

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.000340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human DHFR by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398391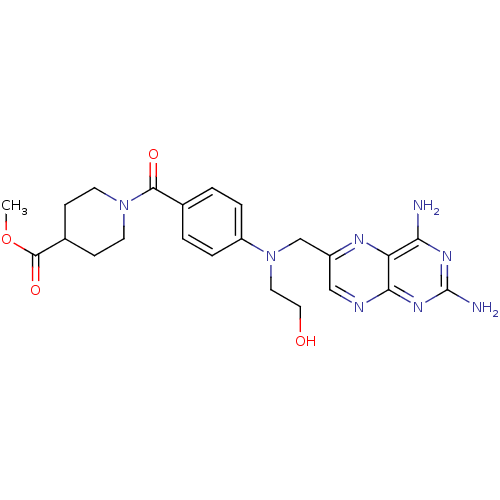 (CHEMBL2178602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398394 (CHEMBL1232702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398392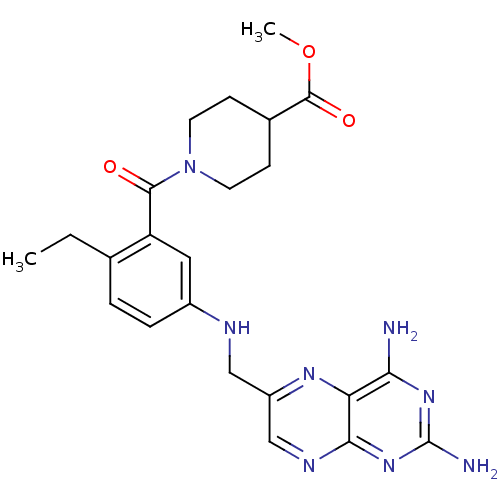 (CHEMBL2178603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398390 (CHEMBL2177120) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398395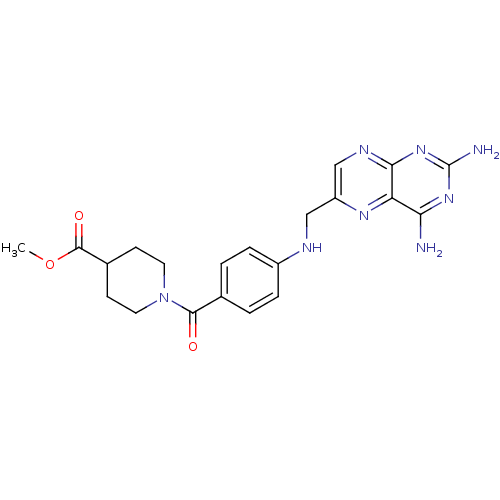 (CHEMBL1232399) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398389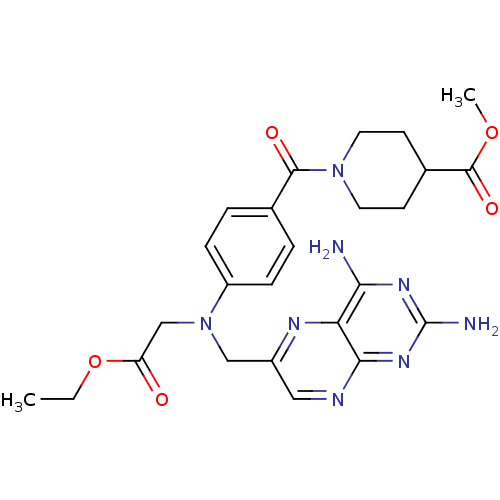 (CHEMBL2178601) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398396 (CHEMBL2178600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398393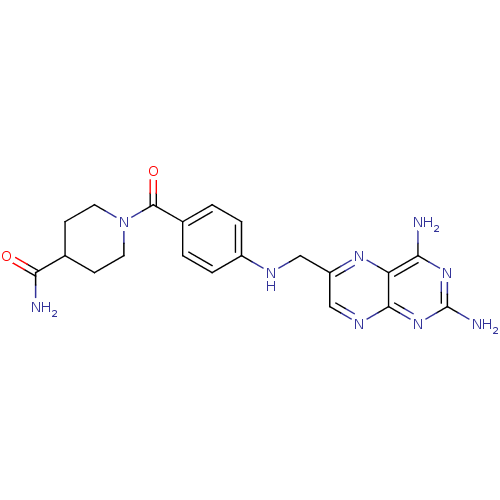 (CHEMBL2178599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50398388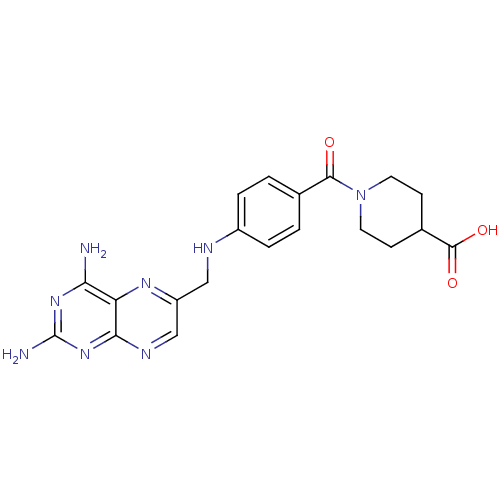 (CHEMBL2178604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human DHFR by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human TS by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50398394 (CHEMBL1232702) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human DHFR by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43835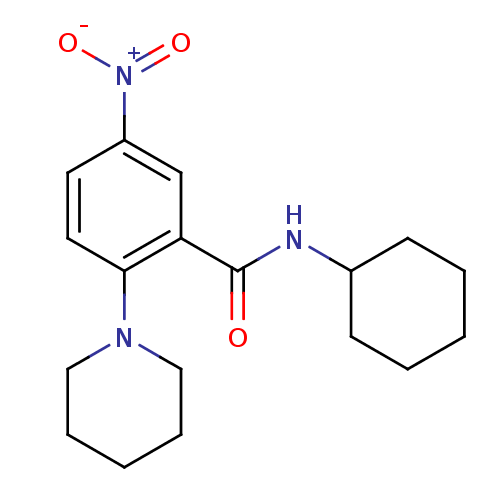 (Glucosamine derivative, 8 | MLS000582359 | N-cyclo...