 Found 55 hits with Last Name = 'haedo' and Initial = 'rj'
Found 55 hits with Last Name = 'haedo' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399552
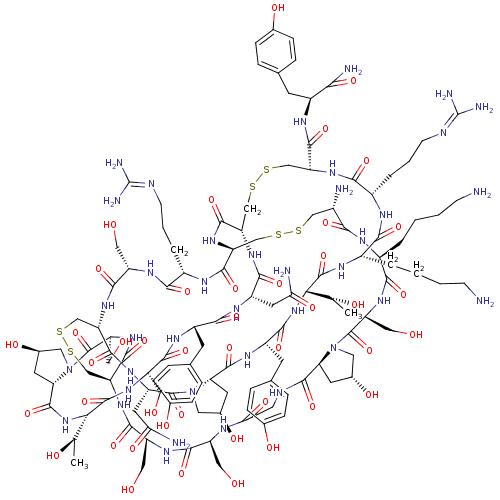
(CHEMBL1651027)Show SMILES [#6]-[#6@@H](-[#8])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6@@H](-[#8])-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@@H]-3-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-4-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-4-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6@@H](-[#8])-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-3=O)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C120H182N38O43S6/c1-53(165)91-114(197)138-66(10-4-6-26-122)95(178)136-68(12-8-28-132-120(129)130)98(181)149-81(107(190)139-69(93(126)176)29-55-13-19-58(167)20-14-55)49-204-207-52-84-110(193)153-80-48-203-202-47-64(123)94(177)135-65(9-3-5-25-121)97(180)147-78(45-163)117(200)156-38-61(170)32-85(156)111(194)133-37-90(175)134-74(41-159)102(185)145-76(43-161)105(188)152-83(109(192)148-79(46-164)118(201)158-40-63(172)34-87(158)113(196)155-92(54(2)166)115(198)146-77(44-162)103(186)140-70(30-56-15-21-59(168)22-16-56)99(182)141-72(35-88(124)173)100(183)150-84)51-206-205-50-82(151-104(187)75(42-160)144-96(179)67(137-106(80)189)11-7-27-131-119(127)128)108(191)143-73(36-89(125)174)116(199)157-39-62(171)33-86(157)112(195)142-71(101(184)154-91)31-57-17-23-60(169)24-18-57/h13-24,53-54,61-87,91-92,159-172H,3-12,25-52,121-123H2,1-2H3,(H2,124,173)(H2,125,174)(H2,126,176)(H,133,194)(H,134,175)(H,135,177)(H,136,178)(H,137,189)(H,138,197)(H,139,190)(H,140,186)(H,141,182)(H,142,195)(H,143,191)(H,144,179)(H,145,185)(H,146,198)(H,147,180)(H,148,192)(H,149,181)(H,150,183)(H,151,187)(H,152,188)(H,153,193)(H,154,184)(H,155,196)(H4,127,128,131)(H4,129,130,132)/t53-,54-,61-,62-,63-,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,75+,76+,77+,78+,79+,80+,81+,82+,83+,84+,85+,86+,87+,91+,92+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 4 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399552

(CHEMBL1651027)Show SMILES [#6]-[#6@@H](-[#8])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6@@H](-[#8])-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@@H]-3-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-4-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-4-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6@@H](-[#8])-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-3=O)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C120H182N38O43S6/c1-53(165)91-114(197)138-66(10-4-6-26-122)95(178)136-68(12-8-28-132-120(129)130)98(181)149-81(107(190)139-69(93(126)176)29-55-13-19-58(167)20-14-55)49-204-207-52-84-110(193)153-80-48-203-202-47-64(123)94(177)135-65(9-3-5-25-121)97(180)147-78(45-163)117(200)156-38-61(170)32-85(156)111(194)133-37-90(175)134-74(41-159)102(185)145-76(43-161)105(188)152-83(109(192)148-79(46-164)118(201)158-40-63(172)34-87(158)113(196)155-92(54(2)166)115(198)146-77(44-162)103(186)140-70(30-56-15-21-59(168)22-16-56)99(182)141-72(35-88(124)173)100(183)150-84)51-206-205-50-82(151-104(187)75(42-160)144-96(179)67(137-106(80)189)11-7-27-131-119(127)128)108(191)143-73(36-89(125)174)116(199)157-39-62(171)33-86(157)112(195)142-71(101(184)154-91)31-57-17-23-60(169)24-18-57/h13-24,53-54,61-87,91-92,159-172H,3-12,25-52,121-123H2,1-2H3,(H2,124,173)(H2,125,174)(H2,126,176)(H,133,194)(H,134,175)(H,135,177)(H,136,178)(H,137,189)(H,138,197)(H,139,190)(H,140,186)(H,141,182)(H,142,195)(H,143,191)(H,144,179)(H,145,185)(H,146,198)(H,147,180)(H,148,192)(H,149,181)(H,150,183)(H,151,187)(H,152,188)(H,153,193)(H,154,184)(H,155,196)(H4,127,128,131)(H4,129,130,132)/t53-,54-,61-,62-,63-,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,75+,76+,77+,78+,79+,80+,81+,82+,83+,84+,85+,86+,87+,91+,92+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399551
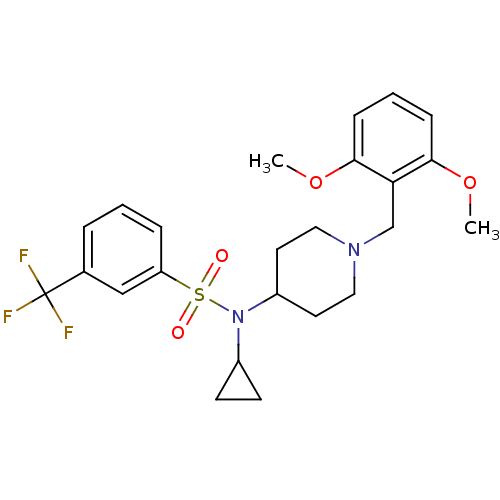
(CHEMBL2180892)Show SMILES COc1cccc(OC)c1CN1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H29F3N2O4S/c1-32-22-7-4-8-23(33-2)21(22)16-28-13-11-19(12-14-28)29(18-9-10-18)34(30,31)20-6-3-5-17(15-20)24(25,26)27/h3-8,15,18-19H,9-14,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399541

