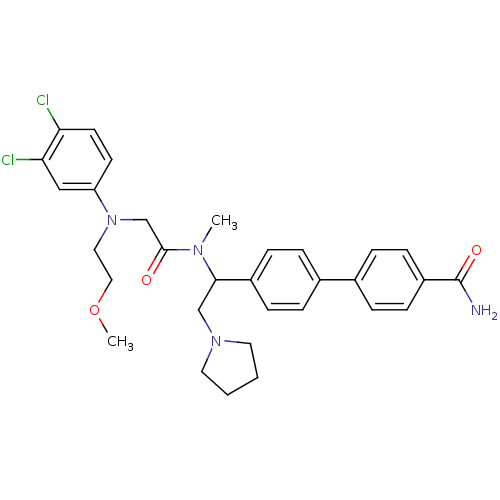
(4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...)Show SMILES COCCN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(30(38)21-37(17-18-40-2)26-13-14-27(32)28(33)19-26)29(20-36-15-3-4-16-36)24-9-5-22(6-10-24)23-7-11-25(12-8-23)31(34)39/h5-14,19,29H,3-4,15-18,20-21H2,1-2H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566910
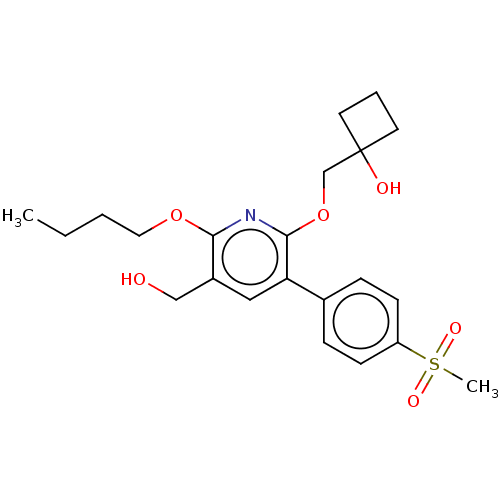
(CHEMBL4870569)Show SMILES CCCCOc1nc(OCC2(O)CCC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359013
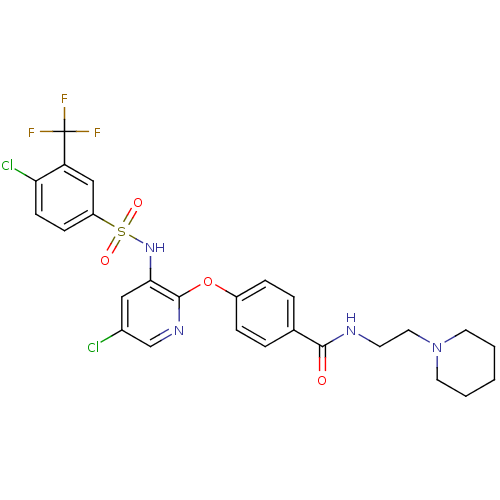
(CHEMBL1924014)Show SMILES FC(F)(F)c1cc(ccc1Cl)S(=O)(=O)Nc1cc(Cl)cnc1Oc1ccc(cc1)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C26H25Cl2F3N4O4S/c27-18-14-23(34-40(37,38)20-8-9-22(28)21(15-20)26(29,30)31)25(33-16-18)39-19-6-4-17(5-7-19)24(36)32-10-13-35-11-2-1-3-12-35/h4-9,14-16,34H,1-3,10-13H2,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244022
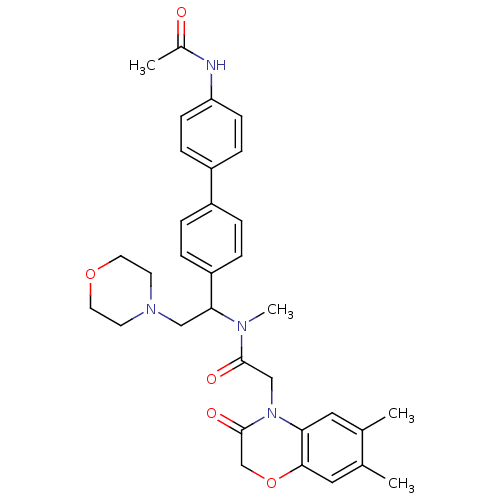
(CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccc(NC(C)=O)cc1)C(=O)CN1C(=O)COc2cc(C)c(C)cc12 Show InChI InChI=1S/C33H38N4O5/c1-22-17-29-31(18-23(22)2)42-21-33(40)37(29)20-32(39)35(4)30(19-36-13-15-41-16-14-36)27-7-5-25(6-8-27)26-9-11-28(12-10-26)34-24(3)38/h5-12,17-18,30H,13-16,19-21H2,1-4H3,(H,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377216
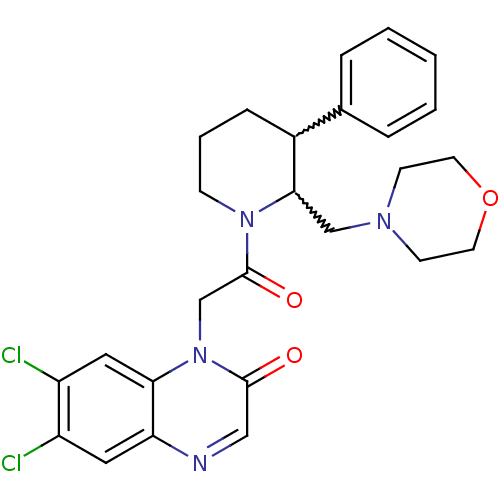
(CHEMBL256988)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1Cl |w:17.18,16.26| Show InChI InChI=1S/C26H28Cl2N4O3/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-8-4-7-19(18-5-2-1-3-6-18)24(31)16-30-9-11-35-12-10-30/h1-3,5-6,13-15,19,24H,4,7-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130157

