

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029704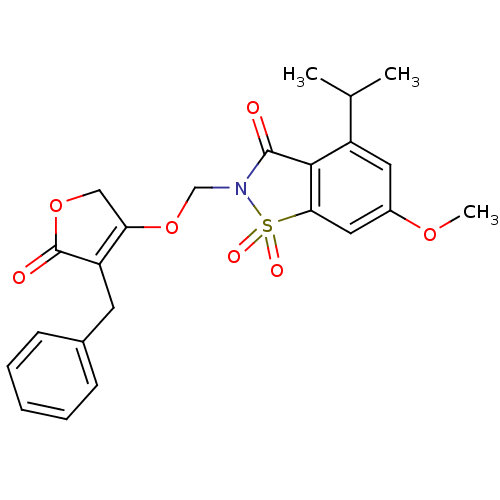 (2-(4-Benzyl-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029709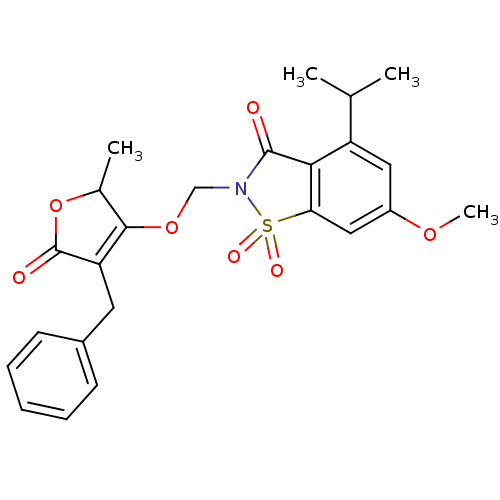 (2-(4-Benzyl-2-methyl-5-oxo-2,5-dihydro-furan-3-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029710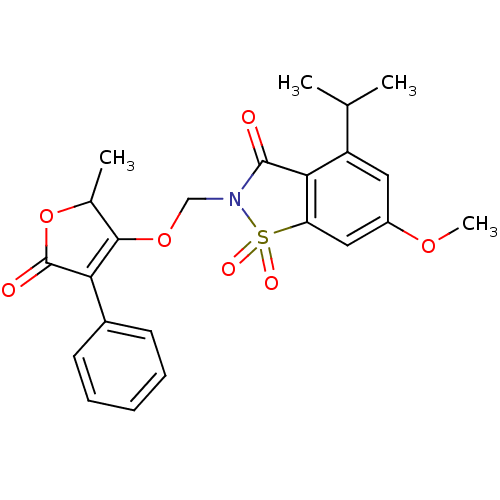 (4-Isopropyl-6-methoxy-2-(2-methyl-5-oxo-4-phenyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285289 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699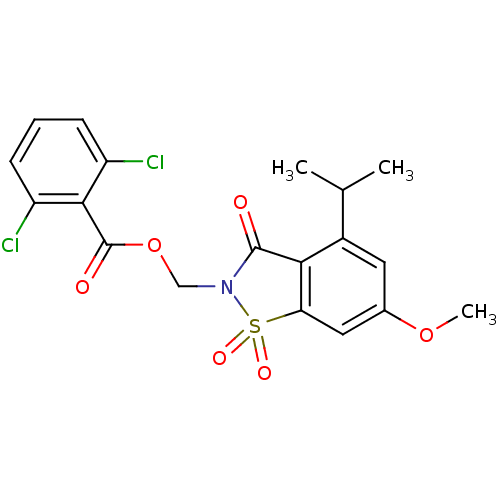 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029717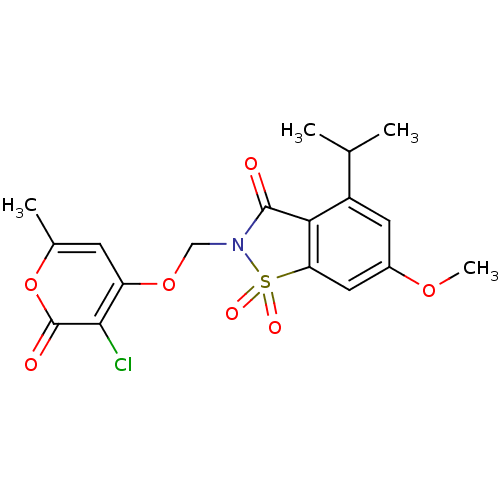 (2-(3-Chloro-6-methyl-2-oxo-2H-pyran-4-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029698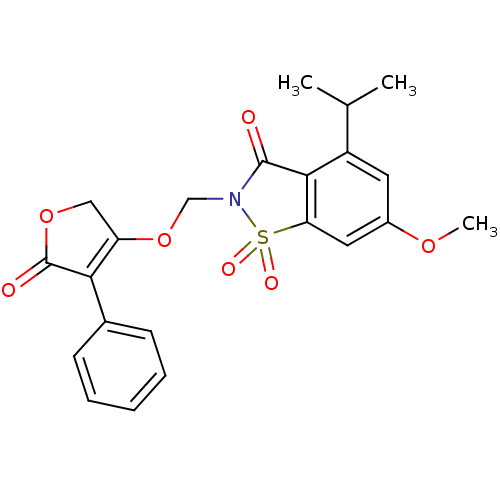 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(5-oxo-4-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029696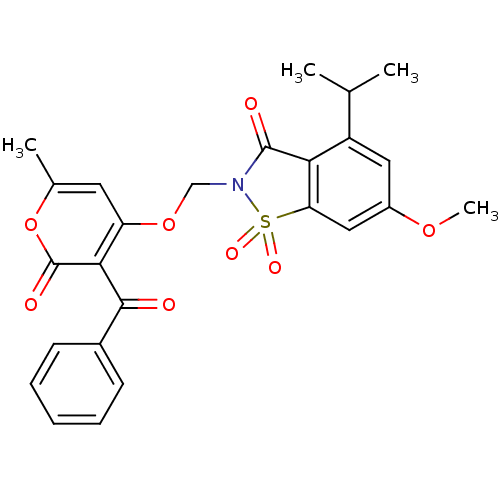 (2-(3-Benzoyl-6-methyl-2-oxo-2H-pyran-4-yloxymethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029712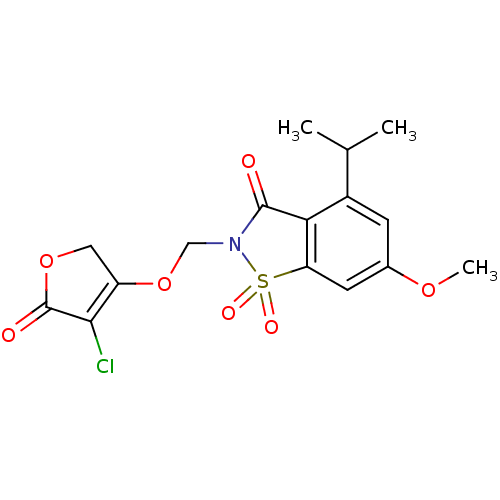 (2-(4-Chloro-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029713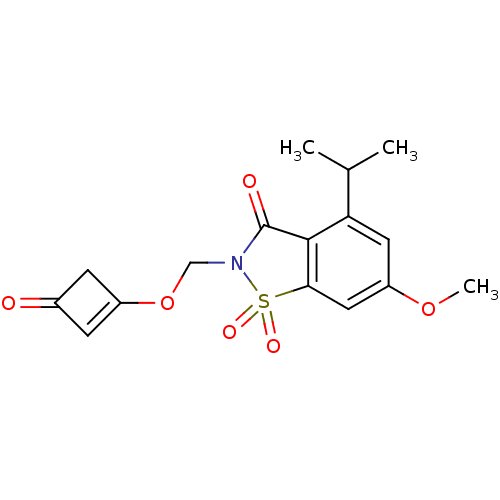 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(3-oxo-cyclobut-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029691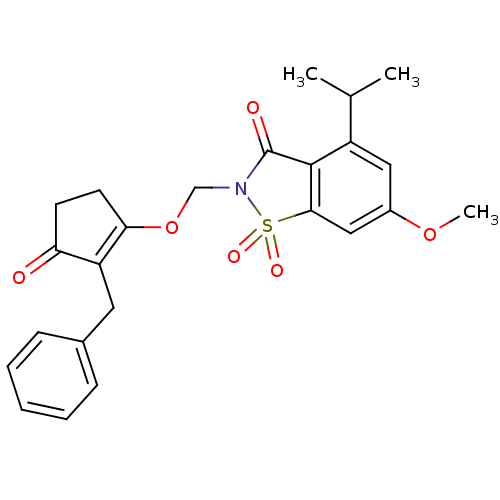 (2-(2-Benzyl-3-oxo-cyclopent-1-enyloxymethyl)-4-iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285280 (2,6-Dichloro-benzoic acid 4-ethoxy-6-methoxy-1,1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029692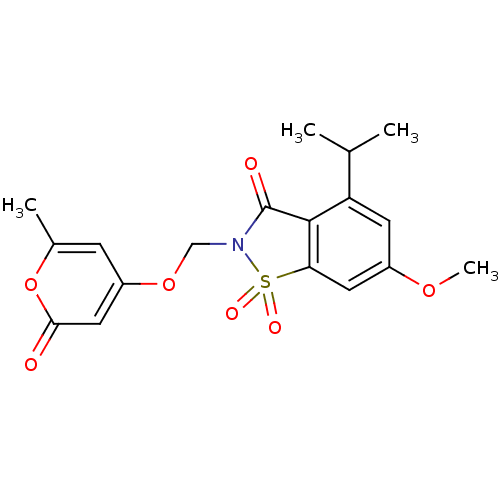 (4-Isopropyl-6-methoxy-2-(6-methyl-2-oxo-2H-pyran-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029695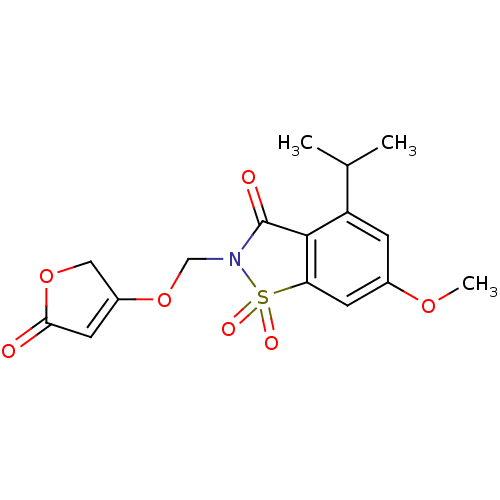 