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | -31.7 | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50398388 (CHEMBL2178604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of Leishmania major PTR1 by spectrophotometric assay | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50398391 (CHEMBL2178602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human DHFR by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50398392 (CHEMBL2178603) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human DHFR by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43833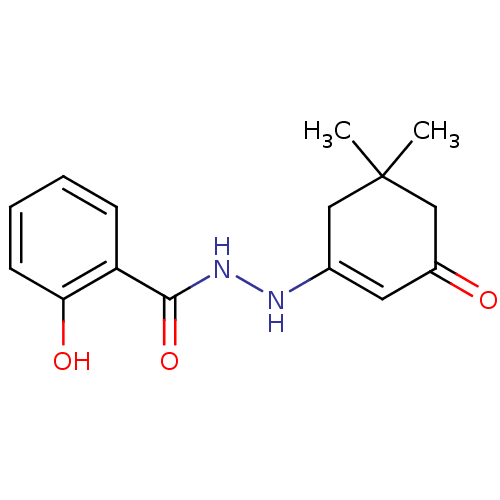 (2-hydroxy-N''''-(3-keto-5,5-dimethyl-cyclohexen-1-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | -29.1 | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent 6-phosphofructokinase (Trypanosoma brucei) | BDBM50320388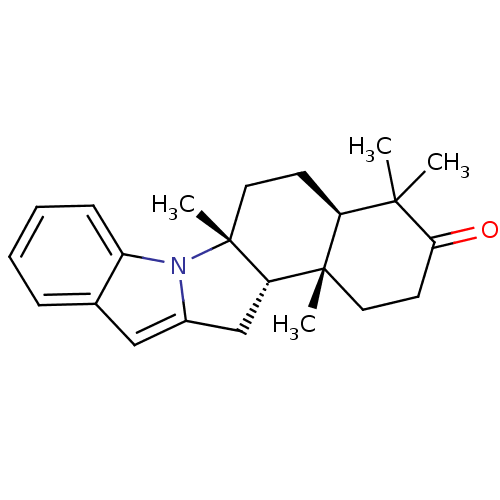 (CHEMBL1083627 | polysin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Yaound£ I Curated by ChEMBL | Assay Description Competitive reversible inhibition of Trypanosoma brucei PFK expressed in Escherichia coli using fructose-6-phosphate by spectrophotometry analysis | Bioorg Med Chem Lett 20: 3495-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.145 BindingDB Entry DOI: 10.7270/Q2HH6K8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent 6-phosphofructokinase (Trypanosoma brucei) | BDBM50320385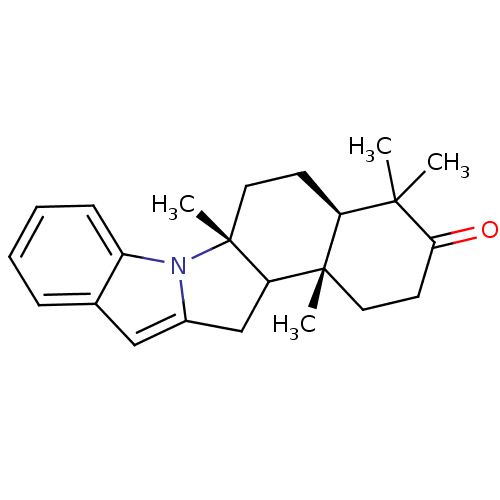 ((4aR,6aR,12bS)-4,4,6a,12b-Tetramethyl-1,4a,5,6,6a,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Yaound£ I Curated by ChEMBL | Assay Description Competitive reversible inhibition of Trypanosoma brucei PFK expressed in Escherichia coli using fructose-6-phosphate