 Found 47 hits with Last Name = 'foster' and Initial = 'm'
Found 47 hits with Last Name = 'foster' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50354849
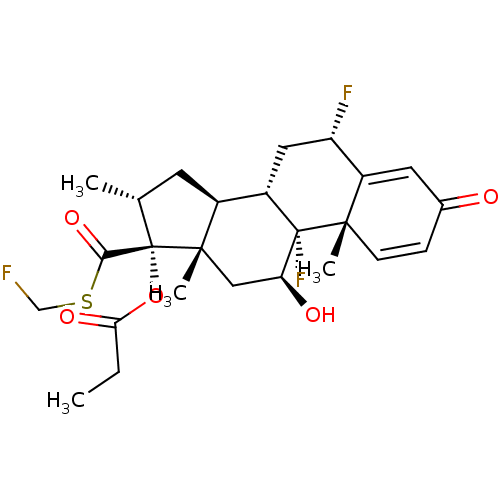
(CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...)Show SMILES CCC(=O)O[C@@]1([C@H](C)C[C@H]2[C@@H]3C[C@H](F)C4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)SCF |r,c:18,t:14| Show InChI InChI=1S/C25H31F3O5S/c1-5-20(31)33-25(21(32)34-12-26)13(2)8-15-16-10-18(27)17-9-14(29)6-7-22(17,3)24(16,28)19(30)11-23(15,25)4/h6-7,9,13,15-16,18-19,30H,5,8,10-12H2,1-4H3/t13-,15+,16+,18+,19+,22+,23+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 568-74 (2005)
Article DOI: 10.1124/jpet.105.085217
BindingDB Entry DOI: 10.7270/Q2CC0Z7J |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM86696
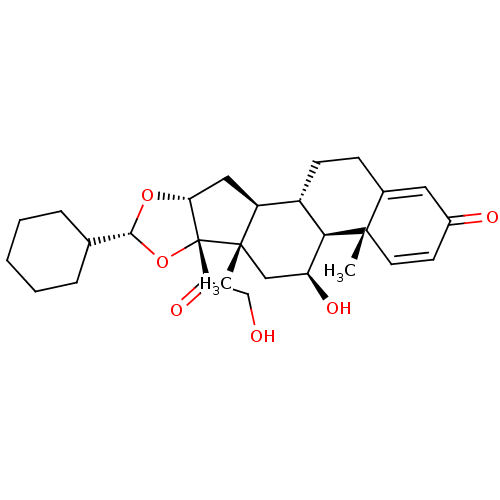
(des-CIC | desisobutyryl-ciclesonide)Show SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=CC(=O)C=C[C@]34C)[C@@H]1C[C@H]1O[C@H](O[C@@]21C(=O)CO)C1CCCCC1 |c:13,t:9| Show InChI InChI=1S/C28H38O6/c1-26-11-10-18(30)12-17(26)8-9-19-20-13-23-28(22(32)15-29,27(20,2)14-21(31)24(19)26)34-25(33-23)16-6-4-3-5-7-16/h10-12,16,19-21,23-25,29,31H,3-9,13-15H2,1-2H3/t19-,20-,21-,23+,24+,25+,26-,27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 568-74 (2005)
Article DOI: 10.1124/jpet.105.085217
BindingDB Entry DOI: 10.7270/Q2CC0Z7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 568-74 (2005)
Article DOI: 10.1124/jpet.105.085217
BindingDB Entry DOI: 10.7270/Q2CC0Z7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50219254

