

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430060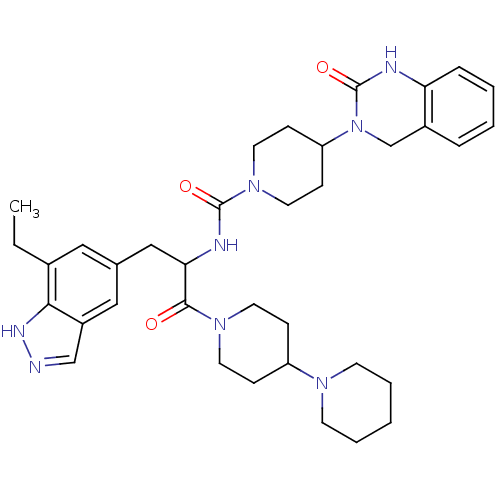 (CHEMBL2336421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50273292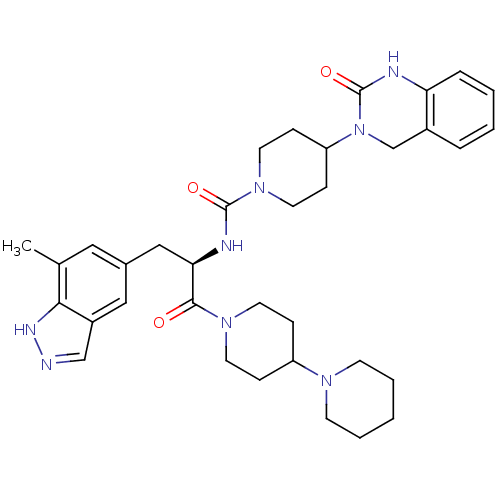 ((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108297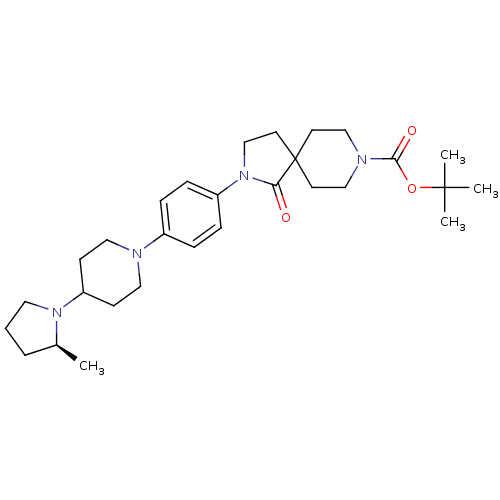 (US8604046, 77) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50273292 ((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [I125]CGRP from human CGRP receptor in SK-N-MC cells | J Med Chem 51: 4858-61 (2008) Article DOI: 10.1021/jm800546t BindingDB Entry DOI: 10.7270/Q2N016BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50184069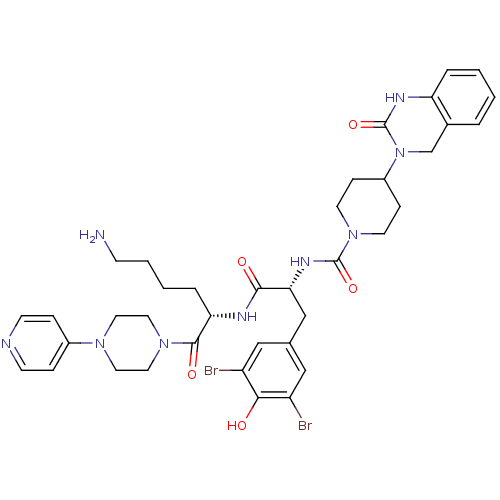 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Antagonist activity at human CGRP receptor | Bioorg Med Chem Lett 23: 3157-61 (2013) Article DOI: 10.1016/j.bmcl.2013.04.012 BindingDB Entry DOI: 10.7270/Q2348MSQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50184069 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells | ACS Med Chem Lett 3: 337-341 (2012) Article DOI: 10.1021/ml300021s BindingDB Entry DOI: 