(CHEMBL2180891)Show SMILES CCCN(C1CCN(Cc2c(OC)cccc2OC)CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H31F3N2O4S/c1-4-13-29(34(30,31)20-8-5-7-18(16-20)24(25,26)27)19-11-14-28(15-12-19)17-21-22(32-2)9-6-10-23(21)33-3/h5-10,16,19H,4,11-15,17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399550
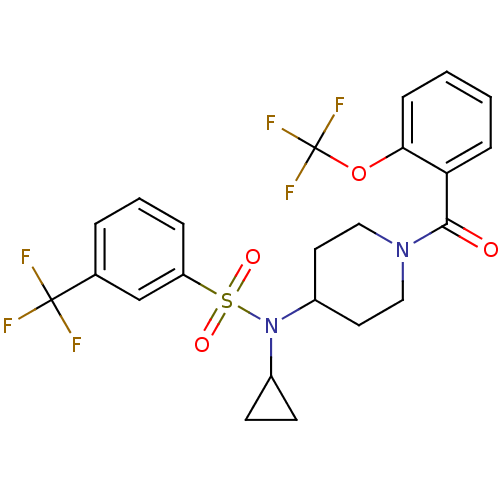
(CHEMBL2180894)Show SMILES FC(F)(F)Oc1ccccc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H22F6N2O4S/c24-22(25,26)15-4-3-5-18(14-15)36(33,34)31(16-8-9-16)17-10-12-30(13-11-17)21(32)19-6-1-2-7-20(19)35-23(27,28)29/h1-7,14,16-17H,8-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333703
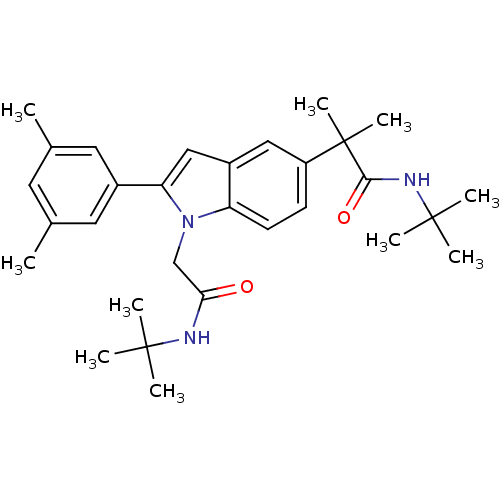
(CHEMBL1643724 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES Cc1cc(C)cc(c1)-c1cc2cc(ccc2n1CC(=O)NC(C)(C)C)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H41N3O2/c1-19-13-20(2)15-21(14-19)25-17-22-16-23(30(9,10)27(35)32-29(6,7)8)11-12-24(22)33(25)18-26(34)31-28(3,4)5/h11-17H,18H2,1-10H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399546
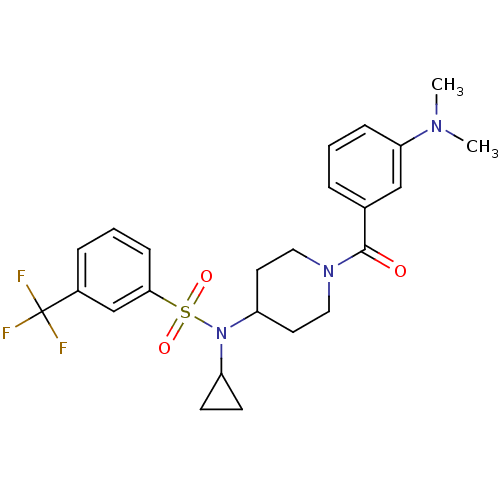
(CHEMBL2180898)Show SMILES CN(C)c1cccc(c1)C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H28F3N3O3S/c1-28(2)21-7-3-5-17(15-21)23(31)29-13-11-20(12-14-29)30(19-9-10-19)34(32,33)22-8-4-6-18(16-22)24(25,26)27/h3-8,15-16,19-20H,9-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399548
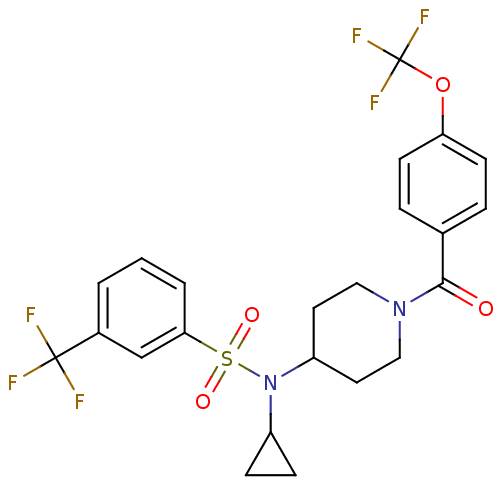
(CHEMBL2180896)Show SMILES FC(F)(F)Oc1ccc(cc1)C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H22F6N2O4S/c24-22(25,26)16-2-1-3-20(14-16)36(33,34)31(17-6-7-17)18-10-12-30(13-11-18)21(32)15-4-8-19(9-5-15)35-23(27,28)29/h1-5,8-9,14,17-18H,6-7,10-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399549
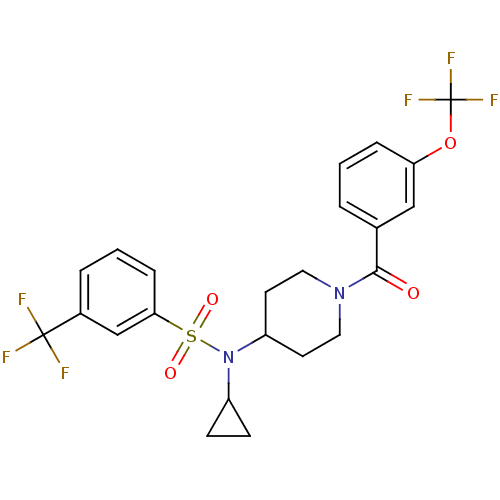
(CHEMBL2180895)Show SMILES FC(F)(F)Oc1cccc(c1)C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H22F6N2O4S/c24-22(25,26)16-4-2-6-20(14-16)36(33,34)31(17-7-8-17)18-9-11-30(12-10-18)21(32)15-3-1-5-19(13-15)35-23(27,28)29/h1-6,13-14,17-18H,7-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399547
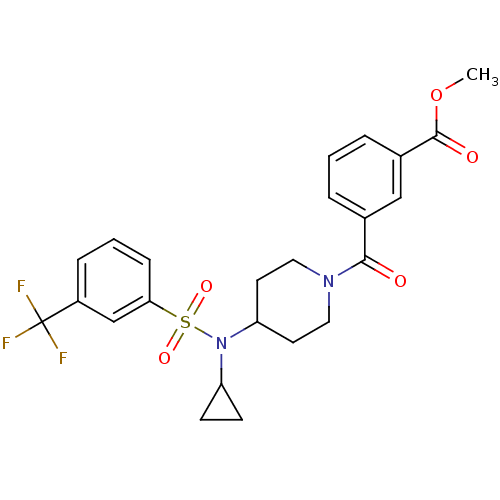
(CHEMBL2180897)Show SMILES COC(=O)c1cccc(c1)C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H25F3N2O5S/c1-34-23(31)17-5-2-4-16(14-17)22(30)28-12-10-20(11-13-28)29(19-8-9-19)35(32,33)21-7-3-6-18(15-21)24(25,26)27/h2-7,14-15,19-20H,8-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333709
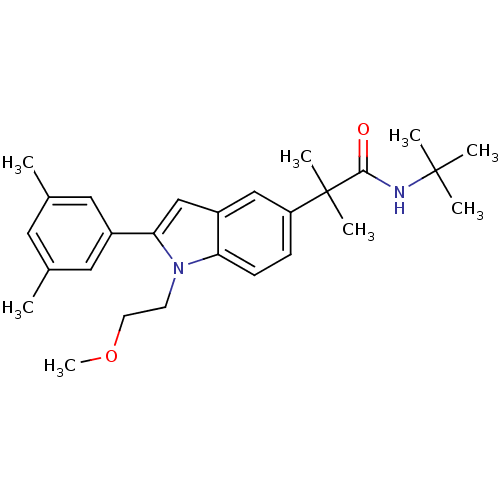
(CHEMBL1643725 | N-tert-butyl-2-(2-(3,5-dimethylphe...)Show SMILES COCCn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C27H36N2O2/c1-18-13-19(2)15-20(14-18)24-17-21-16-22(9-10-23(21)29(24)11-12-31-8)27(6,7)25(30)28-26(3,4)5/h9-10,13-17H,11-12H2,1-8H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333710
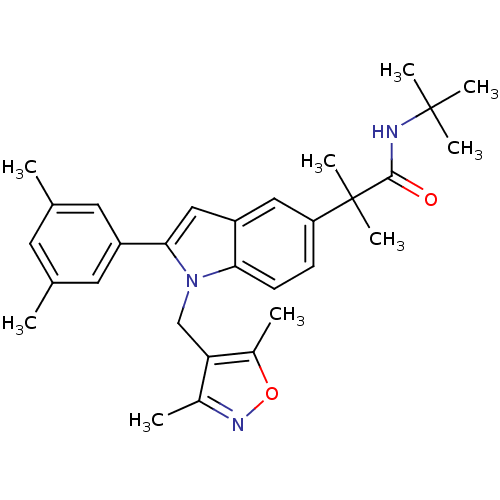
(CHEMBL1643726 | N-tert-butyl-2-(1-((3,5-dimethylis...)Show SMILES Cc1noc(C)c1Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C30H37N3O2/c1-18-12-19(2)14-22(13-18)27-16-23-15-24(30(8,9)28(34)31-29(5,6)7)10-11-26(23)33(27)17-25-20(3)32-35-21(25)4/h10-16H,17H2,1-9H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399544
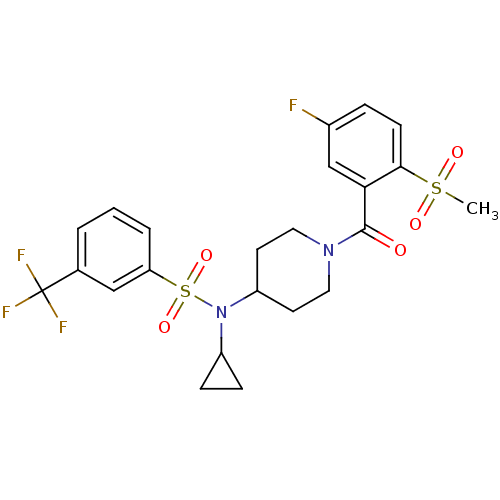
(CHEMBL2180905)Show SMILES CS(=O)(=O)c1ccc(F)cc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H24F4N2O5S2/c1-35(31,32)21-8-5-16(24)14-20(21)22(30)28-11-9-18(10-12-28)29(17-6-7-17)36(33,34)19-4-2-3-15(13-19)23(25,26)27/h2-5,8,13-14,17-18H,6-7,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333712