(CHEMBL347592)Show SMILES C[C@@]1(CO[C@@H](OC1)c1nc(c([nH]1)-c1ccnc(NCc2ccccc2)n1)-c1ccc(F)cc1)C(=O)N1CCOCC1 |wU:4.7,1.0,wD:1.37,(10.24,-.19,;9.47,-1.52,;8.56,-.28,;7.03,-.44,;6.42,-1.85,;7.31,-3.09,;8.84,-2.94,;4.88,-2.01,;4.11,-3.34,;2.61,-3.03,;2.45,-1.5,;3.85,-.87,;1.1,-.73,;1.1,.81,;-.23,1.58,;-1.56,.81,;-1.56,-.73,;-2.89,-1.5,;-4.22,-.73,;-4.22,.81,;-5.55,1.58,;-5.55,3.1,;-4.22,3.89,;-2.89,3.1,;-2.89,1.56,;-.23,-1.5,;1.47,-4.07,;,-3.59,;-1.14,-4.62,;-.82,-6.12,;-1.96,-7.16,;.65,-6.59,;1.8,-5.56,;10.8,-2.29,;10.8,-3.83,;12.13,-1.52,;13.46,-2.31,;14.79,-1.54,;14.8,,;13.47,.77,;12.13,.02,)| Show InChI InChI=1S/C30H31FN6O4/c1-30(28(38)37-13-15-39-16-14-37)18-40-27(41-19-30)26-35-24(21-7-9-22(31)10-8-21)25(36-26)23-11-12-32-29(34-23)33-17-20-5-3-2-4-6-20/h2-12,27H,13-19H2,1H3,(H,35,36)(H,32,33,34)/t27-,30- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibition of murine Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 11: 693-6 (2001)
BindingDB Entry DOI: 10.7270/Q2F191WG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50219252
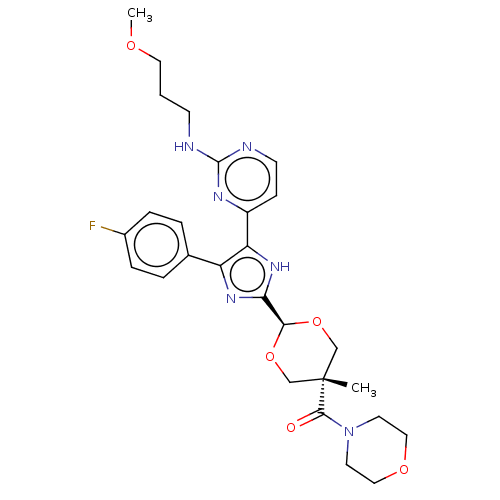
(CHEMBL159932)Show SMILES COCCCNc1nccc(n1)-c1[nH]c(nc1-c1ccc(F)cc1)[C@H]1OC[C@@](C)(CO1)C(=O)N1CCOCC1 |wU:24.26,27.30,wD:27.34,(-7.57,.44,;-6.24,-.34,;-4.9,.42,;-3.57,-.35,;-2.24,.42,;-.9,-.35,;.43,.42,;.43,1.96,;1.76,2.74,;3.12,1.96,;3.12,.42,;1.76,-.35,;4.45,-.35,;5.85,.28,;6.91,-.87,;6.13,-2.2,;4.61,-1.88,;3.47,-2.92,;3.8,-4.42,;2.65,-5.45,;1.18,-4.98,;.03,-6.02,;.85,-3.47,;2,-2.44,;8.43,-.7,;9.06,.72,;10.58,.88,;11.49,-.37,;12.26,.96,;10.86,-1.78,;9.34,-1.94,;12.82,-1.15,;12.82,-2.69,;14.15,-.37,;14.15,1.17,;15.51,1.93,;16.84,1.16,;16.82,-.38,;15.49,-1.15,)| Show InChI InChI=1S/C27H33FN6O5/c1-27(25(35)34-11-14-37-15-12-34)16-38-24(39-17-27)23-32-21(18-4-6-19(28)7-5-18)22(33-23)20-8-10-30-26(31-20)29-9-3-13-36-2/h4-8,10,24H,3,9,11-17H2,1-2H3,(H,32,33)(H,29,30,31)/t24-,27- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibition of murine Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 11: 693-6 (2001)
BindingDB Entry DOI: 10.7270/Q2F191WG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50219253

(CHEMBL161197)Show SMILES C[C@@]1(CO[C@@H](OC1)c1nc(c([nH]1)-c1ccnc(NC2CC2)n1)-c1ccc(F)cc1)C(=O)N1CCOCC1 |wU:4.7,1.0,wD:1.33,(10.32,.96,;9.56,-.37,;8.65,.88,;7.12,.72,;6.51,-.7,;7.4,-1.94,;8.93,-1.78,;4.98,-.86,;4.21,-2.19,;2.69,-1.87,;2.53,-.35,;3.94,.28,;1.2,.42,;1.2,1.96,;-.14,2.73,;-1.47,1.96,;-1.47,.42,;-2.81,-.35,;-4.14,.42,;-5.64,.02,;-4.73,1.97,;-.14,-.35,;1.55,-2.92,;1.87,-4.41,;.75,-5.43,;-.72,-4.97,;-1.87,-6,;-1.05,-3.46,;.09,-2.43,;10.89,-1.14,;10.89,-2.68,;12.22,-.37,;13.55,-1.15,;14.88,-.38,;14.89,1.16,;13.56,1.93,;12.21,1.17,)| Show InChI InChI=1S/C26H29FN6O4/c1-26(24(34)33-10-12-35-13-11-33)14-36-23(37-15-26)22-31-20(16-2-4-17(27)5-3-16)21(32-22)19-8-9-28-25(30-19)29-18-6-7-18/h2-5,8-9,18,23H,6-7,10-15H2,1H3,(H,31,32)(H,28,29,30)/t23-,26- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibition of murine Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 11: 693-6 (2001)
BindingDB Entry DOI: 10.7270/Q2F191WG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Mus musculus-Mus musculus (mouse)) | BDBM50219251