by spectrophotometry analysis | Bioorg Med Chem Lett 20: 3495-8 (2010) Article DOI: 10.1016/j.bmcl.2010.04.145 BindingDB Entry DOI: 10.7270/Q2HH6K8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50398395 (CHEMBL1232399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human DHFR by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50398393 (CHEMBL2178599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human DHFR by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43832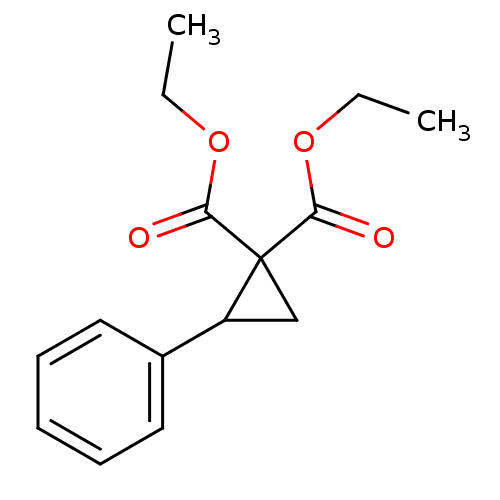 (2-phenylcyclopropane-1,1-dicarboxylic acid diethyl...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | -26.9 | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43834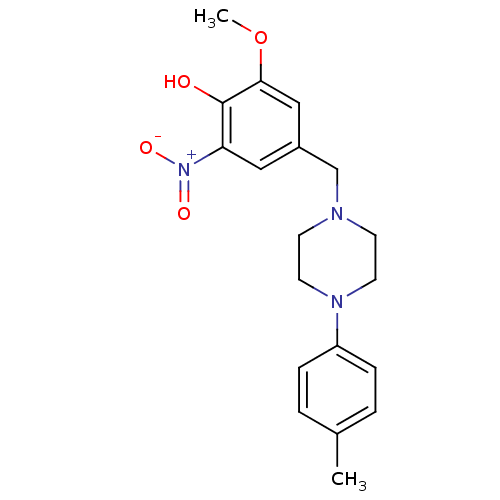 (2-methoxy-4-[[4-(4-methylphenyl)-1-piperazinyl]met...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | -26.3 | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43838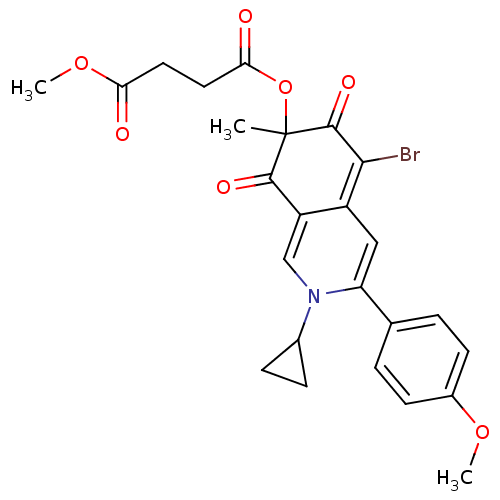 (4-O-[5-bromo-2-cyclopropyl-3-(4-methoxyphenyl)-7-m...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | -25.8 | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50398391 (CHEMBL2178602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human TS by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43837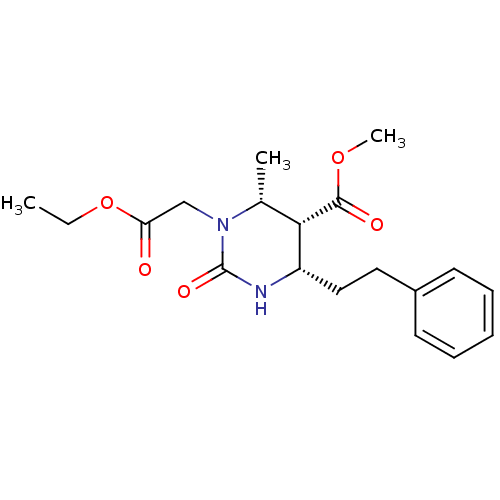 ((4S,5R,6R)-1-(2-ethoxy-2-keto-ethyl)-2-keto-6-meth...