((S)-tert-butyl 2-(3,5-dimethylphenyl)-5-(1-ethoxy-...)Show SMILES CCOC(=O)C(C)(C)c1ccc2n(C(=O)OC(C)(C)C)c(c([C@H](C)CO)c2c1)-c1cc(C)cc(C)c1 |r| Show InChI InChI=1S/C30H39NO5/c1-10-35-27(33)30(8,9)22-11-12-24-23(16-22)25(20(4)17-32)26(21-14-18(2)13-19(3)15-21)31(24)28(34)36-29(5,6)7/h11-16,20,32H,10,17H2,1-9H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333714
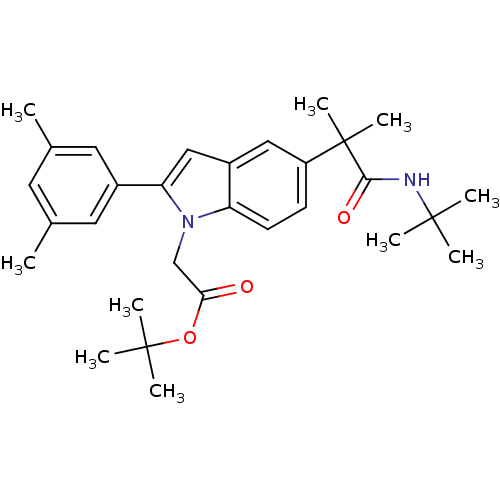
(CHEMBL1643723 | tert-butyl 2-(5-(1-(tert-butylamin...)Show SMILES Cc1cc(C)cc(c1)-c1cc2cc(ccc2n1CC(=O)OC(C)(C)C)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C30H40N2O3/c1-19-13-20(2)15-21(14-19)25-17-22-16-23(30(9,10)27(34)31-28(3,4)5)11-12-24(22)32(25)18-26(33)35-29(6,7)8/h11-17H,18H2,1-10H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399545
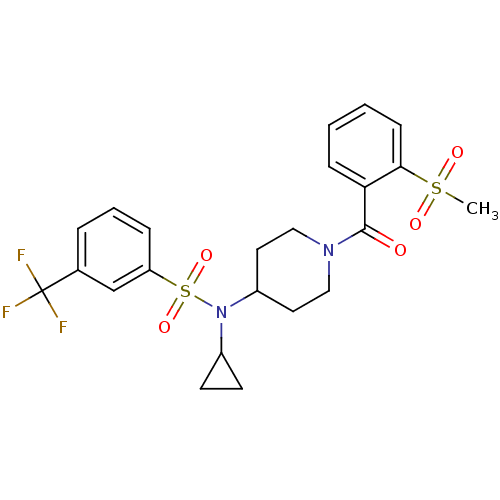
(CHEMBL2180902)Show SMILES CS(=O)(=O)c1ccccc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H25F3N2O5S2/c1-34(30,31)21-8-3-2-7-20(21)22(29)27-13-11-18(12-14-27)28(17-9-10-17)35(32,33)19-6-4-5-16(15-19)23(24,25)26/h2-8,15,17-18H,9-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333715