(CHEMBL423071)Show SMILES C[C@@]1(CO[C@@H](OC1)c1nc(c([nH]1)-c1ccnc(Nc2ccccc2)n1)-c1ccc(F)cc1)C(=O)N1CCOCC1 |wU:4.7,1.0,wD:1.36,(10.99,1.09,;10.19,-.24,;9.3,1.03,;7.76,.9,;7.1,-.5,;7.99,-1.76,;9.53,-1.65,;5.58,-.63,;4.85,-1.97,;3.33,-1.72,;3.12,-.17,;4.5,.49,;1.79,.59,;1.79,2.15,;.43,2.92,;-.9,2.13,;-.9,.59,;-2.24,-.21,;-3.6,.55,;-3.62,2.08,;-4.95,2.83,;-6.29,2.04,;-6.26,.51,;-4.91,-.24,;.43,-.17,;2.28,-2.84,;2.72,-4.31,;1.65,-5.43,;.17,-5.08,;-.88,-6.21,;-.27,-3.61,;.78,-2.49,;11.5,-1.04,;11.48,-2.61,;12.86,-.29,;14.19,-1.08,;15.53,-.31,;15.55,1.24,;14.22,2.03,;12.88,1.26,)| Show InChI InChI=1S/C29H29FN6O4/c1-29(27(37)36-13-15-38-16-14-36)17-39-26(40-18-29)25-34-23(19-7-9-20(30)10-8-19)24(35-25)22-11-12-31-28(33-22)32-21-5-3-2-4-6-21/h2-12,26H,13-18H2,1H3,(H,34,35)(H,31,32,33)/t26-,29- | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38 kinase (40 ng/well) was determined in mouse |
Bioorg Med Chem Lett 11: 693-6 (2001)
BindingDB Entry DOI: 10.7270/Q2F191WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132421
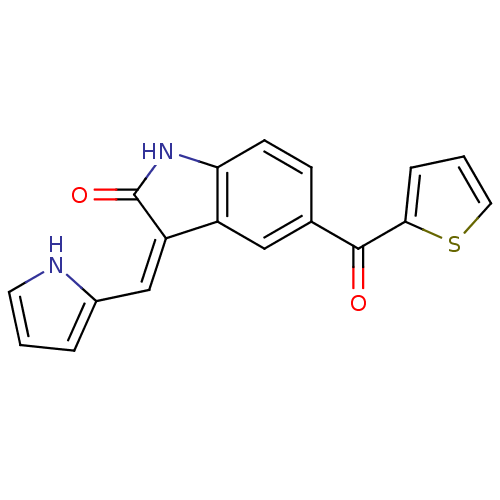
(3-[1-(1H-Pyrrol-2-yl)-meth-(Z)-ylidene]-5-(thiophe...)Show SMILES O=C(c1cccs1)c1ccc2NC(=O)\C(=C/c3ccc[nH]3)c2c1 Show InChI InChI=1S/C18H12N2O2S/c21-17(16-4-2-8-23-16)11-5-6-15-13(9-11)14(18(22)20-15)10-12-3-1-7-19-12/h1-10,19H,(H,20,22)/b14-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132420
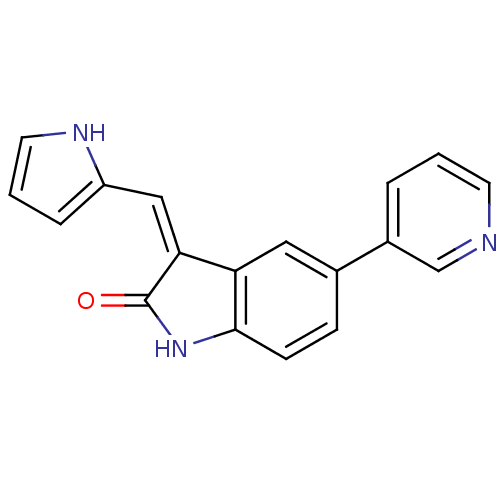
(3-((1H-pyrrol-2-yl)methylene)-5-(pyridin-3-yl)indo...)Show InChI InChI=1S/C18H13N3O/c22-18-16(10-14-4-2-8-20-14)15-9-12(5-6-17(15)21-18)13-3-1-7-19-11-13/h1-11,20H,(H,21,22)/b16-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
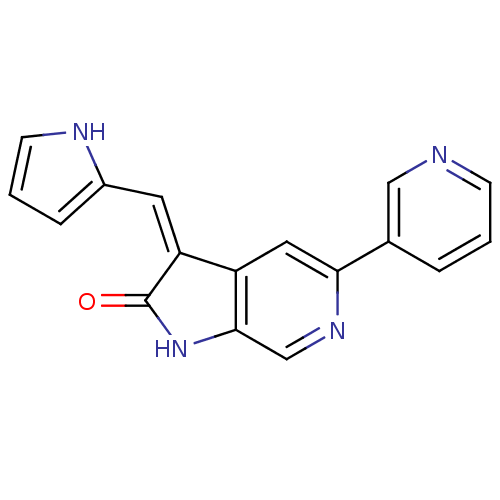
(Homo sapiens (Human)) | BDBM50132427
(5-Pyridin-3-yl-3-[1-(1H-pyrrol-2-yl)-meth-(Z)-ylid...)Show InChI InChI=1S/C17H12N4O/c22-17-14(7-12-4-2-6-19-12)13-8-15(20-10-16(13)21-17)11-3-1-5-18-9-11/h1-10,19H,(H,21,22)/b14-7- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50174302

(CHEMBL200283 | N-((S)-1-((S)-1-(4-bromophenyl)-4-(...)Show SMILES CCNC(=O)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)[C@@H](NC(=O)CCCCC1CCSS1)C(C)C Show InChI InChI=1S/C25H36BrN3O4S2/c1-4-27-25(33)23(31)20(15-17-9-11-18(26)12-10-17)28-24(32)22(16(2)3)29-21(30)8-6-5-7-19-13-14-34-35-19/h9-12,16,19-20,22H,4-8,13-15H2,1-3H3,(H,27,33)(H,28,32)(H,29,30)/t19?,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B |
Bioorg Med Chem Lett 15: 5176-81 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.064
BindingDB Entry DOI: 10.7270/Q23N2466 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50547626