10.7270/Q26D5V2R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430061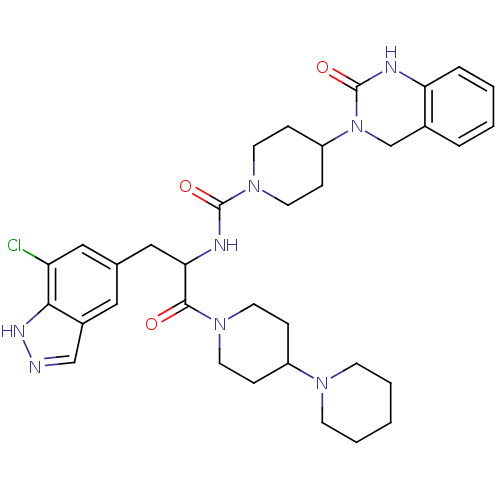 (CHEMBL2336422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50273292 ((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268484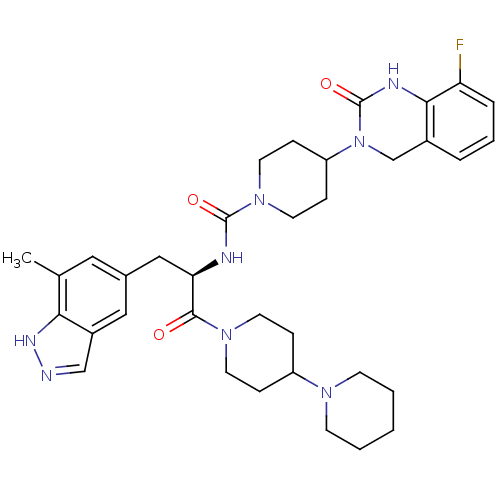 ((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Displacement of [I125]CGRP from human CGRP receptor in SK-N-MC cells | J Med Chem 51: 4858-61 (2008) Article DOI: 10.1021/jm800546t BindingDB Entry DOI: 10.7270/Q2N016BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268484 ((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50268484 ((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Antagonist activity at human CGRP receptor | Bioorg Med Chem Lett 23: 3157-61 (2013) Article DOI: 10.1016/j.bmcl.2013.04.012 BindingDB Entry DOI: 10.7270/Q2348MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576175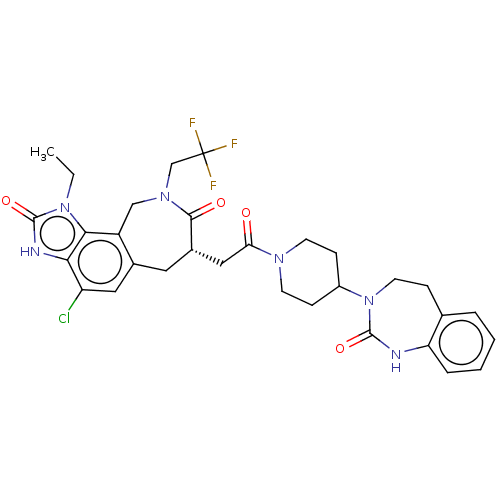 (CHEMBL4859941) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576173 (CHEMBL4848032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430062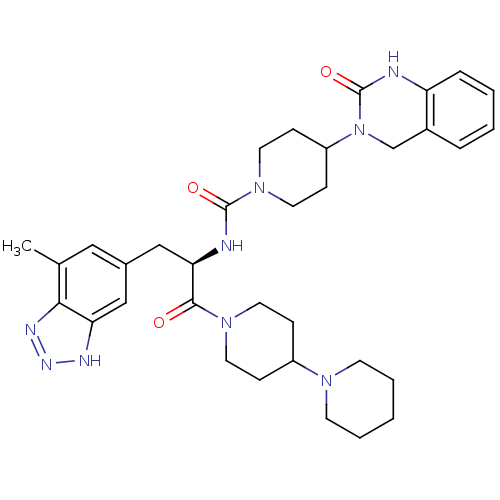 (CHEMBL2336411) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576172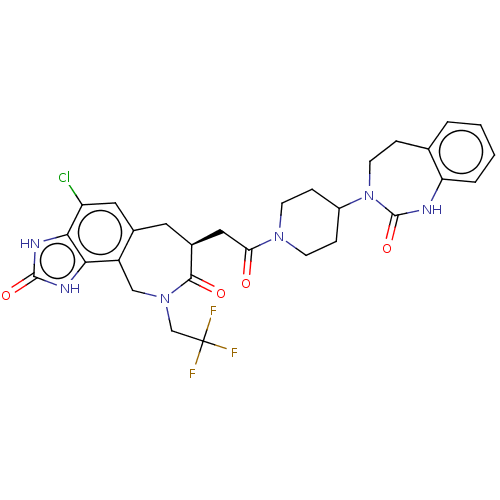 (CHEMBL4848486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576174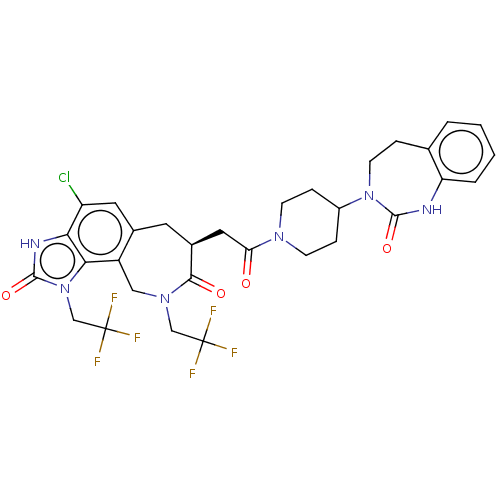 (CHEMBL4857649) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50356282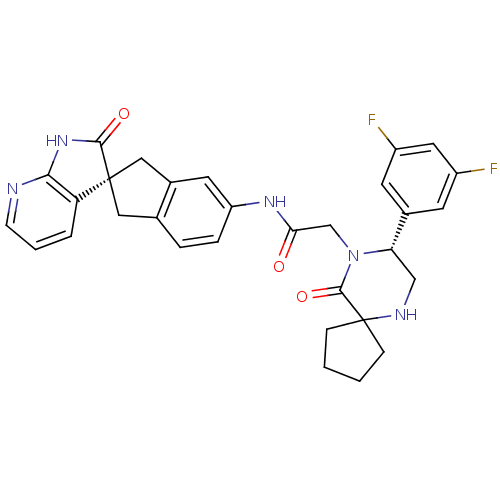 (CHEMBL1910936) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Antagonist activity at human CGRP receptor | Bioorg Med Chem Lett 23: 3157-61 (2013) Article DOI: 10.1016/j.bmcl.2013.04.012 BindingDB Entry DOI: 10.7270/Q2348MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430066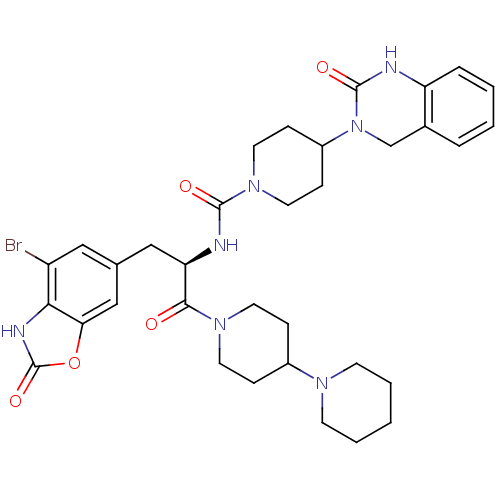 (CHEMBL2336416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50436107 (CHEMBL2397415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cell membranes after 2 hrs by scintillation counting analysis | Bioorg Med Chem Lett 23: 3157-61 (2013) Article DOI: 10.1016/j.bmcl.2013.04.012 BindingDB Entry DOI: 10.7270/Q2348MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576177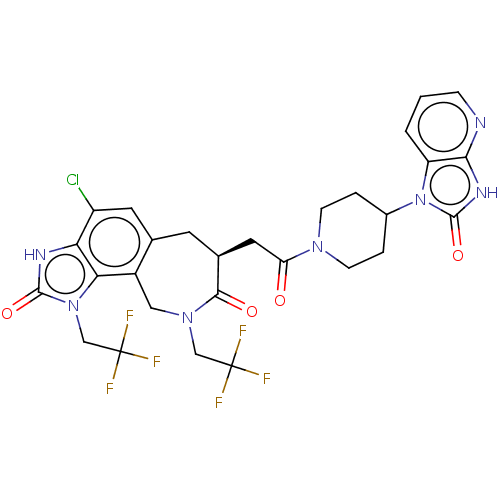 (CHEMBL4875327) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400098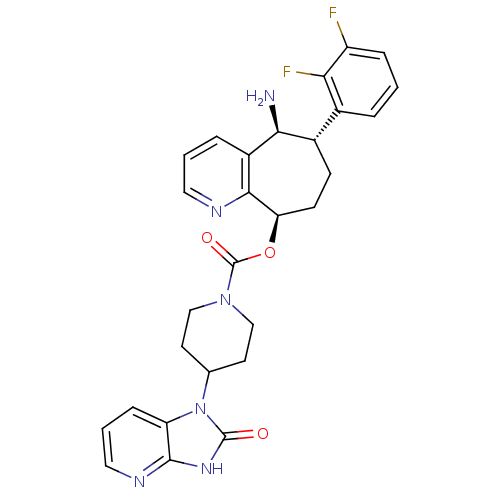 (CHEMBL2178422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108281 (US8604046, 61) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430058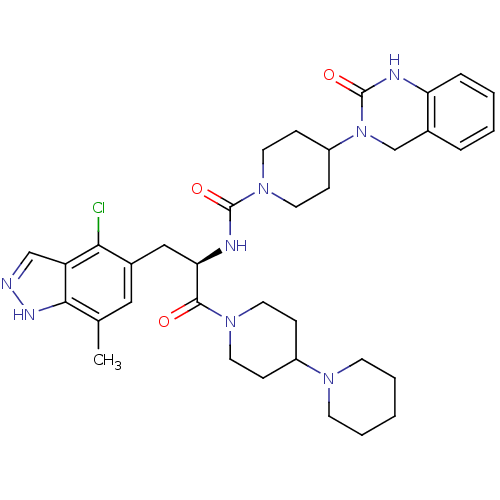 (CHEMBL2336418) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430057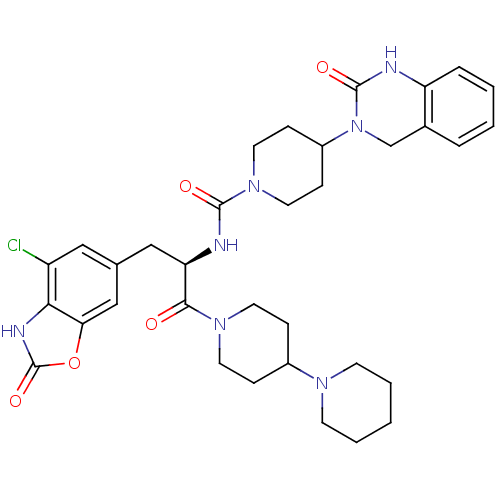 (CHEMBL2336417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108294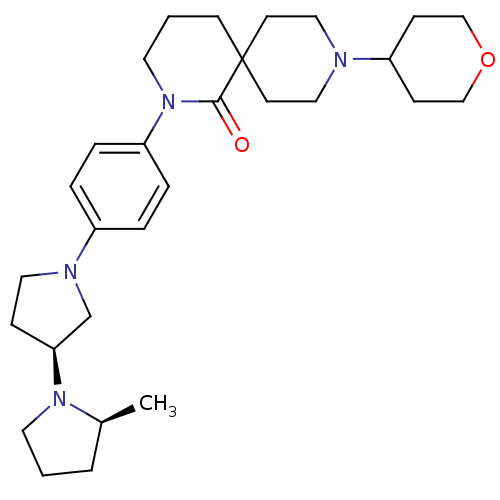 (US8604046, 74) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108315 (US8604046, 95) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576176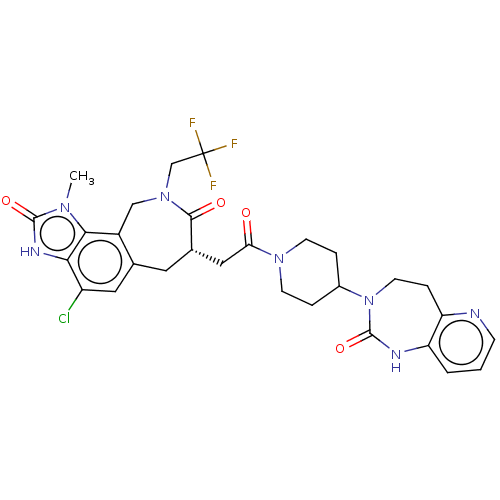 (CHEMBL4874157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50548647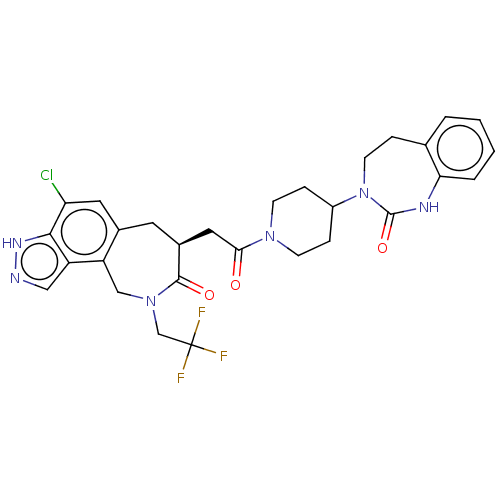 (CHEMBL4746351) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430059 (CHEMBL2336419) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430065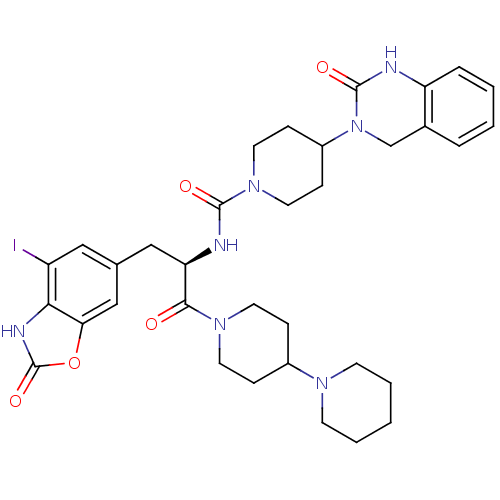 (CHEMBL2336415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108295 (US8604046, 75) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108284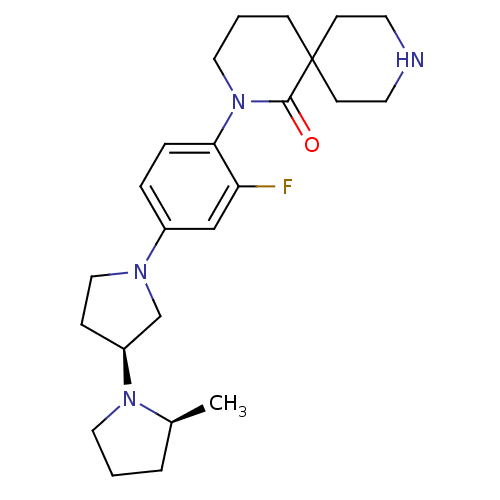 (US8604046, 64) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576182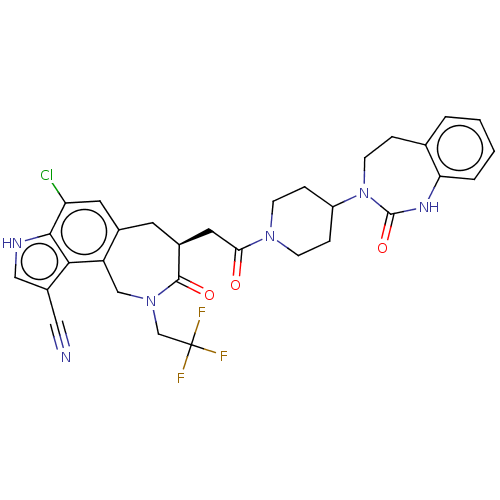 (CHEMBL4874451) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576183 (CHEMBL4871223) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400099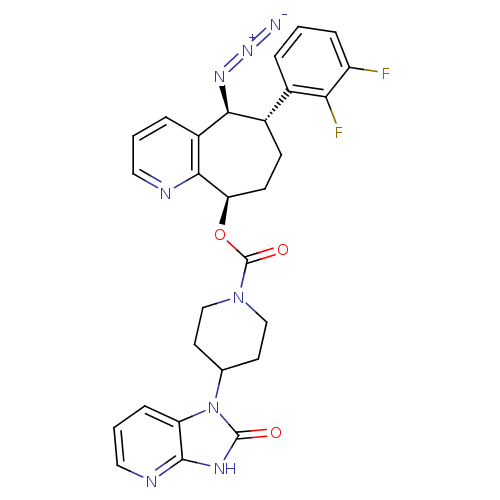 (CHEMBL2178420) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50576185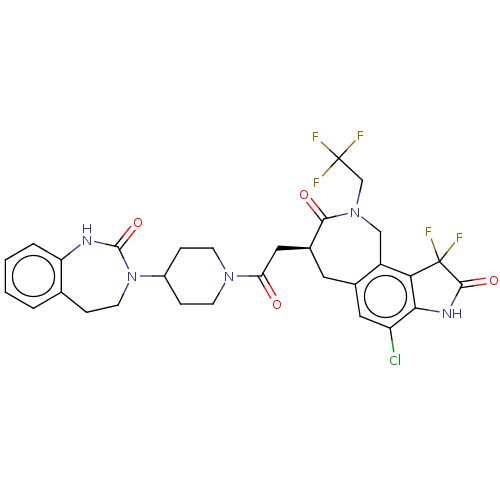 (CHEMBL4873378) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]CGRP from human CGRP receptor in human SK-N-MC cells measured after 2 hrs by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128077 BindingDB Entry DOI: 10.7270/Q23N276F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108282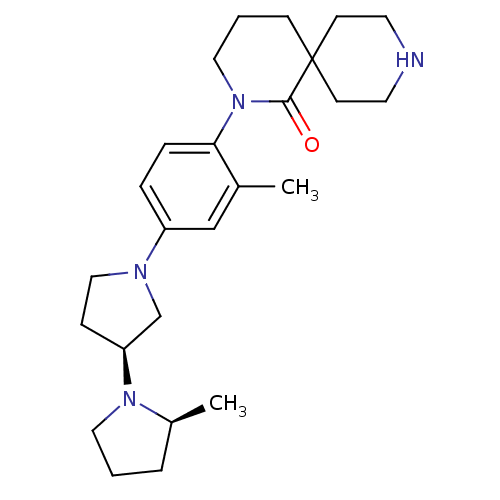 (US8604046, 62) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50388882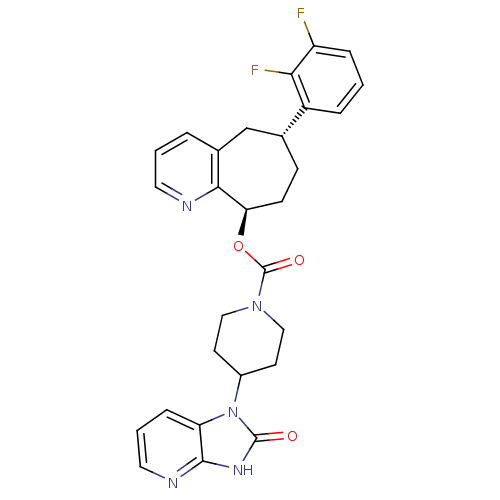 (CHEMBL2063115) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells | ACS Med Chem Lett 3: 337-341 (2012) Article DOI: 10.1021/ml300021s BindingDB Entry DOI: 10.7270/Q26D5V2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM82288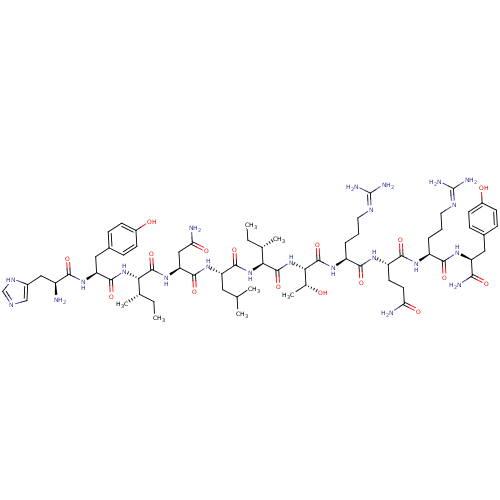 (NPY, porcine | NPY26-36, porcine) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | EMBO J 14: 2806-15 (1995) BindingDB Entry DOI: 10.7270/Q2BK19VW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50382794 (CHEMBL2023191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells | ACS Med Chem Lett 3: 337-341 (2012) Article DOI: 10.1021/ml300021s BindingDB Entry DOI: 10.7270/Q26D5V2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108310 (US8604046, 90) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50400102 (CHEMBL2178424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis | J Med Chem 55: 10644-51 (2012) Article DOI: 10.1021/jm3013147 BindingDB Entry DOI: 10.7270/Q2M046M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108328 (US8604046, 108) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50548648 (CHEMBL4746329) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human CGRP receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127624 BindingDB Entry DOI: 10.7270/Q2959N5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490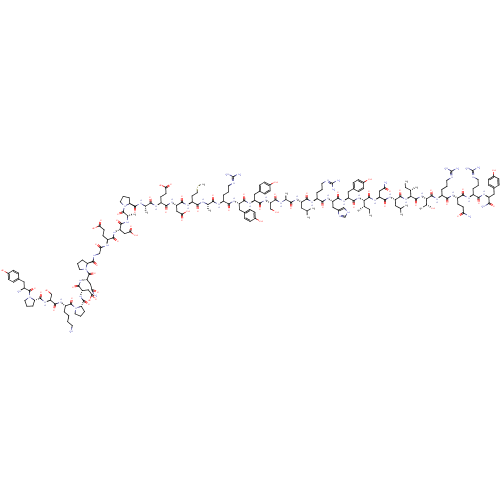 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | EMBO J 14: 