(CHEMBL1643720 | N-tert-butyl-2-(3,5-dimethylphenyl...)Show SMILES CC(O)Cn1c(cc2cc(ccc12)C(=O)NC(C)(C)C)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C24H30N2O2/c1-15-9-16(2)11-19(10-15)22-13-20-12-18(23(28)25-24(4,5)6)7-8-21(20)26(22)14-17(3)27/h7-13,17,27H,14H2,1-6H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333717
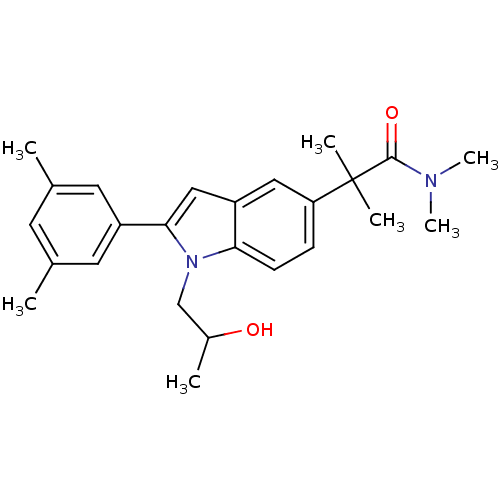
(2-(2-(3,5-dimethylphenyl)-1-(2-hydroxypropyl)-1H-i...)Show SMILES CC(O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)N(C)C)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C25H32N2O2/c1-16-10-17(2)12-19(11-16)23-14-20-13-21(25(4,5)24(29)26(6)7)8-9-22(20)27(23)15-18(3)28/h8-14,18,28H,15H2,1-7H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333718

((S)-1-(7-azabicyclo[2.2.1]heptan-7-yl)-2-(2-(3,5-d...)Show SMILES CCCN[C@@H](C)c1c([nH]c2ccc(cc12)C(C)(C)C(=O)N1C2CCC1CC2)-c1cc(C)cc(C)c1 |r,THB:18:20:22.23:25.26| Show InChI InChI=1S/C31H41N3O/c1-7-14-32-21(4)28-26-18-23(31(5,6)30(35)34-24-9-10-25(34)12-11-24)8-13-27(26)33-29(28)22-16-19(2)15-20(3)17-22/h8,13,15-18,21,24-25,32-33H,7,9-12,14H2,1-6H3/t21-,24?,25?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399542
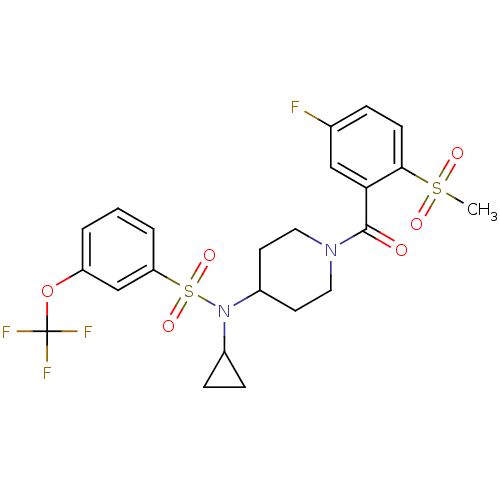
(CHEMBL2180889)Show SMILES CS(=O)(=O)c1ccc(F)cc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C23H24F4N2O6S2/c1-36(31,32)21-8-5-15(24)13-20(21)22(30)28-11-9-17(10-12-28)29(16-6-7-16)37(33,34)19-4-2-3-18(14-19)35-23(25,26)27/h2-5,8,13-14,16-17H,6-7,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333720
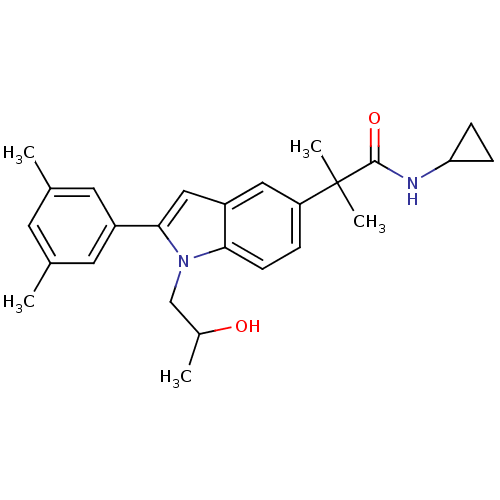
(CHEMBL1643718 | N-cyclopropyl-2-(2-(3,5-dimethylph...)Show SMILES CC(O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC1CC1)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C26H32N2O2/c1-16-10-17(2)12-19(11-16)24-14-20-13-21(6-9-23(20)28(24)15-18(3)29)26(4,5)25(30)27-22-7-8-22/h6,9-14,18,22,29H,7-8,15H2,1-5H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50333721