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(5-oxo-2,5-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029711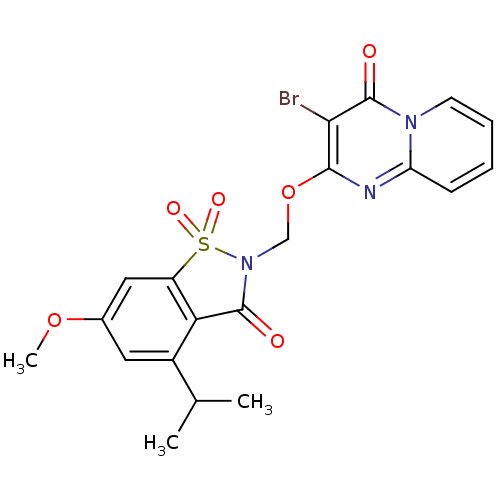 (3-Bromo-2-(4-isopropyl-6-methoxy-1,1,3-trioxo-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029720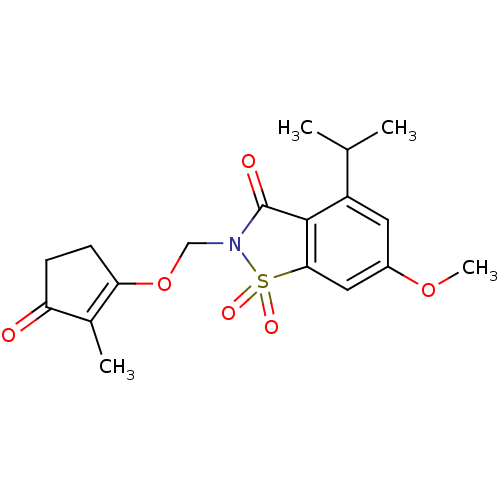 (4-Isopropyl-6-methoxy-2-(2-methyl-3-oxo-cyclopent-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029707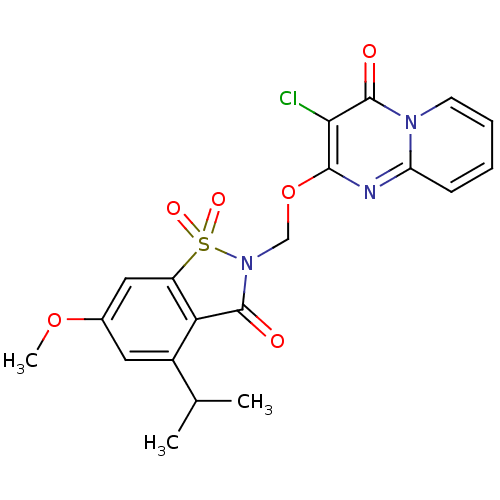 (3-Chloro-2-(4-isopropyl-6-methoxy-1,1,3-trioxo-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285286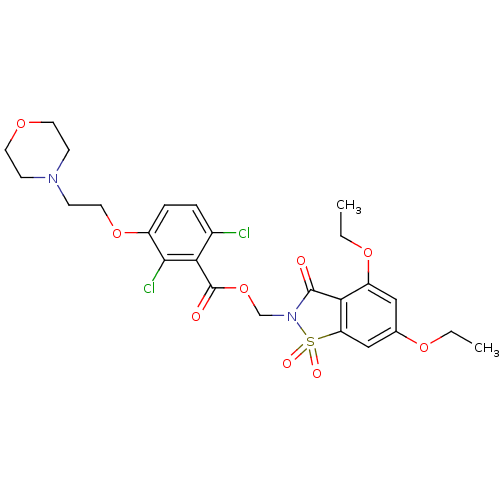 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029721 (2-(4-Isopropyl-6-methoxy-1,1,3-trioxo-1,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285284 (2,6-Dichloro-benzoic acid 4,6-diethoxy-1,1,3-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285283 (2,6-Dichloro-benzoic acid 