(CHEMBL4760821)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(=O)NCCOCCOCC(=O)N[C@@H](CCCCNC(=O)c1ccc3c(c1)C(=O)OC31c3ccc4cc(O)ccc4c3Oc3c1ccc1cc(O)ccc31)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(C)C |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Grb2-SH2 domain (unknown origin) expressed in Escherichia coli BL21 (DE23) after 1 hr by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115711
BindingDB Entry DOI: 10.7270/Q2FB56JN |
More data for this
Ligand-Target Pair | |
Cathepsin B
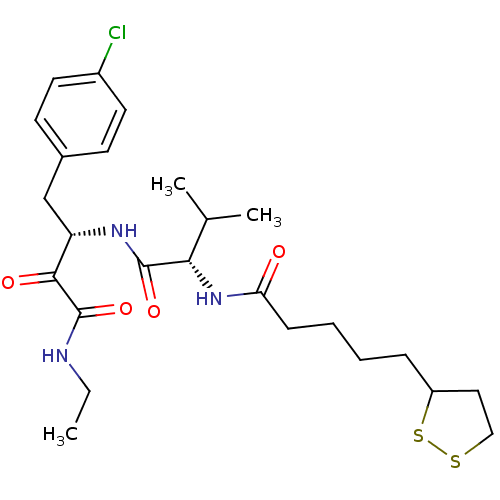
(Homo sapiens (Human)) | BDBM50174304
(CHEMBL198962 | N-((S)-1-((S)-1-(4-chlorophenyl)-4-...)Show SMILES CCNC(=O)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@@H](NC(=O)CCCCC1CCSS1)C(C)C Show InChI InChI=1S/C25H36ClN3O4S2/c1-4-27-25(33)23(31)20(15-17-9-11-18(26)12-10-17)28-24(32)22(16(2)3)29-21(30)8-6-5-7-19-13-14-34-35-19/h9-12,16,19-20,22H,4-8,13-15H2,1-3H3,(H,27,33)(H,28,32)(H,29,30)/t19?,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B |
Bioorg Med Chem Lett 15: 5176-81 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.064
BindingDB Entry DOI: 10.7270/Q23N2466 |
More data for this
Ligand-Target Pair | |
Cathepsin B
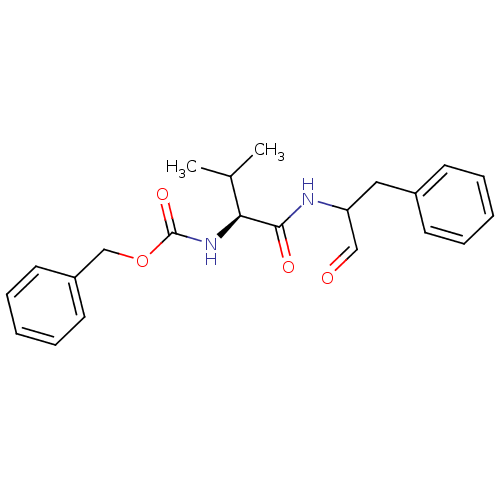
(Homo sapiens (Human)) | BDBM50073850
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B |
Bioorg Med Chem Lett 15: 5176-81 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.064
BindingDB Entry DOI: 10.7270/Q23N2466 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50174313

((S)-2-(5-[1,2]dithiolan-3-yl-pentanoylamino)-4-met...)Show SMILES CCNC(=O)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CCCCC1CCSS1 Show InChI InChI=1S/C26H38ClN3O4S2/c1-4-28-26(34)24(32)21(16-18-9-11-19(27)12-10-18)30-25(33)22(15-17(2)3)29-23(31)8-6-5-7-20-13-14-35-36-20/h9-12,17,20-22H,4-8,13-16H2,1-3H3,(H,28,34)(H,29,31)(H,30,33)/t20?,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B |
Bioorg Med Chem Lett 15: 5176-81 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.064
BindingDB Entry DOI: 10.7270/Q23N2466 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132422
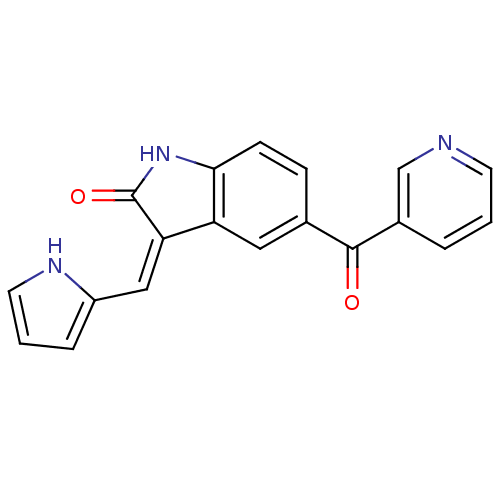
(5-(Pyridine-3-carbonyl)-3-[1-(1H-pyrrol-2-yl)-meth...)Show SMILES O=C(c1cccnc1)c1ccc2NC(=O)\C(=C/c3ccc[nH]3)c2c1 Show InChI InChI=1S/C19H13N3O2/c23-18(13-3-1-7-20-11-13)12-5-6-17-15(9-12)16(19(24)22-17)10-14-4-2-8-21-14/h1-11,21H,(H,22,24)/b16-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132428
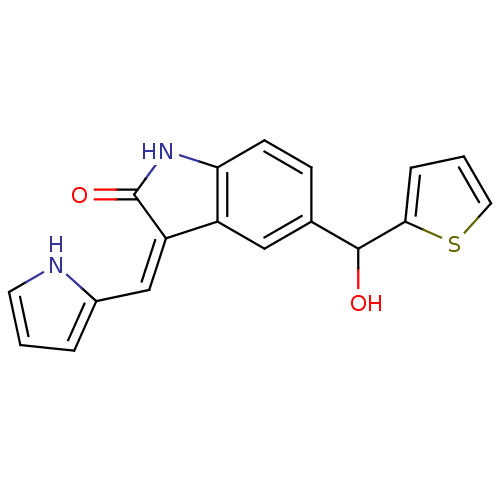
(5-(Hydroxy-thiophen-2-yl-methyl)-3-[1-(1H-pyrrol-2...)Show SMILES OC(c1cccs1)c1ccc2NC(=O)\C(=C/c3ccc[nH]3)c2c1 Show InChI InChI=1S/C18H14N2O2S/c21-17(16-4-2-8-23-16)11-5-6-15-13(9-11)14(18(22)20-15)10-12-3-1-7-19-12/h1-10,17,19,21H,(H,20,22)/b14-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132417
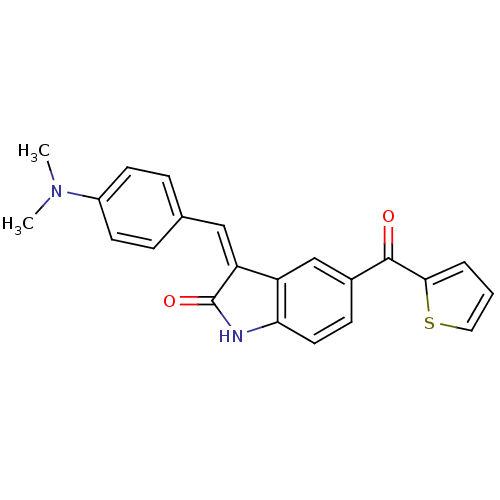
(3-[1-(4-Dimethylamino-phenyl)-meth-(Z)-ylidene]-5-...)Show SMILES CN(C)c1ccc(\C=C2/C(=O)Nc3ccc(cc23)C(=O)c2cccs2)cc1 Show InChI InChI=1S/C22H18N2O2S/c1-24(2)16-8-5-14(6-9-16)12-18-17-13-15(7-10-19(17)23-22(18)26)21(25)20-4-3-11-27-20/h3-13H,1-2H3,(H,23,26)/b18-12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132423