(2-(2-(3,5-dimethylphenyl)-1-(2-hydroxypropyl)-1H-i...)Show SMILES CC(O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NCC(F)(F)F)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C25H29F3N2O2/c1-15-8-16(2)10-18(9-15)22-12-19-11-20(6-7-21(19)30(22)13-17(3)31)24(4,5)23(32)29-14-25(26,27)28/h6-12,17,31H,13-14H2,1-5H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of Cav2.2 channel by fluorescent calcium-influx assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399543
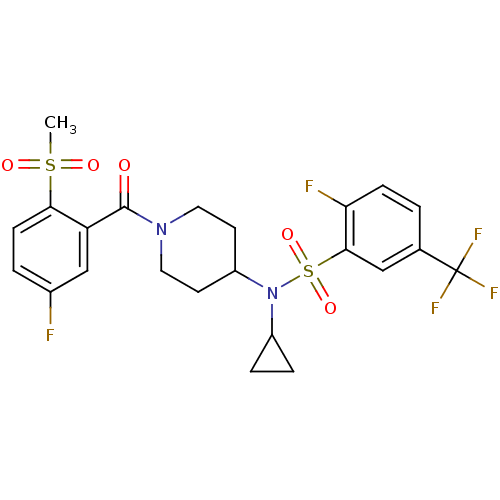
(CHEMBL2180887)Show SMILES CS(=O)(=O)c1ccc(F)cc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cc(ccc1F)C(F)(F)F Show InChI InChI=1S/C23H23F5N2O5S2/c1-36(32,33)20-7-3-15(24)13-18(20)22(31)29-10-8-17(9-11-29)30(16-4-5-16)37(34,35)21-12-14(23(26,27)28)2-6-19(21)25/h2-3,6-7,12-13,16-17H,4-5,8-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 30 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50399541

(CHEMBL2180891)Show SMILES CCCN(C1CCN(Cc2c(OC)cccc2OC)CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H31F3N2O4S/c1-4-13-29(34(30,31)20-8-5-7-18(16-20)24(25,26)27)19-11-14-28(15-12-19)17-21-22(32-2)9-6-10-23(21)33-3/h5-10,16,19H,4,11-15,17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav1.2 assessed as inhibition of calcium flux by cell based FLIPR assay |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Homo sapiens (Human)) | BDBM50399541

(CHEMBL2180891)Show SMILES CCCN(C1CCN(Cc2c(OC)cccc2OC)CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H31F3N2O4S/c1-4-13-29(34(30,31)20-8-5-7-18(16-20)24(25,26)27)19-11-14-28(15-12-19)17-21-22(32-2)9-6-10-23(21)33-3/h5-10,16,19H,4,11-15,17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.2 expressed HEK293 cells assessed as inhibition of calcium flux by FLIPR assay in presence of 4 mM of potassium |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399544

(CHEMBL2180905)Show SMILES CS(=O)(=O)c1ccc(F)cc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H24F4N2O5S2/c1-35(31,32)21-8-5-16(24)14-20(21)22(30)28-11-9-18(10-12-28)29(17-6-7-17)36(33,34)19-4-2-3-15(13-19)23(25,26)27/h2-5,8,13-14,17-18H,6-7,9-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50333708
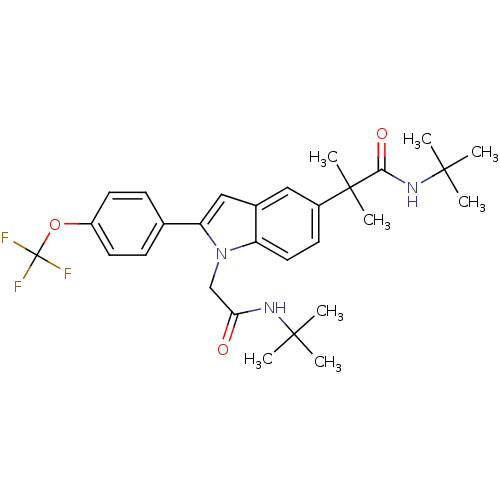
(CHEMBL1643735 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-14-11-20(28(7,8)25(37)34-27(4,5)6)15-19(22)16-23(35)18-9-12-21(13-10-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50333708

(CHEMBL1643735 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-14-11-20(28(7,8)25(37)34-27(4,5)6)15-19(22)16-23(35)18-9-12-21(13-10-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50333708

(CHEMBL1643735 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-14-11-20(28(7,8)25(37)34-27(4,5)6)15-19(22)16-23(35)18-9-12-21(13-10-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50399544

(CHEMBL2180905)Show SMILES CS(=O)(=O)c1ccc(F)cc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H24F4N2O5S2/c1-35(31,32)21-8-5-16(24)14-20(21)22(30)28-11-9-18(10-12-28)29(17-6-7-17)36(33,34)19-4-2-3-15(13-19)23(25,26)27/h2-5,8,13-14,17-18H,6-7,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR |
J Med Chem 55: 9847-55 (2012)
Article DOI: 10.1021/jm301056k
BindingDB Entry DOI: 10.7270/Q26W9C7J |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443607

(CHEMBL3092207)Show SMILES NC(=O)c1ccc(C(=O)N2CCC(CC2)N(C2CC2)S(=O)(=O)c2cccc(c2)C(F)(F)F)c(c1)C(F)(F)F Show InChI InChI=1S/C24H23F6N3O4S/c25-23(26,27)15-2-1-3-18(13-15)38(36,37)33(16-5-6-16)17-8-10-32(11-9-17)22(35)19-7-4-14(21(31)34)12-20(19)24(28,29)30/h1-4,7,12-13,16-17H,5-6,8-11H2,(H2,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443606
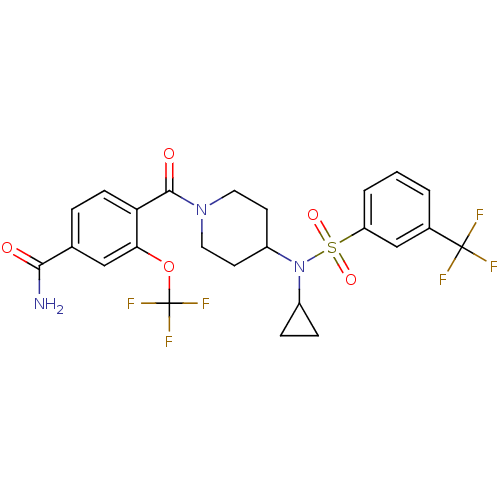
(CHEMBL3092208)Show SMILES NC(=O)c1ccc(C(=O)N2CCC(CC2)N(C2CC2)S(=O)(=O)c2cccc(c2)C(F)(F)F)c(OC(F)(F)F)c1 Show InChI InChI=1S/C24H23F6N3O5S/c25-23(26,27)15-2-1-3-18(13-15)39(36,37)33(16-5-6-16)17-8-10-32(11-9-17)22(35)19-7-4-14(21(31)34)12-20(19)38-24(28,29)30/h1-4,7,12-13,16-17H,5-6,8-11H2,(H2,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443615