4,6-dimethoxy-1,1,3-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029715 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(3-oxo-3H-inden-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029718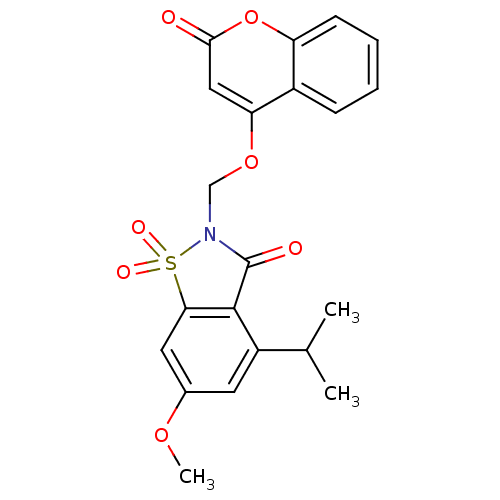 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(2-oxo-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029700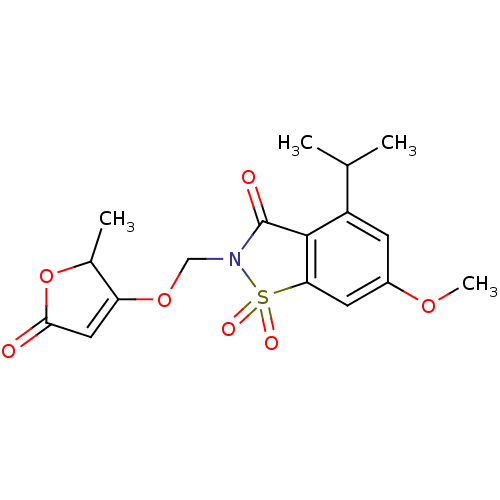 (4-Isopropyl-6-methoxy-2-(2-methyl-5-oxo-2,5-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029706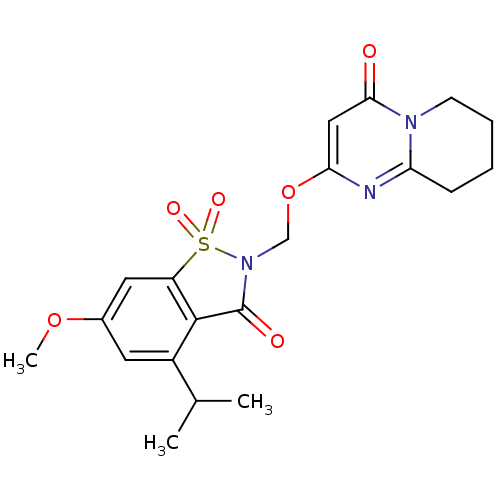 (2-(4-Isopropyl-6-methoxy-1,1,3-trioxo-1,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285287 (2,6-Dichloro-3-(2-pyrrolidin-1-yl-ethoxy)-benzoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029716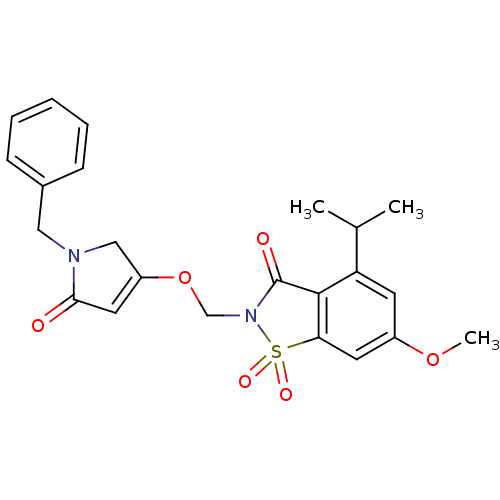 (2-(1-Benzyl-5-oxo-2,5-dihydro-1H-pyrrol-3-yloxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285290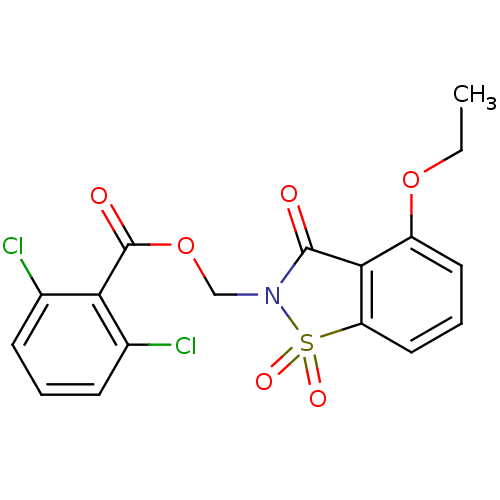 (2,6-Dichloro-benzoic acid 4-ethoxy-1,1,3-trioxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029708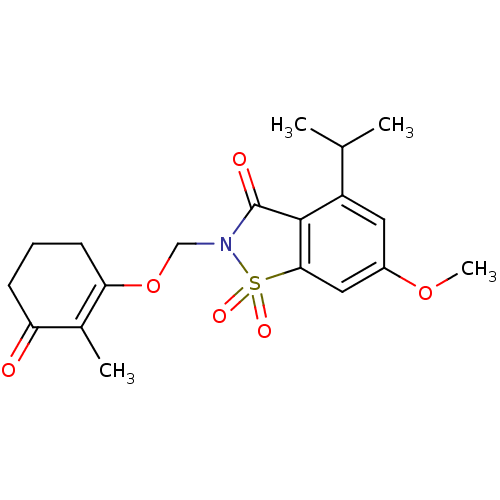 (4-Isopropyl-6-methoxy-2-(2-methyl-3-oxo-cyclohex-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029702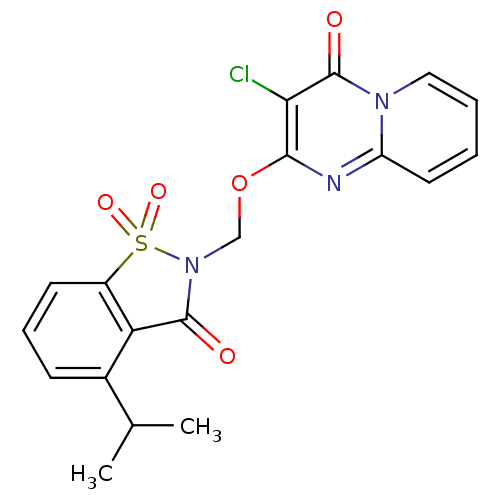 (3-Chloro-2-(4-isopropyl-1,1,3-trioxo-1,3-dihydro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029693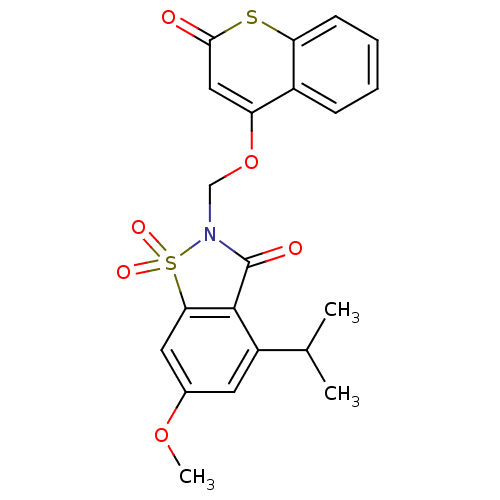 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(2-oxo-2H-thioch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285282 (2,6-Dichloro-benzoic acid 4-isopropoxy-1,1,3-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029714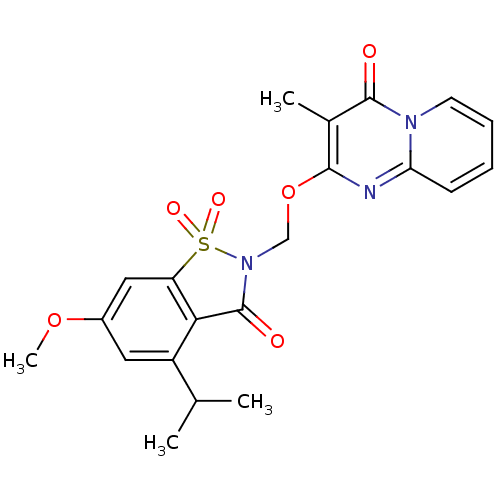 (2-(4-Isopropyl-6-methoxy-1,1,3-trioxo-1,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029703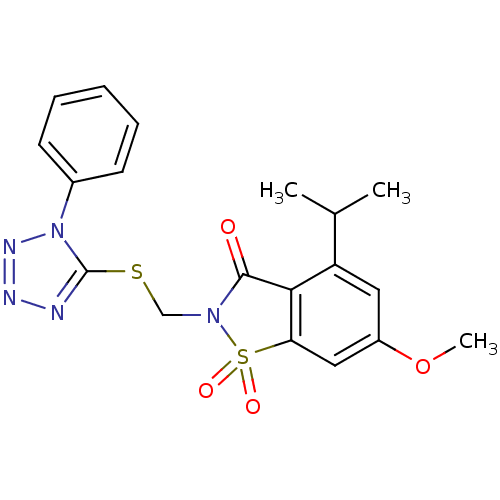 