2806-15 (1995) BindingDB Entry DOI: 10.7270/Q2BK19VW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108296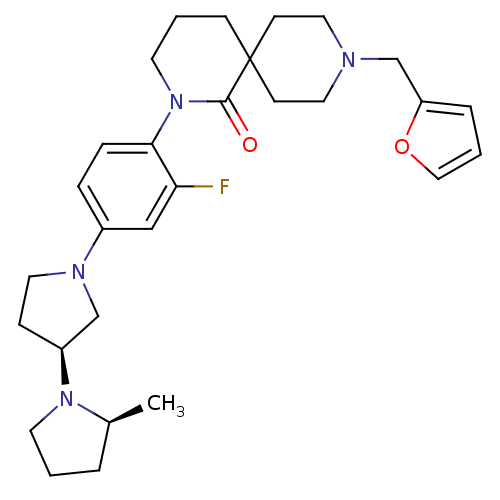 (US8604046, 76) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108303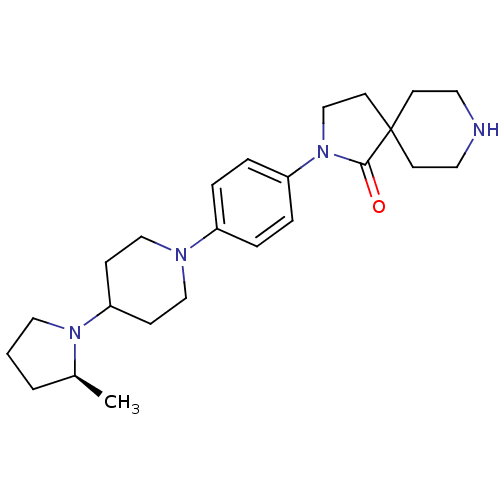 (US8604046, 83) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50548647 (CHEMBL4746351) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human CGRP receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127624 BindingDB Entry DOI: 10.7270/Q2959N5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108316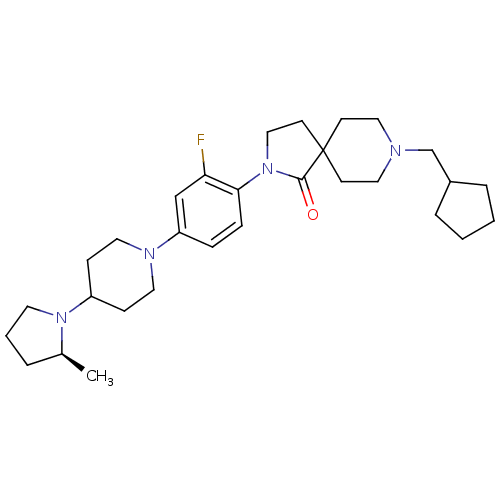 (US8604046, 96) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Macaca mulatta (Rhesus macaque)) | BDBM108283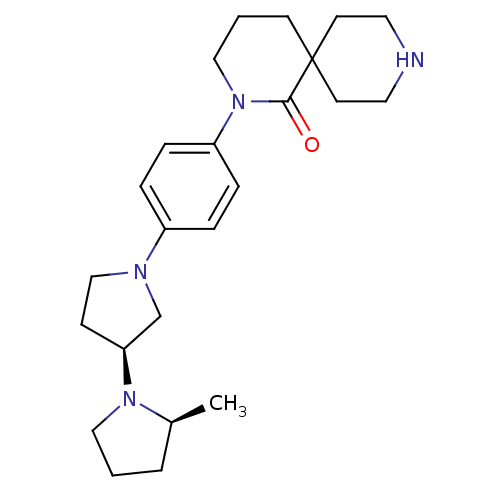 (US8604046, 63) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description The compounds of this invention have been demonstrated to displace [3H]- methylhistamine radioligand binding to mammalian cell membranes expressing r... | US Patent US8604046 (2013) BindingDB Entry DOI: 10.7270/Q2W957TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7025 total ) | Next | Last >> |