(CHEMBL3092199)Show SMILES CS(=O)(=O)c1ccc(cc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F)C#N Show InChI InChI=1S/C24H24F3N3O5S2/c1-36(32,33)22-8-5-16(15-28)13-21(22)23(31)29-11-9-19(10-12-29)30(18-6-7-18)37(34,35)20-4-2-3-17(14-20)24(25,26)27/h2-5,8,13-14,18-19H,6-7,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443614
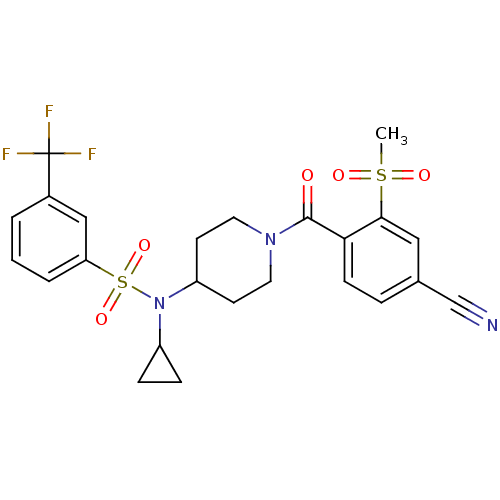
(CHEMBL3092200)Show SMILES CS(=O)(=O)c1cc(ccc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F)C#N Show InChI InChI=1S/C24H24F3N3O5S2/c1-36(32,33)22-13-16(15-28)5-8-21(22)23(31)29-11-9-19(10-12-29)30(18-6-7-18)37(34,35)20-4-2-3-17(14-20)24(25,26)27/h2-5,8,13-14,18-19H,6-7,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443613