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.00E+4 | -23.7 | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43836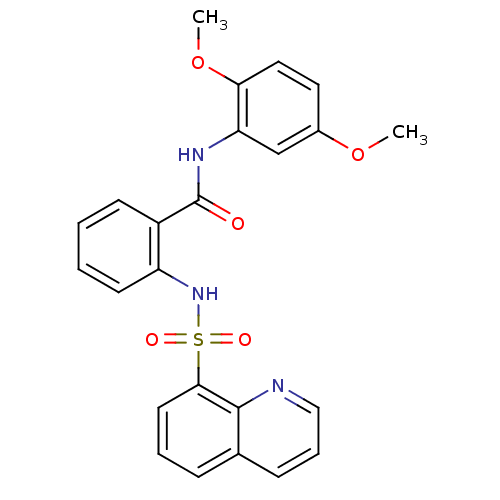 (Glucosamine derivative, 9 | MLS000580208 | N-(2,5-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | -22.8 | 4.00E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43829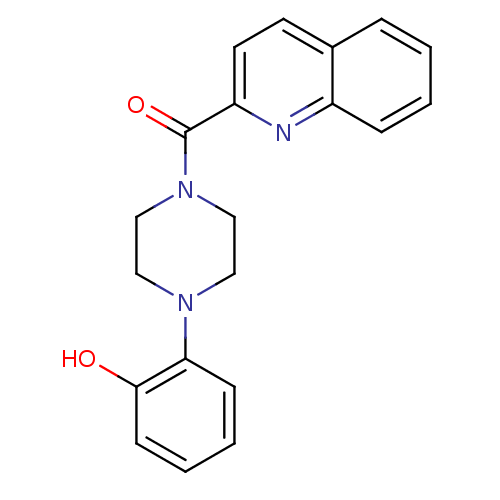 (Glucosamine derivative, 2 | MLS000568543 | SMR0001...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | -22.8 | 6.00E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43830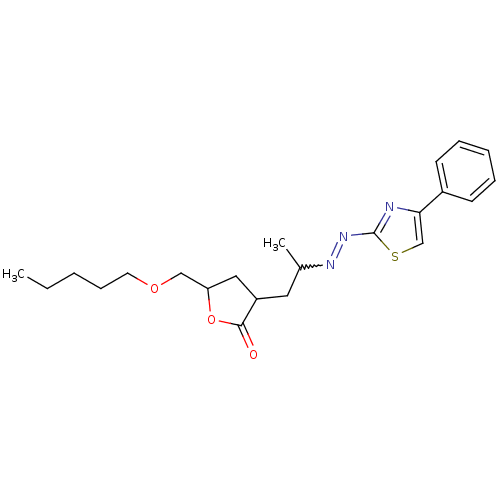 (5-(amoxymethyl)-3-[2-[(4-phenylthiazol-2-yl)hydraz...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | -22.7 | 4.50E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphotransferase (Trypanosoma brucei) | BDBM43831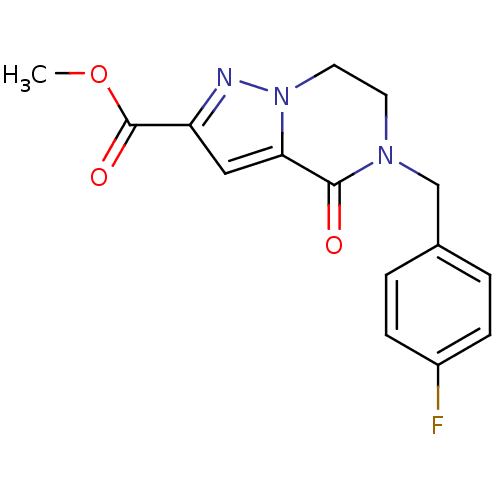 (5-(4-fluorobenzyl)-4-keto-6,7-dihydropyrazolo[1,5-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.14E+5 | -22.5 | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | 25 |
Groupe de Chimie Organique Biologique, Laboratoire Synthése Physico-Chimie des Molécules d'Inérêt Biologique | Assay Description Competitive inhibition assay for glucosamine derivatives on hexokinase from trypanosoma brucei. The inhibition of hexokinase by compounds was measur... | Chem Biol 9: 839-47 (2002) Article DOI: 10.1016/S1074-5521(02)00169-2 BindingDB Entry DOI: 10.7270/Q2MC8XF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50030947 (CHEMBL331845 | N-[2-(6-Amino-purin-9-yl)-4-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Leishmania mexicana | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50030949 ((2R,3R,4S,5R)-2-(6-Amino-8-thiophen-2-yl-purin-9-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Leishmania