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(1-phenyl-1H-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285285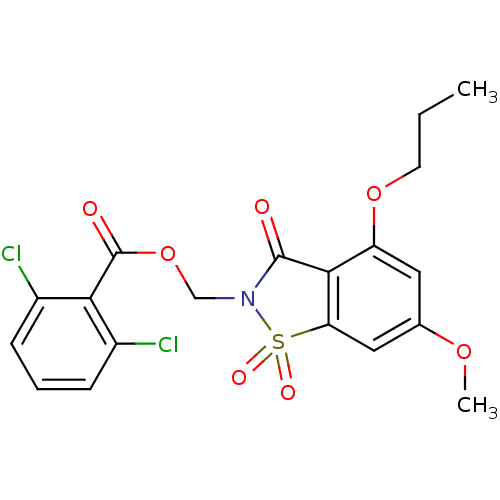 (2,6-Dichloro-benzoic acid 6-methoxy-1,1,3-trioxo-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283355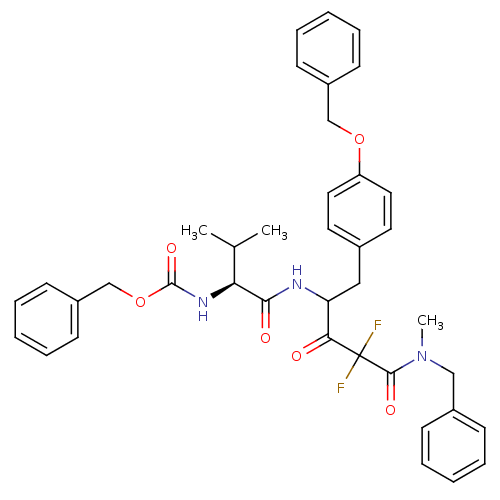 (CHEMBL311418 | {(S)-1-[3-(Benzyl-methyl-carbamoyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285288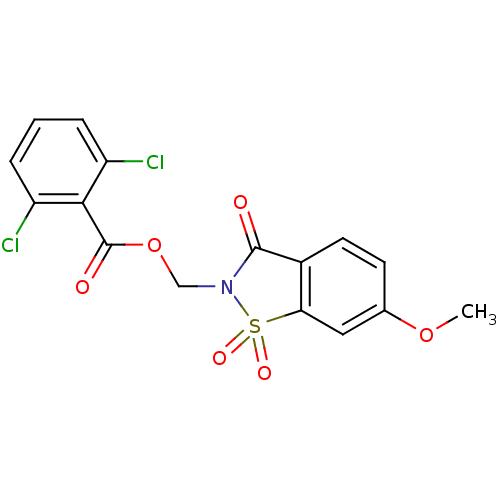 (2,6-Dichloro-benzoic acid 6-methoxy-1,1,3-trioxo-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029705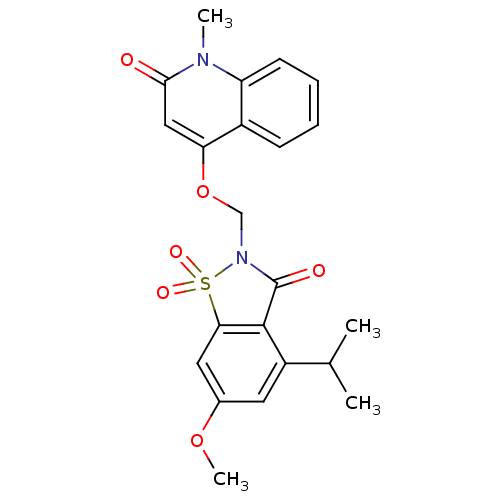 (4-(4-Isopropyl-6-methoxy-1,1,3-trioxo-1,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029694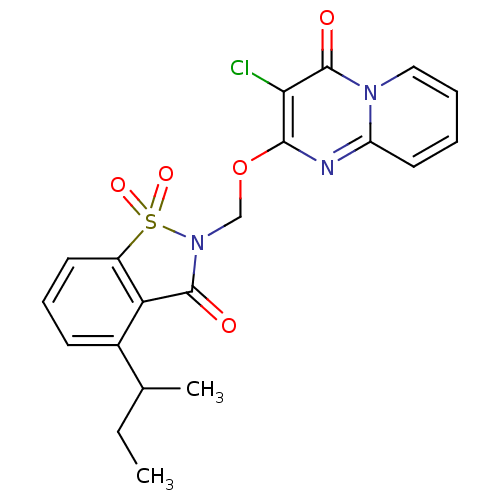 (2-(4-sec-Butyl-1,1,3-trioxo-1,3-dihydro-1lambda*6*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029719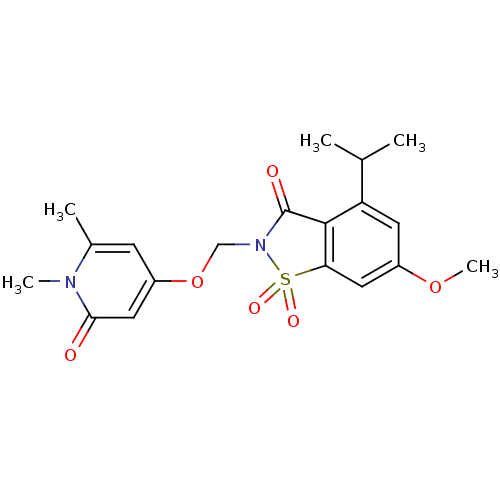 (2-(1,6-Dimethyl-2-oxo-1,2-dihydro-pyridin-4-yloxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029701 (3-Benzyl-2-(4-isopropyl-6-methoxy-1,1,3-trioxo-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283358 (CHEMBL79799 | [(S)-1-(1-Benzyl-3-benzylcarbamoyl-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285281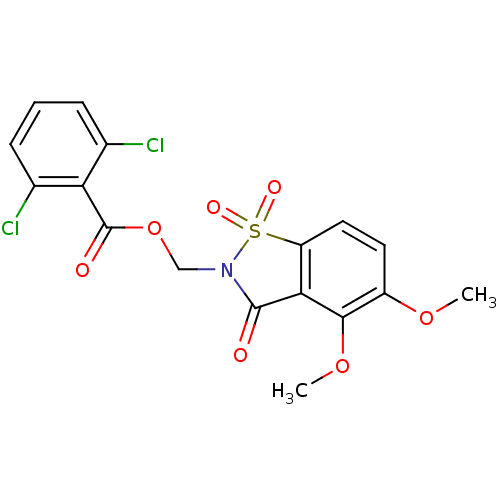 (2,6-Dichloro-benzoic acid 4,5-dimethoxy-1,1,3-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039636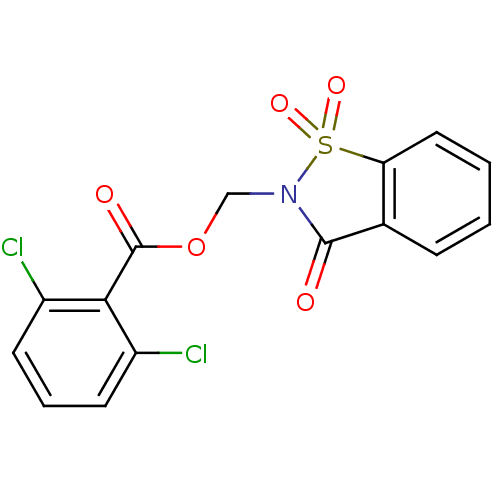 (2,6-Dichloro-benzoic acid 1,1,3-trioxo-1,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283362 (CHEMBL311049 | {(S)-1-[1-(4-Benzyloxy-benzyl)-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037811 (CHEMBL123016 | {(S)-1-[(S)-3-Benzylcarbamoyl-1-(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the ability to inhibit HIV-protease. | J Med Chem 37: 3684-92 (1994) BindingDB Entry DOI: 