((S)-1-((4S,6R)-4-Benzo[b]thiophen-2-yl-2-methyl-pi...)Show SMILES C[C@H]1C[C@@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2ccccc2s1 Show InChI InChI=1S/C25H28N2O2S/c1-17-13-19(25-14-18-5-2-3-8-24(18)30-25)10-12-27(17)15-20(28)16-29-23-7-4-6-22-21(23)9-11-26-22/h2-9,11,14,17,19-20,26,28H,10,12-13,15-16H2,1H3/t17-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130163

((S)-1-(1H-Indol-4-yloxy)-3-[(4S,6R)-4-(4-methoxy-b...)Show SMILES COc1cccc2sc(cc12)[C@@H]1CCN(C[C@H](O)COc2cccc3[nH]ccc23)[C@@H](C)C1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-18(26-14-21-23(30-2)6-4-8-25(21)32-26)10-12-28(17)15-19(29)16-31-24-7-3-5-22-20(24)9-11-27-22/h3-9,11,14,17-19,27,29H,10,12-13,15-16H2,1-2H3/t17-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50128380

((S)-1-(1H-indol-4-yloxy)-3-(4-(benzo[b]thiophen-2-...)Show SMILES O[C@H](COc1cccc2[nH]ccc12)CN1CCC(CC1)c1cc2ccccc2s1 Show InChI InChI=1S/C24H26N2O2S/c27-19(16-28-22-6-3-5-21-20(22)8-11-25-21)15-26-12-9-17(10-13-26)24-14-18-4-1-2-7-23(18)29-24/h1-8,11,14,17,19,25,27H,9-10,12-13,15-16H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Neuropharmacology 45: 935-44 (2003)
Article DOI: 10.1016/s0028-3908(03)00268-5
BindingDB Entry DOI: 10.7270/Q2VQ318R |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50359018