(2-Oxo-3-[1-(1H-pyrrol-2-yl)-meth-(Z)-ylidene]-2,3-...)Show SMILES CN(C)C(=O)c1ccc2NC(=O)\C(=C/c3ccc[nH]3)c2c1 Show InChI InChI=1S/C16H15N3O2/c1-19(2)16(21)10-5-6-14-12(8-10)13(15(20)18-14)9-11-4-3-7-17-11/h3-9,17H,1-2H3,(H,18,20)/b13-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM17032

((3Z)-2-oxo-3-(1H-pyrrol-2-ylmethylidene)-2,3-dihyd...)Show InChI InChI=1S/C14H10N2O3/c17-13-11(7-9-2-1-5-15-9)10-6-8(14(18)19)3-4-12(10)16-13/h1-7,15H,(H,16,17)(H,18,19)/b11-7- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132419

(5-Pyridin-3-yl-3-[1-(1H-pyrrol-2-yl)-meth-(Z)-ylid...)Show InChI InChI=1S/C17H12N4O/c22-17-13(9-12-4-2-8-19-12)16-15(21-17)6-5-14(20-16)11-3-1-7-18-10-11/h1-10,19H,(H,21,22)/b13-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132424

(3-[2-Oxo-2-(1H-pyrrol-2-yl)-eth-(Z)-ylidene]-5-(th...)Show SMILES O=C(\C=C1/C(=O)Nc2ccc(cc12)C(=O)c1cccs1)c1ccc[nH]1 Show InChI InChI=1S/C19H12N2O3S/c22-16(15-3-1-7-20-15)10-13-12-9-11(5-6-14(12)21-19(13)24)18(23)17-4-2-8-25-17/h1-10,20H,(H,21,24)/b13-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132418

(5-Pyridin-3-yl-3-[1-(1H-pyrrol-2-yl)-meth-(Z)-ylid...)Show InChI InChI=1S/C17H12N4O/c22-17-15(8-13-4-2-6-19-13)14-7-12(10-20-16(14)21-17)11-3-1-5-18-9-11/h1-10,19H,(H,20,21,22)/b15-8- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50547624
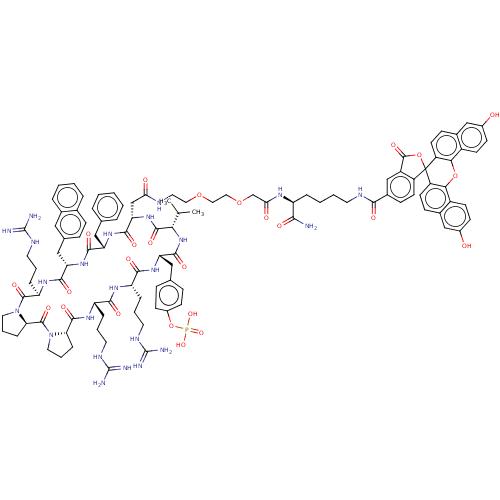
(CHEMBL4761634)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc3ccccc3c1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(=O)NCCOCCOCC(=O)N[C@@H](CCCCNC(=O)c1ccc3c(c1)C(=O)OC31c3ccc4cc(O)ccc4c3Oc3c1ccc1cc(O)ccc31)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(C)C |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Grb2-SH2 domain (unknown origin) expressed in Escherichia coli BL21 (DE23) after 1 hr by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115711
BindingDB Entry DOI: 10.7270/Q2FB56JN |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50174317