(CHEMBL3092201)Show SMILES CS(=O)(=O)c1cc(ccc1C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H24F6N2O5S2/c1-38(34,35)21-14-16(24(28,29)30)5-8-20(21)22(33)31-11-9-18(10-12-31)32(17-6-7-17)39(36,37)19-4-2-3-15(13-19)23(25,26)27/h2-5,8,13-14,17-18H,6-7,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443612
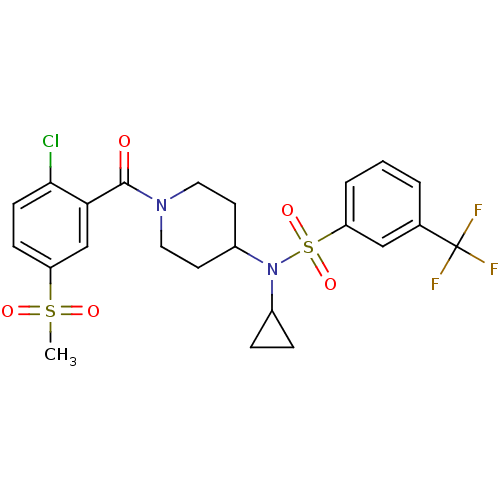
(CHEMBL3092202)Show SMILES CS(=O)(=O)c1ccc(Cl)c(c1)C(=O)N1CCC(CC1)N(C1CC1)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H24ClF3N2O5S2/c1-35(31,32)18-7-8-21(24)20(14-18)22(30)28-11-9-17(10-12-28)29(16-5-6-16)36(33,34)19-4-2-3-15(13-19)23(25,26)27/h2-4,7-8,13-14,16-17H,5-6,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443611
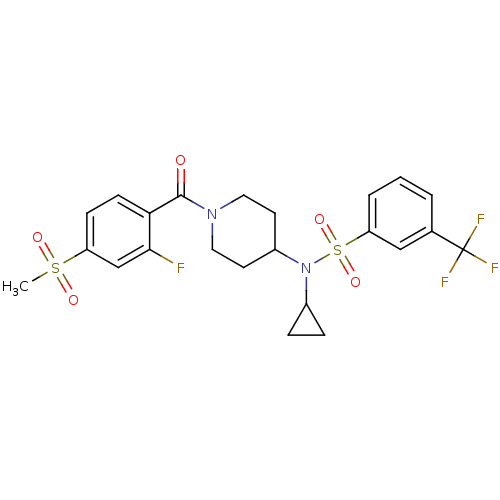
(CHEMBL3092203)Show SMILES CS(=O)(=O)c1ccc(C(=O)N2CCC(CC2)N(C2CC2)S(=O)(=O)c2cccc(c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C23H24F4N2O5S2/c1-35(31,32)18-7-8-20(21(24)14-18)22(30)28-11-9-17(10-12-28)29(16-5-6-16)36(33,34)19-4-2-3-15(13-19)23(25,26)27/h2-4,7-8,13-14,16-17H,5-6,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443610
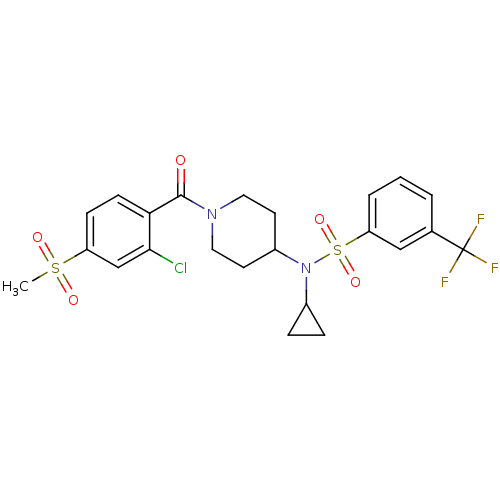
(CHEMBL3092204)Show SMILES CS(=O)(=O)c1ccc(C(=O)N2CCC(CC2)N(C2CC2)S(=O)(=O)c2cccc(c2)C(F)(F)F)c(Cl)c1 Show InChI InChI=1S/C23H24ClF3N2O5S2/c1-35(31,32)18-7-8-20(21(24)14-18)22(30)28-11-9-17(10-12-28)29(16-5-6-16)36(33,34)19-4-2-3-15(13-19)23(25,26)27/h2-4,7-8,13-14,16-17H,5-6,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443609
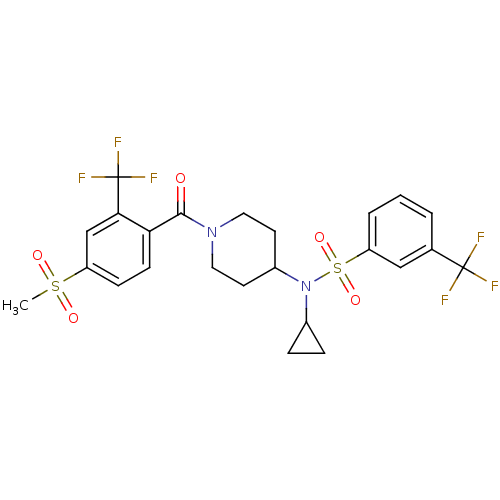
(CHEMBL3092205)Show SMILES CS(=O)(=O)c1ccc(C(=O)N2CCC(CC2)N(C2CC2)S(=O)(=O)c2cccc(c2)C(F)(F)F)c(c1)C(F)(F)F Show InChI InChI=1S/C24H24F6N2O5S2/c1-38(34,35)18-7-8-20(21(14-18)24(28,29)30)22(33)31-11-9-17(10-12-31)32(16-5-6-16)39(36,37)19-4-2-3-15(13-19)23(25,26)27/h2-4,7-8,13-14,16-17H,5-6,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443608
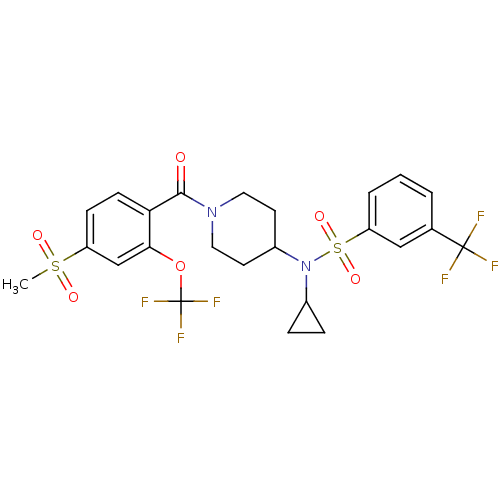
(CHEMBL3092206)Show SMILES CS(=O)(=O)c1ccc(C(=O)N2CCC(CC2)N(C2CC2)S(=O)(=O)c2cccc(c2)C(F)(F)F)c(OC(F)(F)F)c1 Show InChI InChI=1S/C24H24F6N2O6S2/c1-39(34,35)18-7-8-20(21(14-18)38-24(28,29)30)22(33)31-11-9-17(10-12-31)32(16-5-6-16)40(36,37)19-4-2-3-15(13-19)23(25,26)27/h2-4,7-8,13-14,16-17H,5-6,9-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443605
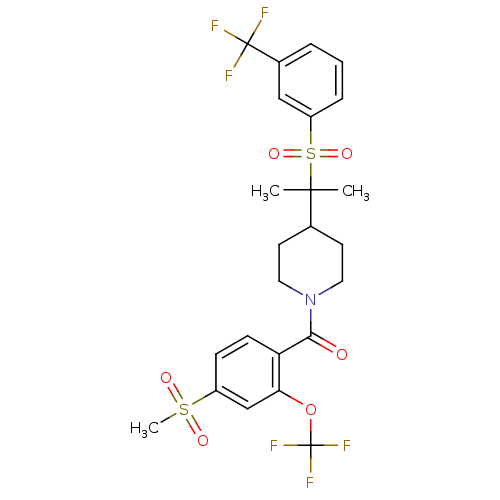
(CHEMBL3092197)Show SMILES CC(C)(C1CCN(CC1)C(=O)c1ccc(cc1OC(F)(F)F)S(C)(=O)=O)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H25F6NO6S2/c1-22(2,39(35,36)18-6-4-5-16(13-18)23(25,26)27)15-9-11-31(12-10-15)21(32)19-8-7-17(38(3,33)34)14-20(19)37-24(28,29)30/h4-8,13-15H,9-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50443604
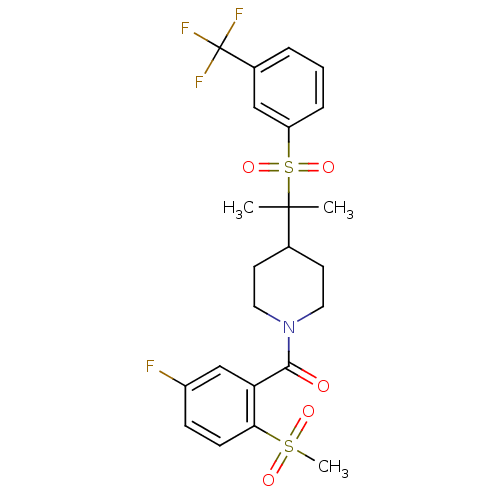
(CHEMBL3092198)Show SMILES CC(C)(C1CCN(CC1)C(=O)c1cc(F)ccc1S(C)(=O)=O)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H25F4NO5S2/c1-22(2,35(32,33)18-6-4-5-16(13-18)23(25,26)27)15-9-11-28(12-10-15)21(29)19-14-17(24)7-8-20(19)34(3,30)31/h4-8,13-15H,9-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) |
ACS Med Chem Lett 4: 1064-8 (2013)
Article DOI: 10.1021/ml4002612
BindingDB Entry DOI: 10.7270/Q2FT8NHJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333724
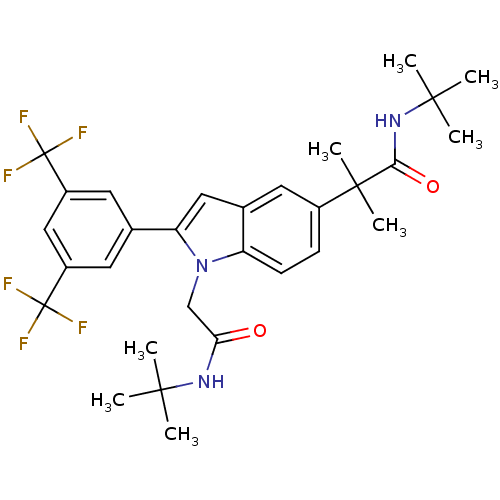
(2-(2-(3,5-bis(trifluoromethyl)phenyl)-1-(2-(tert-b...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C30H35F6N3O2/c1-26(2,3)37-24(40)16-39-22-10-9-19(28(7,8)25(41)38-27(4,5)6)11-18(22)14-23(39)17-12-20(29(31,32)33)15-21(13-17)30(34,35)36/h9-15H,16H2,1-8H3,(H,37,40)(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333723
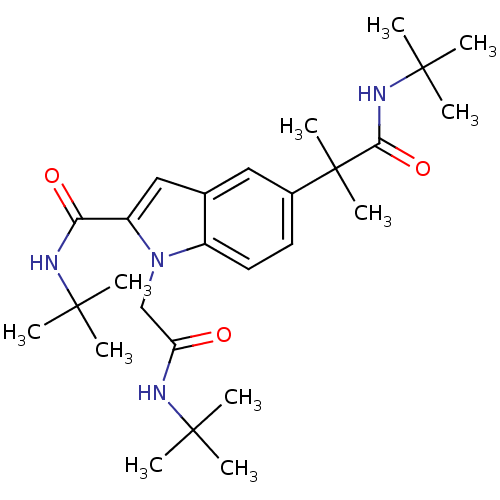
(CHEMBL1643738 | N-tert-butyl-5-(1-(tert-butylamino...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)C(=O)NC(C)(C)C Show InChI InChI=1S/C27H42N4O3/c1-24(2,3)28-21(32)16-31-19-13-12-18(27(10,11)23(34)30-26(7,8)9)14-17(19)15-20(31)22(33)29-25(4,5)6/h12-15H,16H2,1-11H3,(H,28,32)(H,29,33)(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.64E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333722