(CHEMBL1924019)Show SMILES COc1cc(Oc2ccc(cc2)C(=O)NCCN2CCCC2)c(NS(=O)(=O)c2ccc(Cl)c(c2)C(F)(F)F)cc1Cl Show InChI InChI=1S/C27H26Cl2F3N3O5S/c1-39-24-16-25(40-18-6-4-17(5-7-18)26(36)33-10-13-35-11-2-3-12-35)23(15-22(24)29)34-41(37,38)19-8-9-21(28)20(14-19)27(30,31)32/h4-9,14-16,34H,2-3,10-13H2,1H3,(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR2 receptor expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs |
Bioorg Med Chem Lett 21: 7291-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.038
BindingDB Entry DOI: 10.7270/Q2KK9C6D |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244021
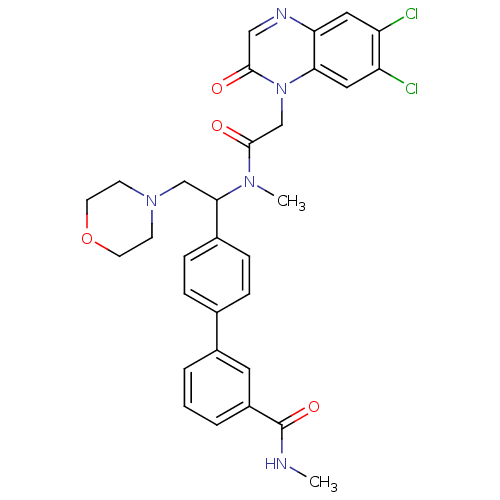
(4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(Cl)c(Cl)cc2ncc1=O Show InChI InChI=1S/C31H31Cl2N5O4/c1-34-31(41)23-5-3-4-22(14-23)20-6-8-21(9-7-20)28(18-37-10-12-42-13-11-37)36(2)30(40)19-38-27-16-25(33)24(32)15-26(27)35-17-29(38)39/h3-9,14-17,28H,10-13,18-19H2,1-2H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50128370
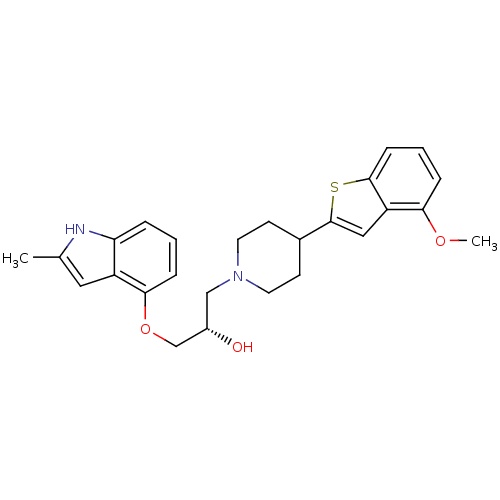
((S)-1-(4-(4-methoxybenzo[b]thiophen-2-yl)piperidin...)Show SMILES COc1cccc2sc(cc12)C1CCN(C[C@H](O)COc2cccc3[nH]c(C)cc23)CC1 Show InChI InChI=1S/C26H30N2O3S/c1-17-13-20-22(27-17)5-3-7-24(20)31-16-19(29)15-28-11-9-18(10-12-28)26-14-21-23(30-2)6-4-8-25(21)32-26/h3-8,13-14,18-19,27,29H,9-12,15-16H2,1-2H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor |
Bioorg Med Chem Lett 16: 2347-51 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.007
BindingDB Entry DOI: 10.7270/Q2W958RV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377214
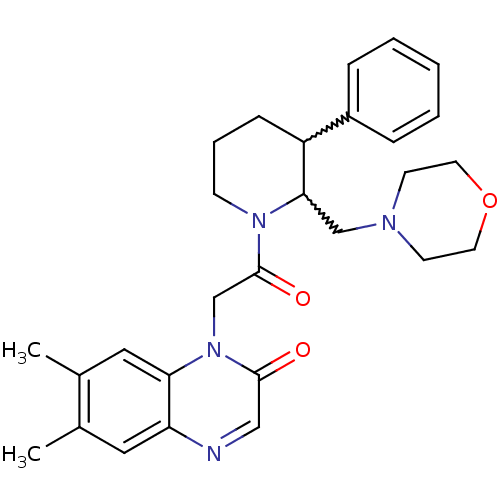
