

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328774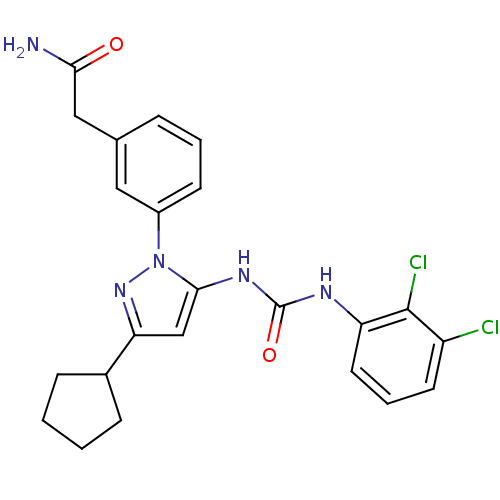 (2-(3-(3-cyclopentyl-5-(3-(2,3-dichlorophenyl)ureid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to unphosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328768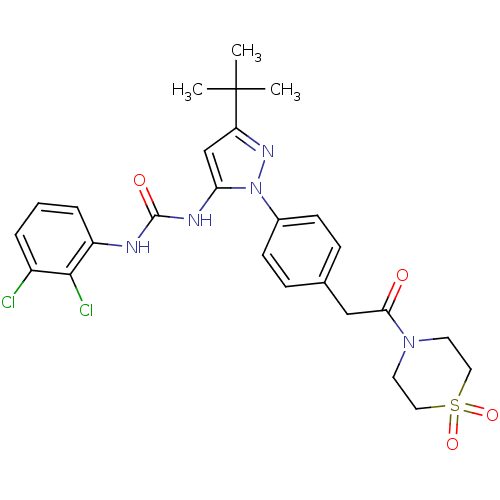 (1-(5-tert-Butyl-2-{4-[2-(1,1-dioxo-1lambda6-thiomo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328755 (2-(3-(3-tert-butyl-5-(3-(2,3-dichlorophenyl)ureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to unphosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328774 (2-(3-(3-cyclopentyl-5-(3-(2,3-dichlorophenyl)ureid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to phosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50328755 (2-(3-(3-tert-butyl-5-(3-(2,3-dichlorophenyl)ureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of CRAF | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328755 (2-(3-(3-tert-butyl-5-(3-(2,3-dichlorophenyl)ureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to phosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328773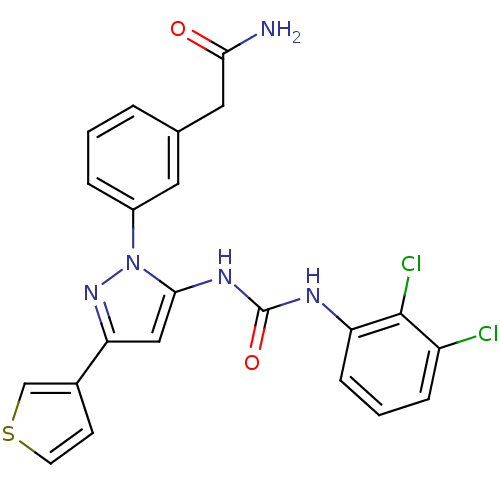 (2-(3-(5-(3-(2,3-dichlorophenyl)ureido)-3-(thiophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to unphosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328769 (2-(3-(3-tert-butyl-5-(3-naphthalen-1-ylureido)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to unphosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50328755 (2-(3-(3-tert-butyl-5-(3-(2,3-dichlorophenyl)ureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of BRAF | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328769 (2-(3-(3-tert-butyl-5-(3-naphthalen-1-ylureido)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to phosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328771 (2-(3-(5-(3-(2,3-dichlorophenyl)ureido)-3-(2-fluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to unphosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328766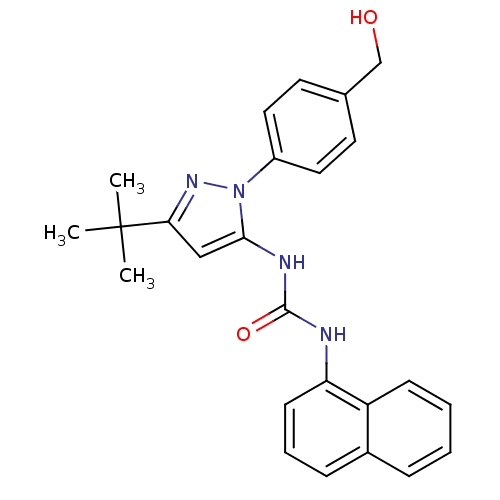 (1-(3-tert-butyl-1-(4-(hydroxymethyl)phenyl)-1H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328773 (2-(3-(5-(3-(2,3-dichlorophenyl)ureido)-3-(thiophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to phosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50073854 (2,10-dihydroxy-12-(3,4,5-trihydroxy-6-hydroxymethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE BioStructures Curated by ChEMBL | Assay