((S)-2-(5-[1,2]dithiolan-3-yl-pentanoylamino)-4-met...)Show SMILES CCNC(=O)C(=O)C(Cc1ccc(Br)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CCCCC1CCSS1 Show InChI InChI=1S/C26H38BrN3O4S2/c1-4-28-26(34)24(32)21(16-18-9-11-19(27)12-10-18)30-25(33)22(15-17(2)3)29-23(31)8-6-5-7-20-13-14-35-36-20/h9-12,17,20-22H,4-8,13-16H2,1-3H3,(H,28,34)(H,29,31)(H,30,33)/t20?,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Santhera Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B |
Bioorg Med Chem Lett 15: 5176-81 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.064
BindingDB Entry DOI: 10.7270/Q23N2466 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132426
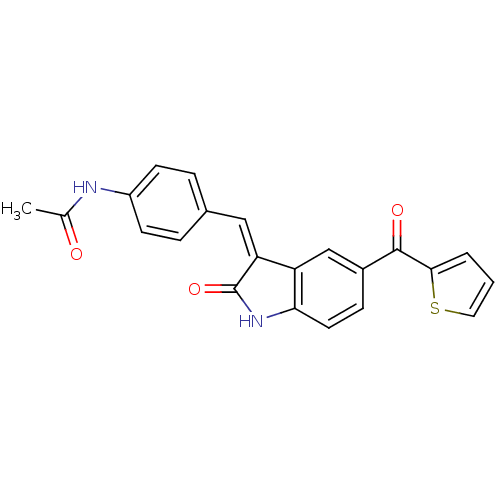
(CHEMBL104062 | N-{4-[2-Oxo-5-(thiophene-2-carbonyl...)Show SMILES CC(=O)Nc1ccc(\C=C2/C(=O)Nc3ccc(cc23)C(=O)c2cccs2)cc1 Show InChI InChI=1S/C22H16N2O3S/c1-13(25)23-16-7-4-14(5-8-16)11-18-17-12-15(6-9-19(17)24-22(18)27)21(26)20-3-2-10-28-20/h2-12H,1H3,(H,23,25)(H,24,27)/b18-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50065287
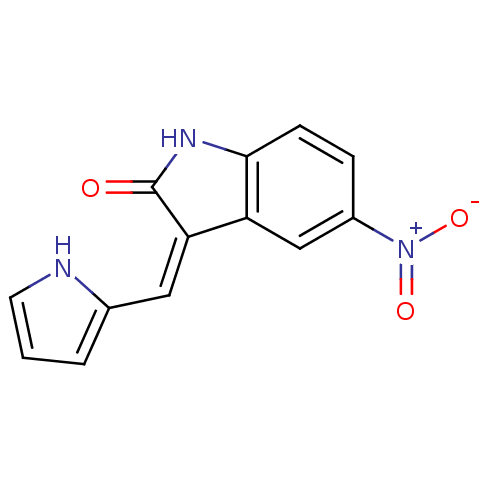
(5-Nitro-3-(1H-pyrrol-2-ylmethylene)-1,3-dihydro-in...)Show SMILES [O-][N+](=O)c1ccc2NC(=O)\C(=C/c3ccc[nH]3)c2c1 Show InChI InChI=1S/C13H9N3O3/c17-13-11(6-8-2-1-5-14-8)10-7-9(16(18)19)3-4-12(10)15-13/h1-7,14H,(H,15,17)/b11-6- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132429

(CHEMBL104409 | N-{2-Oxo-3-[1-(1H-pyrrol-2-yl)-meth...)Show SMILES O=C(Nc1ccc2NC(=O)\C(=C/c3ccc[nH]3)c2c1)c1ccccc1 Show InChI InChI=1S/C20H15N3O2/c24-19(13-5-2-1-3-6-13)22-15-8-9-18-16(12-15)17(20(25)23-18)11-14-7-4-10-21-14/h1-12,21H,(H,22,24)(H,23,25)/b17-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM17015

((3Z)-3-(1H-pyrrol-2-ylmethylidene)-2,3-dihydro-1H-...)Show InChI InChI=1S/C13H10N2O/c16-13-11(8-9-4-3-7-14-9)10-5-1-2-6-12(10)15-13/h1-8,14H,(H,15,16)/b11-8- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491257
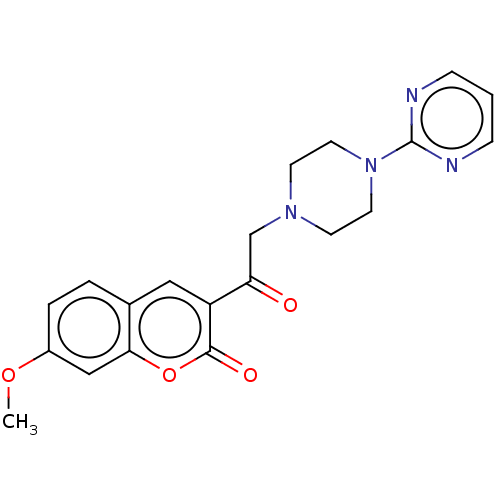
(CHEMBL2381384)Show SMILES COc1ccc2cc(C(=O)CN3CCN(CC3)c3ncccn3)c(=O)oc2c1 Show InChI InChI=1S/C20H20N4O4/c1-27-15-4-3-14-11-16(19(26)28-18(14)12-15)17(25)13-23-7-9-24(10-8-23)20-21-5-2-6-22-20/h2-6,11-12H,7-10,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta ((1 to 42) (unknown origin) oligomer assembly after 30 mins by biotinyl-streptavidin assay |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50491252

(CHEMBL2381401)Show SMILES COc1ccc2cc(C(=O)CN3CCN(Cc4ccccc4)CC3)c(=O)oc2c1 Show InChI InChI=1S/C23H24N2O4/c1-28-19-8-7-18-13-20(23(27)29-22(18)14-19)21(26)16-25-11-9-24(10-12-25)15-17-5-3-2-4-6-17/h2-8,13-14H,9-12,15-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Ellman method |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Ellman method |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132431