(CHEMBL256721)Show SMILES Cc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1C |w:17.18,16.26| Show InChI InChI=1S/C28H34N4O3/c1-20-15-24-25(16-21(20)2)32(27(33)17-29-24)19-28(34)31-10-6-9-23(22-7-4-3-5-8-22)26(31)18-30-11-13-35-14-12-30/h3-5,7-8,15-17,23,26H,6,9-14,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244065
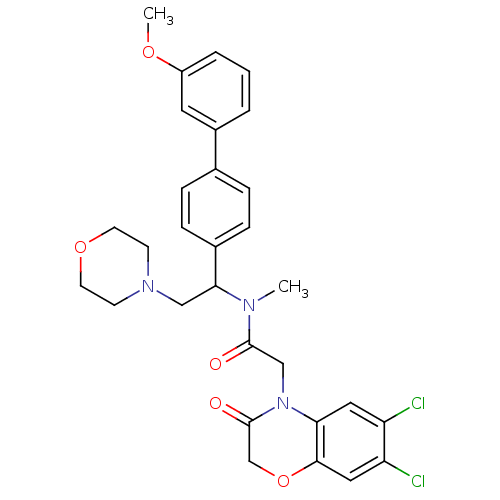
(2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C30H31Cl2N3O5/c1-33(29(36)18-35-26-15-24(31)25(32)16-28(26)40-19-30(35)37)27(17-34-10-12-39-13-11-34)21-8-6-20(7-9-21)22-4-3-5-23(14-22)38-2/h3-9,14-16,27H,10-13,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377229
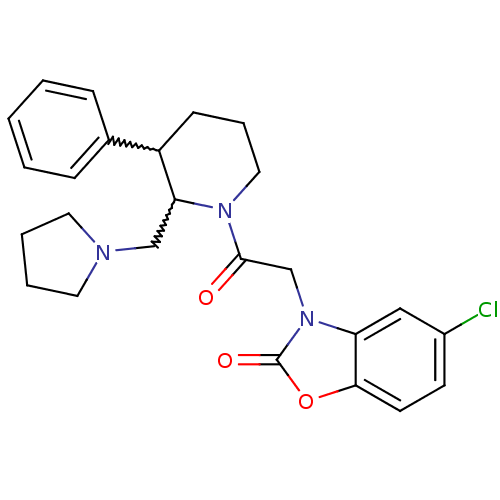
(CHEMBL257150)Show SMILES Clc1ccc2oc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2c1 |w:16.25,17.18| Show InChI InChI=1S/C25H28ClN3O3/c26-19-10-11-23-21(15-19)29(25(31)32-23)17-24(30)28-14-6-9-20(18-7-2-1-3-8-18)22(28)16-27-12-4-5-13-27/h1-3,7-8,10-11,15,20,22H,4-6,9,12-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082407
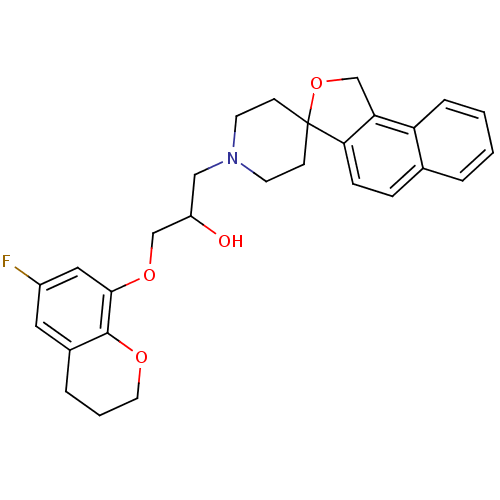
(1-(6-fluoro-3,4-dihydro-2H-8-chromenyloxy)-3-spiro...)Show SMILES OC(COc1cc(F)cc2CCCOc12)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C28H30FNO4/c29-21-14-20-5-3-13-32-27(20)26(15-21)33-17-22(31)16-30-11-9-28(10-12-30)25-8-7-19-4-1-2-6-23(19)24(25)18-34-28/h1-2,4,6-8,14-15,22,31H,3,5,9-13,16-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]- 5-HT binding to cloned human 5-hydroxytryptamine 1A receptor stably expressed in HeLa cells |
Bioorg Med Chem Lett 9: 3243-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W37WVB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data