mexicana | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50030949 ((2R,3R,4S,5R)-2-(6-Amino-8-thiophen-2-yl-purin-9-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyd 3-phosphate dehydrogenase (gGAPDH) of Trypanosoma brucei | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50030949 ((2R,3R,4S,5R)-2-(6-Amino-8-thiophen-2-yl-purin-9-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of human erythrocyte | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase, glycosomal (Trypanosoma brucei brucei) | BDBM50380323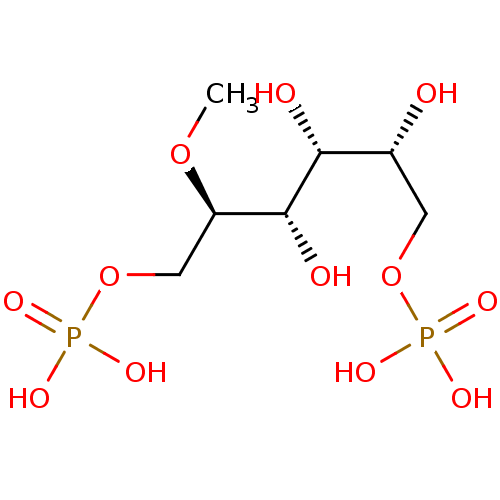 (CHEMBL2017785) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of His-tagged recombinant Trypanosoma brucei fructose bis-phosphate aldolase expressed in Escherichia coli using FBP as substrate after 5 ... | ACS Med Chem Lett 2: 804-808 (2011) Article DOI: 10.1021/ml200129s BindingDB Entry DOI: 10.7270/Q28S4QXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50030947 (CHEMBL331845 | N-[2-(6-Amino-purin-9-yl)-4-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Trypanosoma brucei | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50037775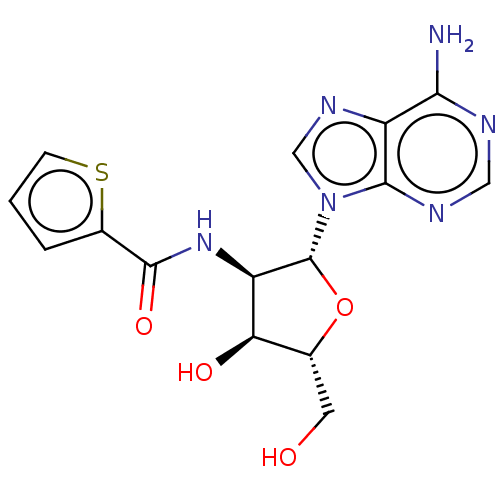 (CHEMBL121204 | Thiophene-2-carboxylic acid [2-(6-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Leishmania mexicana | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50037777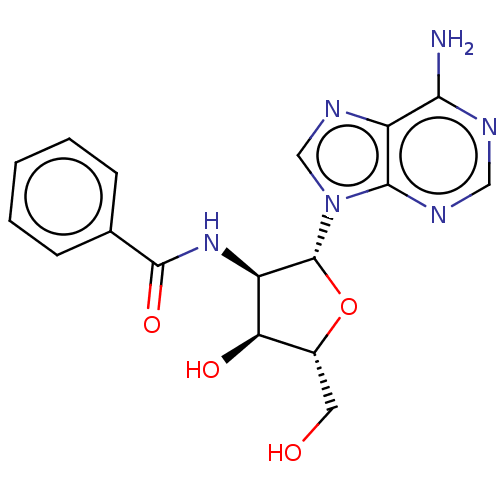 (CHEMBL120653 | N-[2-(6-Amino-purin-9-yl)-4-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Leishmania mexicana | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50037775 (CHEMBL121204 | Thiophene-2-carboxylic acid [2-(6-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Trypanosoma brucei | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50037777 (CHEMBL120653 | N-[2-(6-Amino-purin-9-yl)-4-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Trypanosoma brucei | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50368901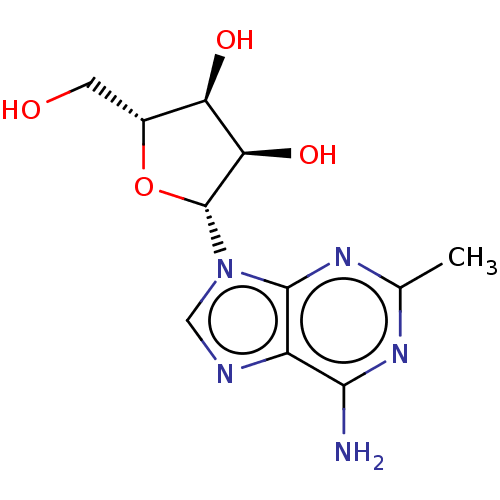 (CHEMBL608348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Leishmania mexicana | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase A (Oryctolagus cuniculus) | BDBM50380323 (CHEMBL2017785) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rabbit muscle FBA assessed as inhibition of FBP cleavage by spectrophotometry | ACS Med Chem Lett 2: 804-808 (2011) Article DOI: 10.1021/ml200129s BindingDB Entry DOI: 10.7270/Q28S4QXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fructose-bisphosphate aldolase A (Oryctolagus cuniculus) | BDBM50380322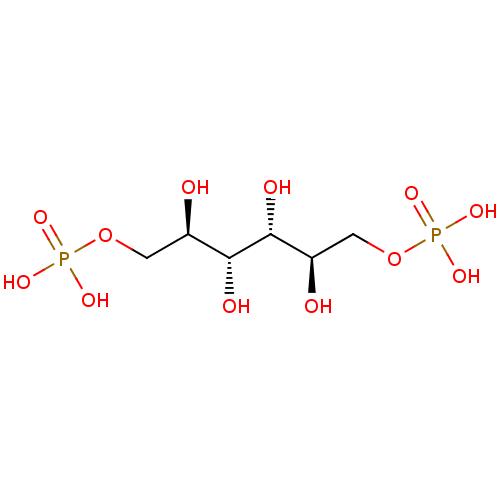 (CHEMBL258208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rabbit muscle FBA assessed as inhibition of FBP cleavage by spectrophotometry | ACS Med Chem Lett 2: 804-808 (2011) Article DOI: 10.1021/ml200129s BindingDB Entry DOI: 10.7270/Q28S4QXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50368901 (CHEMBL608348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of Trypanosoma brucei | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase, glycosomal (Trypanosoma brucei brucei) | BDBM50380322 (CHEMBL258208) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of His-tagged