Description Inhibition of topoisomerase I-DNA complex in trapping assay | J Med Chem 48: 2336-45 (2005) Article DOI: 10.1021/jm049146p BindingDB Entry DOI: 10.7270/Q2CF9QVT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328771 (2-(3-(5-(3-(2,3-dichlorophenyl)ureido)-3-(2-fluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to phosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328762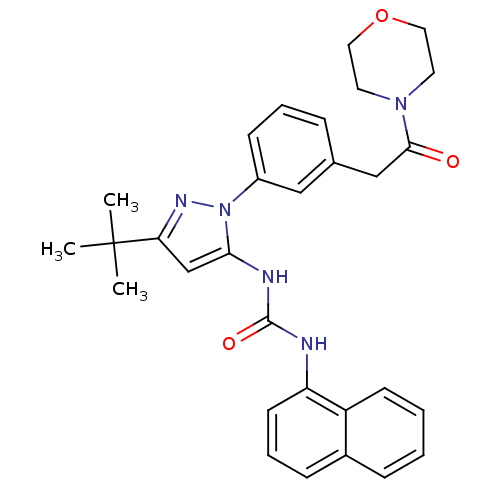 (1-(3-tert-butyl-1-(3-(2-morpholino-2-oxoethyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328772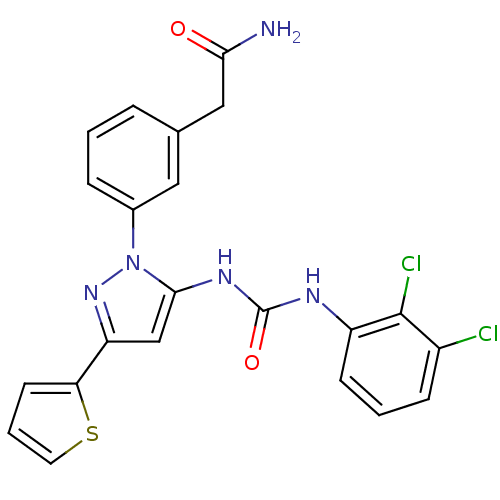 (2-(3-(5-(3-(2,3-dichlorophenyl)ureido)-3-(thiophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to unphosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328767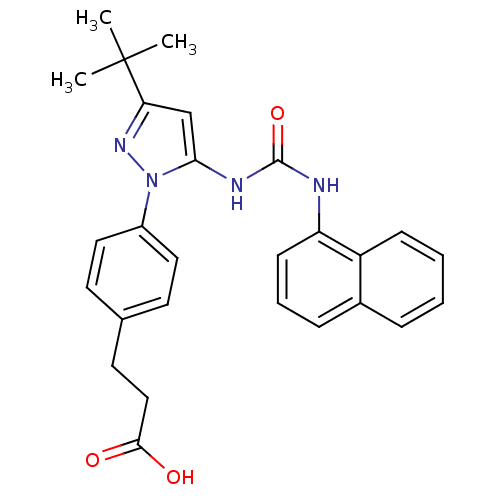 (3-(4-(3-tert-butyl-5-(3-naphthalen-1-ylureido)-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008935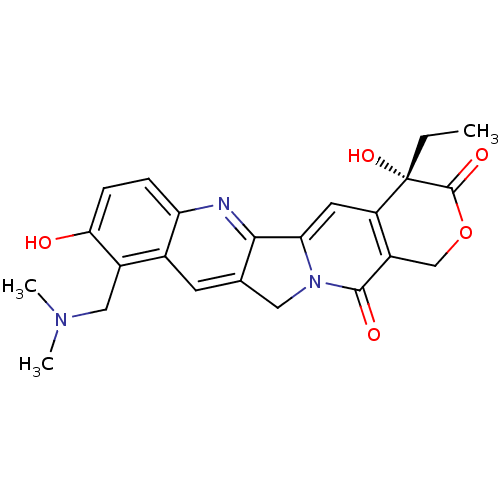 ((20S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE BioStructures Curated by ChEMBL | Assay Description Inhibition of topoisomerase I-DNA complex in trapping assay | J Med Chem 48: 2336-45 (2005) Article DOI: 10.1021/jm049146p BindingDB Entry DOI: 