(CHEMBL1643728 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccccc1 Show InChI InChI=1S/C28H37N3O2/c1-26(2,3)29-24(32)18-31-22-15-14-21(28(7,8)25(33)30-27(4,5)6)16-20(22)17-23(31)19-12-10-9-11-13-19/h9-17H,18H2,1-8H3,(H,29,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333719

(CHEMBL1643732 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(F)cc1F Show InChI InChI=1S/C28H35F2N3O2/c1-26(2,3)31-24(34)16-33-22-12-9-18(28(7,8)25(35)32-27(4,5)6)13-17(22)14-23(33)20-11-10-19(29)15-21(20)30/h9-15H,16H2,1-8H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 940 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333716
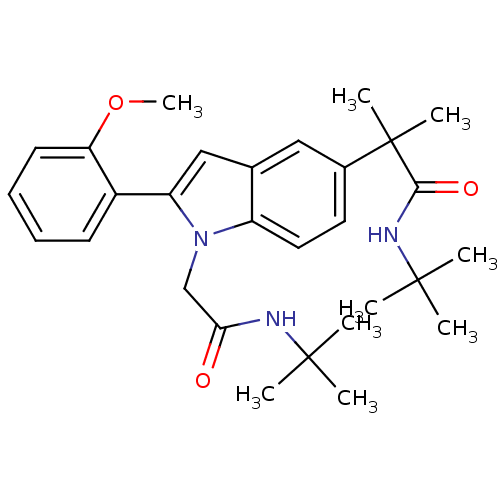
(CHEMBL1643736 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES COc1ccccc1-c1cc2cc(ccc2n1CC(=O)NC(C)(C)C)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C29H39N3O3/c1-27(2,3)30-25(33)18-32-22-15-14-20(29(7,8)26(34)31-28(4,5)6)16-19(22)17-23(32)21-12-10-11-13-24(21)35-9/h10-17H,18H2,1-9H3,(H,30,33)(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333713
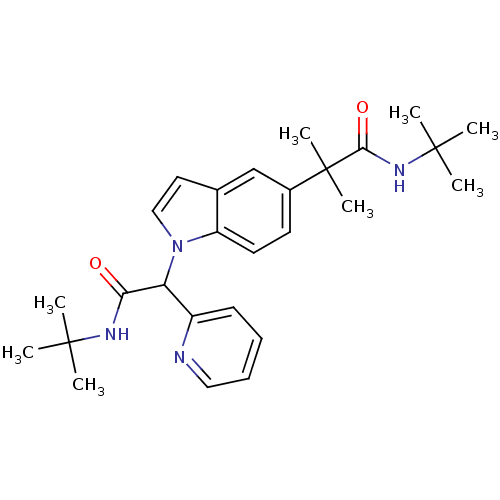
(CHEMBL1643739 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)C(c1ccccn1)n1ccc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N4O2/c1-25(2,3)29-23(32)22(20-11-9-10-15-28-20)31-16-14-18-17-19(12-13-21(18)31)27(7,8)24(33)30-26(4,5)6/h9-17,22H,1-8H3,(H,29,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333711

(CHEMBL1643737 | N-tert-butyl-2-(2-tert-butyl-1-(2-...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H41N3O2/c1-23(2,3)20-15-17-14-18(26(10,11)22(31)28-25(7,8)9)12-13-19(17)29(20)16-21(30)27-24(4,5)6/h12-15H,16H2,1-11H3,(H,27,30)(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50333708

(CHEMBL1643735 | N-tert-butyl-2-(1-(2-(tert-butylam...)Show SMILES CC(C)(C)NC(=O)Cn1c(cc2cc(ccc12)C(C)(C)C(=O)NC(C)(C)C)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H36F3N3O3/c1-26(2,3)33-24(36)17-35-22-14-11-20(28(7,8)25(37)34-27(4,5)6)15-19(22)16-23(35)18-9-12-21(13-10-18)38-29(30,31)32/h9-16H,17H2,1-8H3,(H,33,36)(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Activity at PXR by induction assay |
Bioorg Med Chem Lett 21: 869-73 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.067
BindingDB Entry DOI: 10.7270/Q2B27VJK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data