recombinant Trypanosoma brucei fructose bis-phosphate aldolase expressed in Escherichia coli using FBP as substrate after 5 ... | ACS Med Chem Lett 2: 804-808 (2011) Article DOI: 10.1021/ml200129s BindingDB Entry DOI: 10.7270/Q28S4QXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50368901 (CHEMBL608348) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of human erythrocyte | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50037775 (CHEMBL121204 | Thiophene-2-carboxylic acid [2-(6-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of human erythrocyte | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-bisphosphate aldolase, glycosomal (Trypanosoma brucei brucei) | BDBM50330437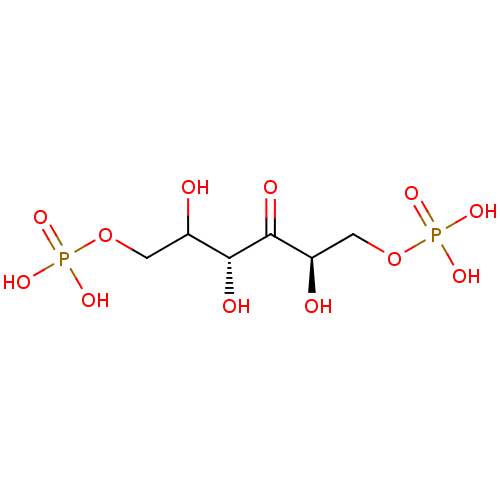 (1,6-di-O-phosphono-D-fructose | 2,3,4-trihydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of His-tagged recombinant Trypanosoma brucei fructose bis-phosphate aldolase expressed in Escherichia coli using FBP as substrate after 5 ... | ACS Med Chem Lett 2: 804-808 (2011) Article DOI: 10.1021/ml200129s BindingDB Entry DOI: 10.7270/Q28S4QXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyceraldehyde-3-phosphate dehydrogenase (Homo sapiens (Human)) | BDBM50030947 (CHEMBL331845 | N-[2-(6-Amino-purin-9-yl)-4-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Tested for the inhibitory activity against Glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH) of human erythrocyte | J Med Chem 37: 3605-13 (1994) BindingDB Entry DOI: 10.7270/Q26W9BQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 96 total ) | Next | Last >> |