10.7270/Q2CF9QVT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50327187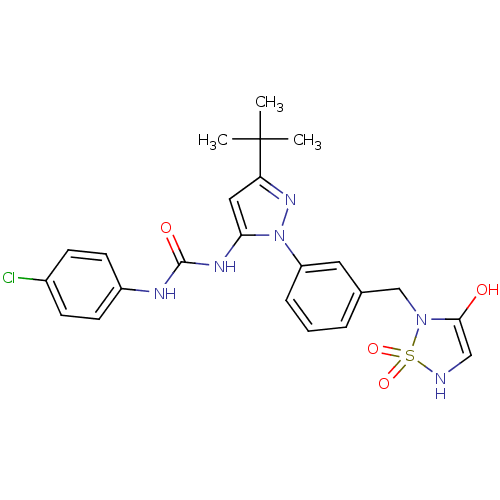 (1-{5-tert-Butyl-2-[3-(1,1,3-trioxo-1lambda*6*-[1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328772 (2-(3-(5-(3-(2,3-dichlorophenyl)ureido)-3-(thiophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to phosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328763 (2-(3-(3-tert-butyl-5-(3-(4-chlorophenyl)ureido)-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328770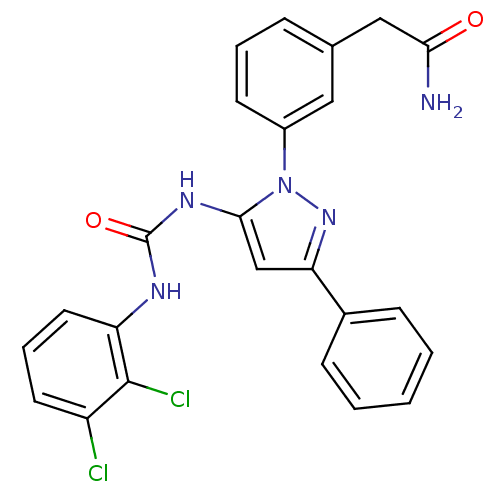 (2-(3-(5-(3-(2,3-dichlorophenyl)ureido)-3-phenyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to phosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 2 (Homo sapiens (Human)) | BDBM50328755 (2-(3-(3-tert-butyl-5-(3-(2,3-dichlorophenyl)ureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of EPHB2 | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486321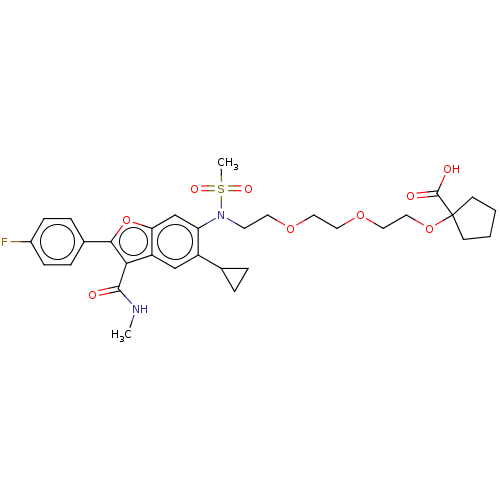 (US10947210, Compound 26-7A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486329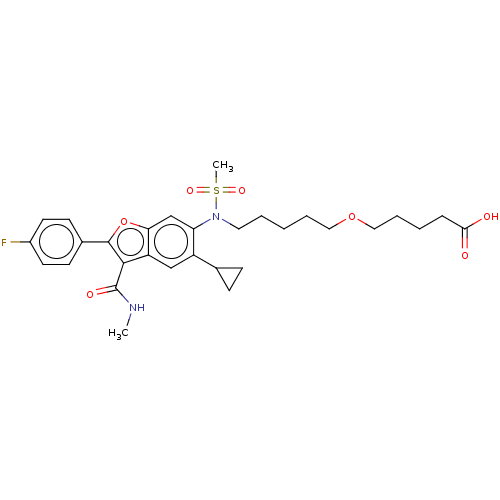 (US10947210, Compound 28-8A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486330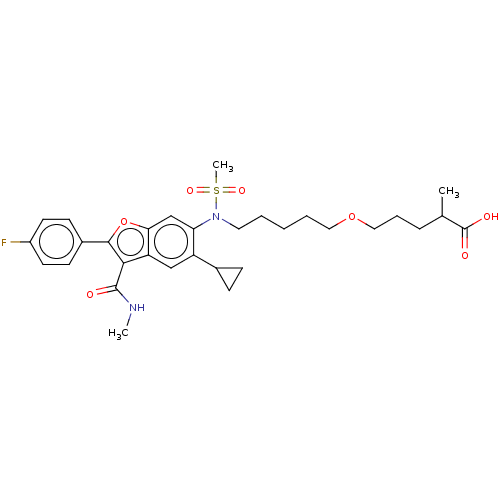 (US10947210, Compound 