10.7270/Q2ZW1JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037811 (CHEMBL123016 | {(S)-1-[(S)-3-Benzylcarbamoyl-1-(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inactivation of purified HIV-1 protease in vitro | Bioorg Med Chem Lett 4: 241-246 (1994) Article DOI: 10.1016/S0960-894X(01)80122-0 BindingDB Entry DOI: 10.7270/Q2HQ3ZVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029697 (3-Chloro-2-(1,1,3-trioxo-1,3-dihydro-1lambda*6*-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065147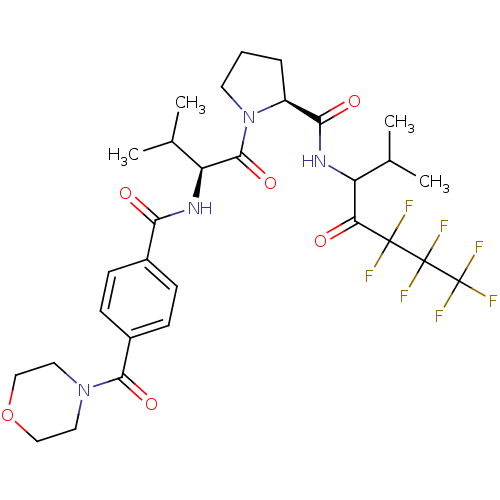 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037812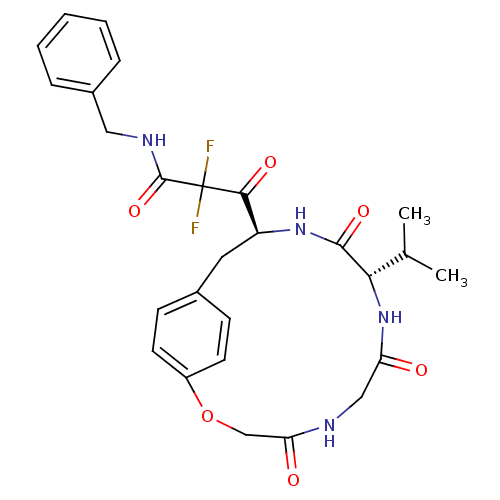 (CHEMBL331994 | MDL-104168 | N-Benzyl-2,2-difluoro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the ability to inhibit HIV-protease. | J Med Chem 37: 3684-92 (1994) BindingDB Entry DOI: 10.7270/Q2ZW1JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1027 total ) | Next | Last >> |