(3-[1-Phenyl-meth-(Z)-ylidene]-5-(thiophene-2-carbo...)Show SMILES O=C(c1cccs1)c1ccc2NC(=O)\C(=C/c3ccccc3)c2c1 Show InChI InChI=1S/C20H13NO2S/c22-19(18-7-4-10-24-18)14-8-9-17-15(12-14)16(20(23)21-17)11-13-5-2-1-3-6-13/h1-12H,(H,21,23)/b16-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491257

(CHEMBL2381384)Show SMILES COc1ccc2cc(C(=O)CN3CCN(CC3)c3ncccn3)c(=O)oc2c1 Show InChI InChI=1S/C20H20N4O4/c1-27-15-4-3-14-11-16(19(26)28-18(14)12-15)17(25)13-23-7-9-24(10-8-23)20-21-5-2-6-22-20/h2-6,11-12H,7-10,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta (1 to 42) (unknown origin) oligomer dissociation after 16 to 18 hrs by ELISA |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132425
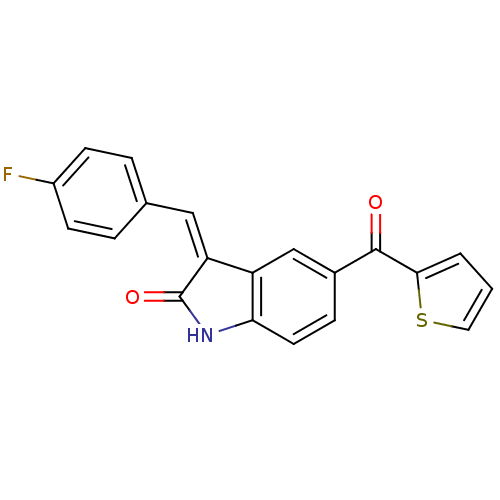
(3-[1-(4-Fluoro-phenyl)-meth-(Z)-ylidene]-5-(thioph...)Show SMILES Fc1ccc(\C=C2/C(=O)Nc3ccc(cc23)C(=O)c2cccs2)cc1 Show InChI InChI=1S/C20H12FNO2S/c21-14-6-3-12(4-7-14)10-16-15-11-13(5-8-17(15)22-20(16)24)19(23)18-2-1-9-25-18/h1-11H,(H,22,24)/b16-10- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491254
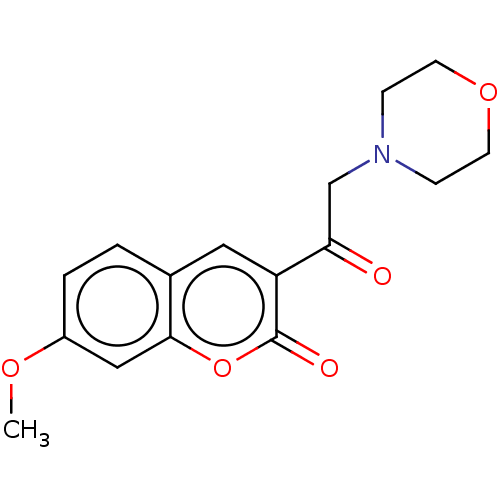
(CHEMBL2381385)Show InChI InChI=1S/C16H17NO5/c1-20-12-3-2-11-8-13(16(19)22-15(11)9-12)14(18)10-17-4-6-21-7-5-17/h2-3,8-9H,4-7,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta (1 to 42) (unknown origin) oligomer dissociation after 16 to 18 hrs by ELISA |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491253
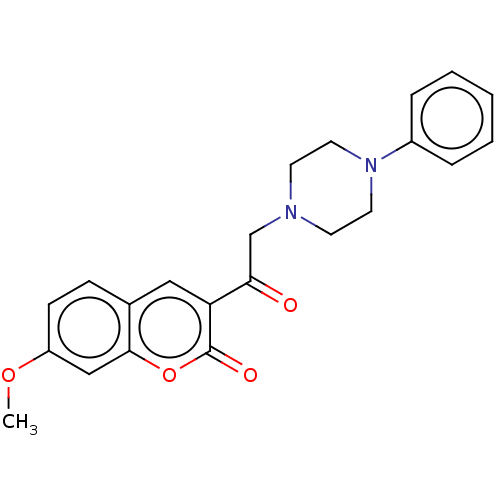
(CHEMBL2381403)Show SMILES COc1ccc2cc(C(=O)CN3CCN(CC3)c3ccccc3)c(=O)oc2c1 Show InChI InChI=1S/C22H22N2O4/c1-27-18-8-7-16-13-19(22(26)28-21(16)14-18)20(25)15-23-9-11-24(12-10-23)17-5-3-2-4-6-17/h2-8,13-14H,9-12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta (1 to 42) (unknown origin) oligomer dissociation after 16 to 18 hrs by ELISA |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50132430

(3-[1-(4-Methanesulfonyl-phenyl)-meth-(Z)-ylidene]-...)Show SMILES CS(=O)(=O)c1ccc(\C=C2/C(=O)Nc3ccc(cc23)C(=O)c2cccs2)cc1 Show InChI InChI=1S/C21H15NO4S2/c1-28(25,26)15-7-4-13(5-8-15)11-17-16-12-14(6-9-18(16)22-21(17)24)20(23)19-3-2-10-27-19/h2-12H,1H3,(H,22,24)/b17-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Janus Kinase 3 protein tyrosine kinase. |
Bioorg Med Chem Lett 13: 3105-10 (2003)
BindingDB Entry DOI: 10.7270/Q2833RDR |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50491256
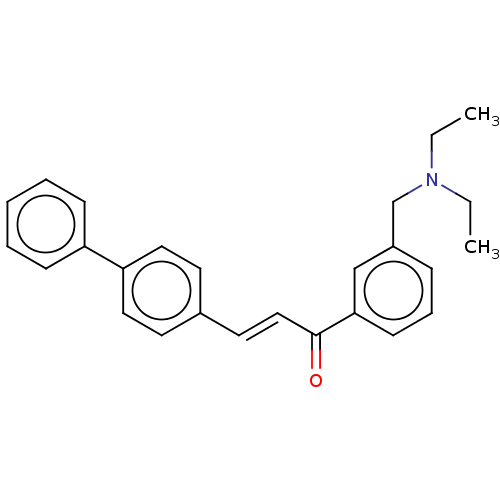
(CHEMBL2381400)Show SMILES CCN(CC)Cc1cccc(c1)C(=O)\C=C\c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H27NO/c1-3-27(4-2)20-22-9-8-12-25(19-22)26(28)18-15-21-13-16-24(17-14-21)23-10-6-5-7-11-23/h5-19H,3-4,20H2,1-2H3/b18-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) by Ellman method |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50547625