28-8B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486076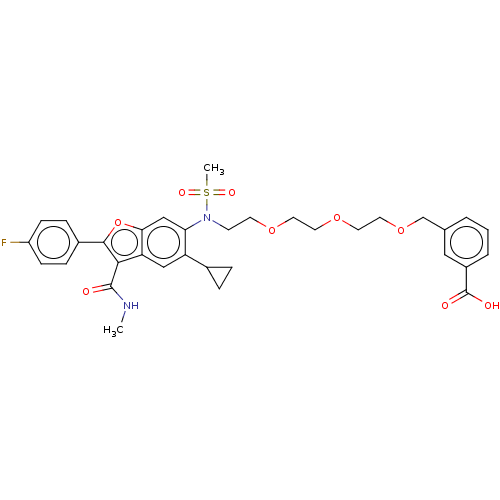 (US10947210, Compound 06-7F) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486194 (US10947210, Compound 11-6A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486200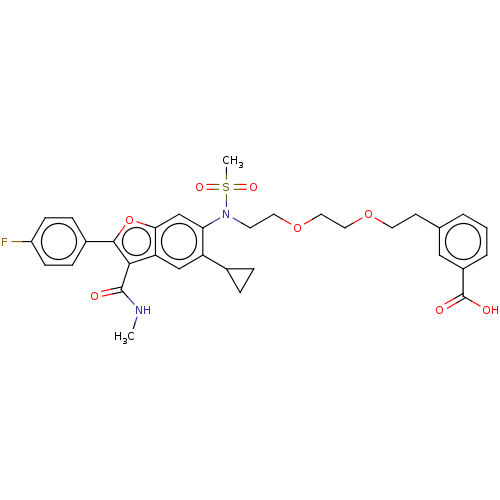 (US10947210, Compound 11-6B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486221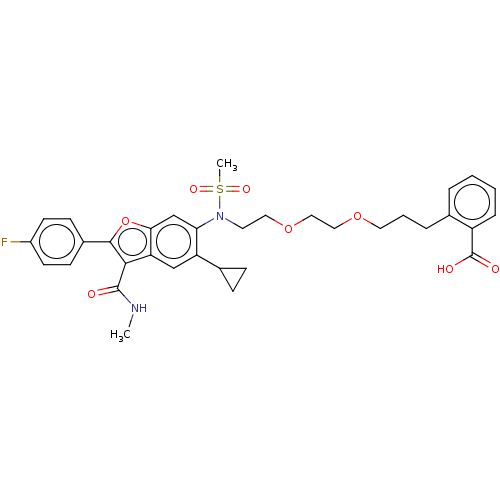 (US10947210, Compound 12-7A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486222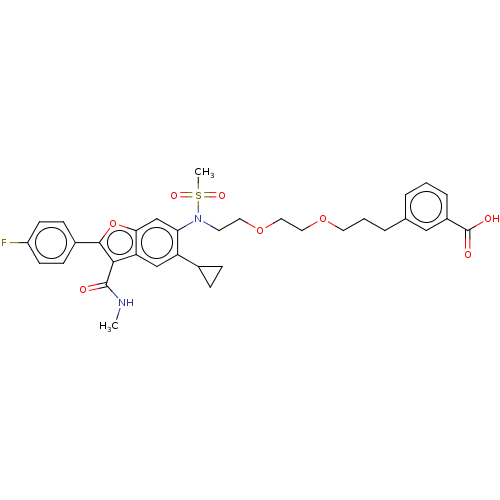 (US10947210, Compound 12-7B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486277 (US10947210, Compound 14-7A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486303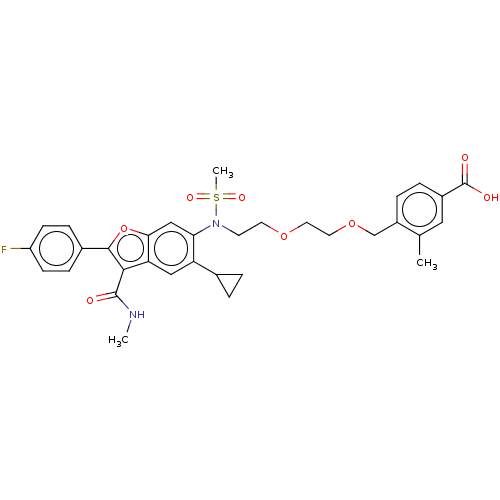 (US10947210, Compound 18-10B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486304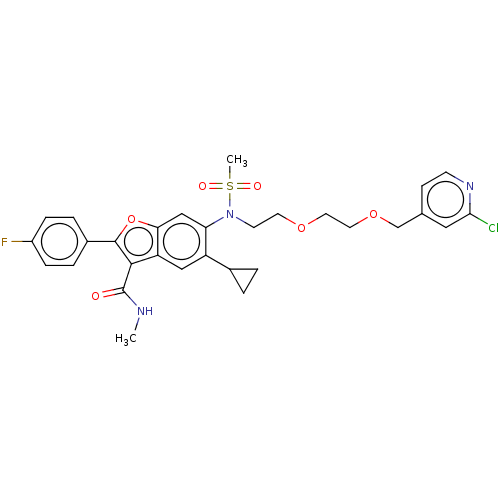 (US10947210, Compound 19-8A) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486305 (US10947210, Compound 19-8B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486307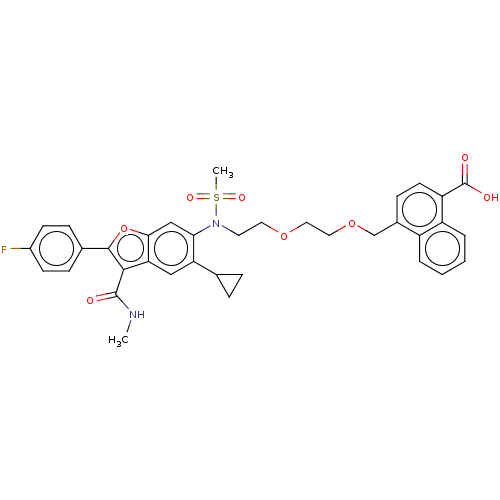 (US10947210, Compound 20-8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486308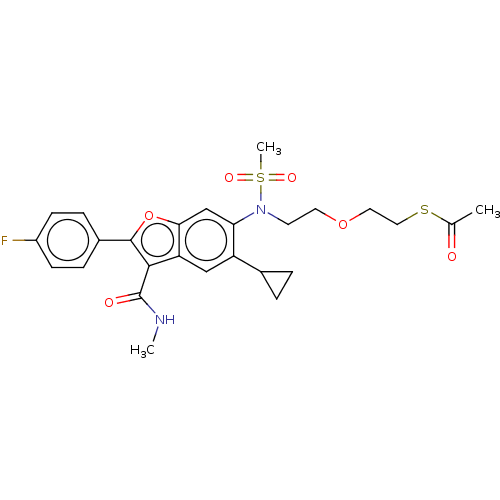 (US10947210, Compound 21-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328770 (2-(3-(5-(3-(2,3-dichlorophenyl)ureido)-3-phenyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Binding affinity to unphosphorylated p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328756 (1-(3-tert-butyl-1-(3-((1-methyl-3,5-dioxo-1,2,4-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008923 ((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE BioStructures Curated by ChEMBL | Assay Description Inhibition of topoisomerase I-DNA complex in trapping assay | J Med Chem 48: 2336-45 (2005) Article DOI: 10.1021/jm049146p BindingDB Entry DOI: 10.7270/Q2CF9QVT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328757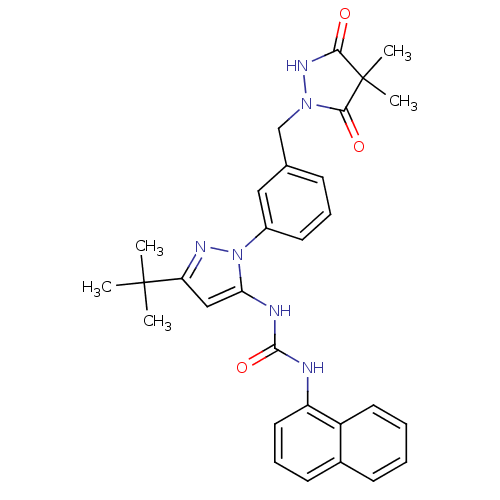 (1-(3-tert-butyl-1-(3-((4,4-dimethyl-3,5-dioxopyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50328759 (1-(1-(3-(2-aminoethyl)phenyl)-3-tert-butyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 508 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of p38alpha by fluoroprobe binding assay | Bioorg Med Chem Lett 20: 5793-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.134 BindingDB Entry DOI: 10.7270/Q2XD11WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486333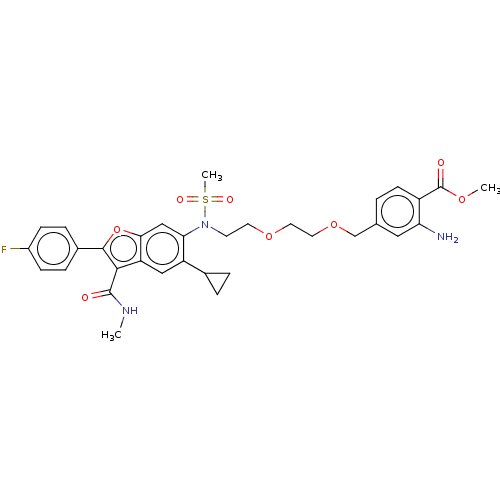 (US10947210, Compound 29-9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486334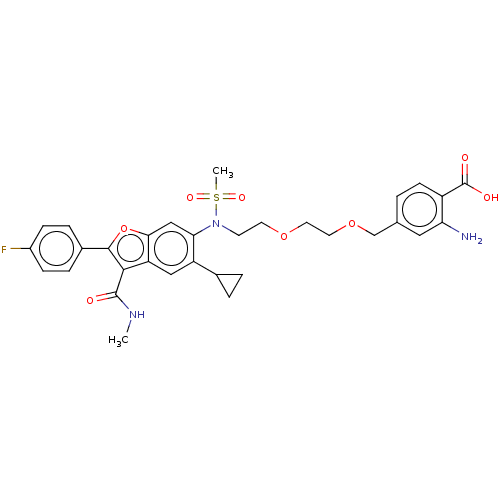 (US10947210, Compound 29-10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486332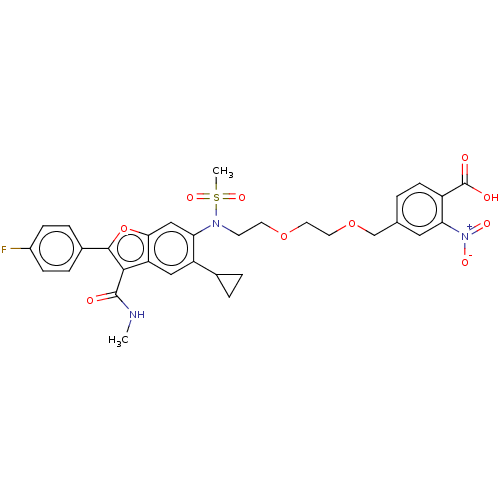 (US10947210, Compound 29-8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486331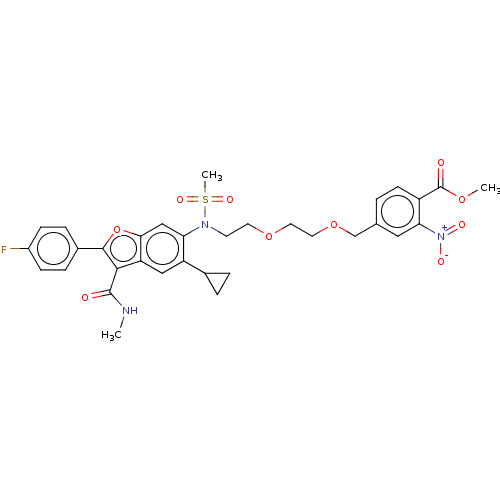 (US10947210, Compound 29-7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486327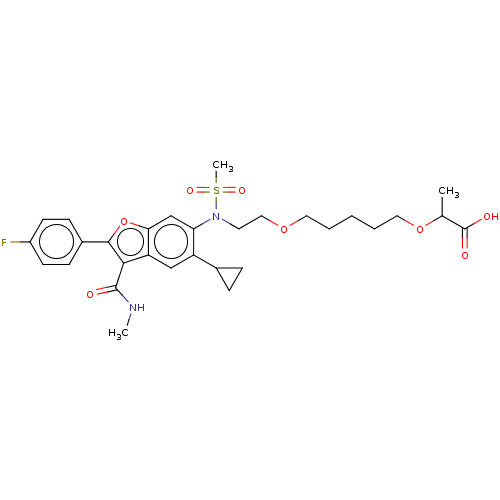 (US10947210, Compound 27-9B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486317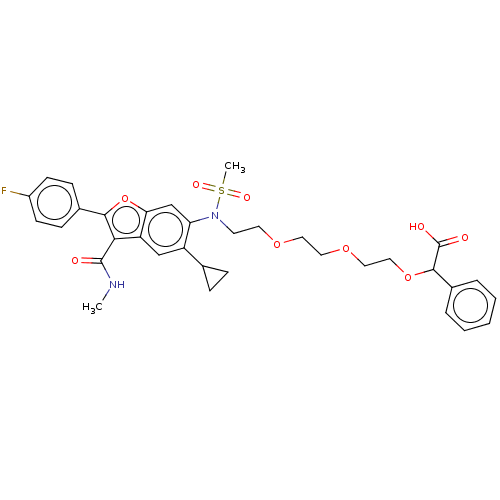 (US10947210, Compound 25-5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM486322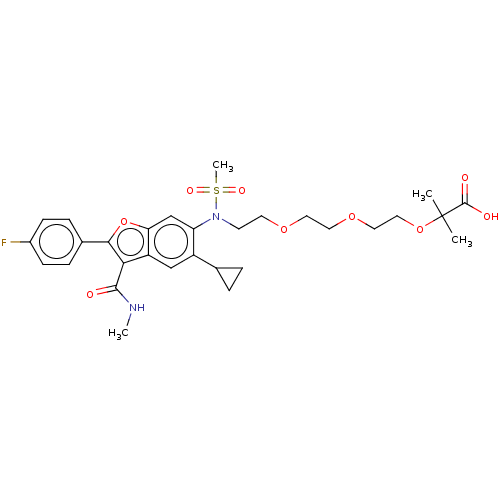 (US10947210, Compound 26-7B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
COCRYSTAL PHARMA, INC. US Patent | Assay Description The reaction mixtures consisted of 50 mM Hepes-KOH, pH 7.5, 5 mM MgCl2, 5 mM DTT, 2% glycerol, 0.01% Triton® X-100, 0.5 uM polyA:U16 substrate, purif... | US Patent US10947210 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 201 total ) | Next | Last >> |