(CHEMBL4751079)Show SMILES [H][C@@]12CCCN1C(=O)[C@@]1([H])CCCN1C(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc3ccccc3c1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(=O)NCCOCCOCC(=O)N[C@@H](CCCCNC(=O)c1ccc3c(c1)C(=O)OC31c3ccc4cc(O)ccc4c3Oc3c1ccc1cc(O)ccc31)C(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(C)C |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Grb2-SH2 domain (unknown origin) expressed in Escherichia coli BL21 (DE23) after 1 hr by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115711
BindingDB Entry DOI: 10.7270/Q2FB56JN |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) by Ellman method |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491258

(CHEMBL2381402)Show SMILES COc1ccc2cc(C(=O)CN3CCC(CC3)c3ccccc3)c(=O)oc2c1 Show InChI InChI=1S/C23H23NO4/c1-27-19-8-7-18-13-20(23(26)28-22(18)14-19)21(25)15-24-11-9-17(10-12-24)16-5-3-2-4-6-16/h2-8,13-14,17H,9-12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta ((1 to 42) (unknown origin) oligomer assembly after 30 mins by biotinyl-streptavidin assay |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491253

(CHEMBL2381403)Show SMILES COc1ccc2cc(C(=O)CN3CCN(CC3)c3ccccc3)c(=O)oc2c1 Show InChI InChI=1S/C22H22N2O4/c1-27-18-8-7-16-13-19(22(26)28-21(16)14-18)20(25)15-23-9-11-24(12-10-23)17-5-3-2-4-6-17/h2-8,13-14H,9-12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta ((1 to 42) (unknown origin) oligomer assembly after 30 mins by biotinyl-streptavidin assay |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491254

(CHEMBL2381385)Show InChI InChI=1S/C16H17NO5/c1-20-12-3-2-11-8-13(16(19)22-15(11)9-12)14(18)10-17-4-6-21-7-5-17/h2-3,8-9H,4-7,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta ((1 to 42) (unknown origin) oligomer assembly after 30 mins by biotinyl-streptavidin assay |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491258

(CHEMBL2381402)Show SMILES COc1ccc2cc(C(=O)CN3CCC(CC3)c3ccccc3)c(=O)oc2c1 Show InChI InChI=1S/C23H23NO4/c1-27-19-8-7-18-13-20(23(26)28-22(18)14-19)21(25)15-24-11-9-17(10-12-24)16-5-3-2-4-6-16/h2-8,13-14,17H,9-12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta (1 to 42) (unknown origin) oligomer dissociation after 16 to 18 hrs by ELISA |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491252

(CHEMBL2381401)Show SMILES COc1ccc2cc(C(=O)CN3CCN(Cc4ccccc4)CC3)c(=O)oc2c1 Show InChI InChI=1S/C23H24N2O4/c1-28-19-8-7-18-13-20(23(27)29-22(18)14-19)21(26)16-25-11-9-24(10-12-25)15-17-5-3-2-4-6-17/h2-8,13-14H,9-12,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta ((1 to 42) (unknown origin) oligomer assembly after 30 mins by biotinyl-streptavidin assay |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50491255
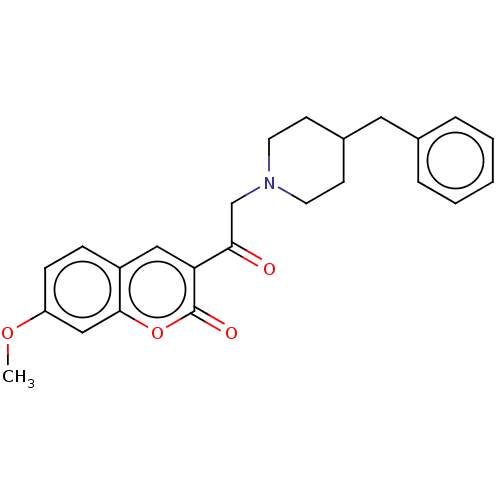
(CHEMBL2381386)Show SMILES COc1ccc2cc(C(=O)CN3CCC(Cc4ccccc4)CC3)c(=O)oc2c1 Show InChI InChI=1S/C24H25NO4/c1-28-20-8-7-19-14-21(24(27)29-23(19)15-20)22(26)16-25-11-9-18(10-12-25)13-17-5-3-2-4-6-17/h2-8,14-15,18H,9-13,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Boston
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta ((1 to 42) (unknown origin) oligomer assembly after 30 mins by biotinyl-streptavidin assay |
Bioorg Med Chem Lett 23: 2614-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.103
BindingDB Entry DOI: 10.7270/Q27M0BWS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data