

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 1 (RAT) | BDBM86211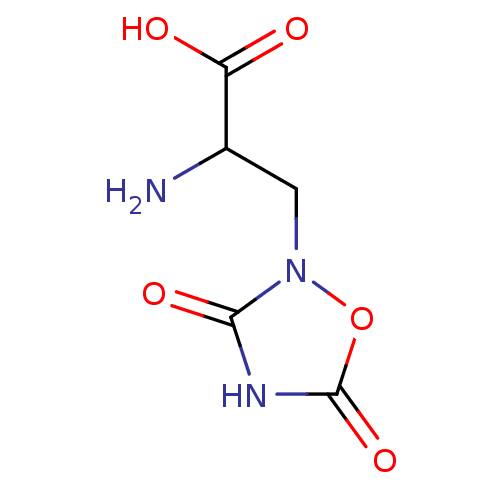 (CAS_52809-07-1 | NSC_40539 | Quisqualate) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50002371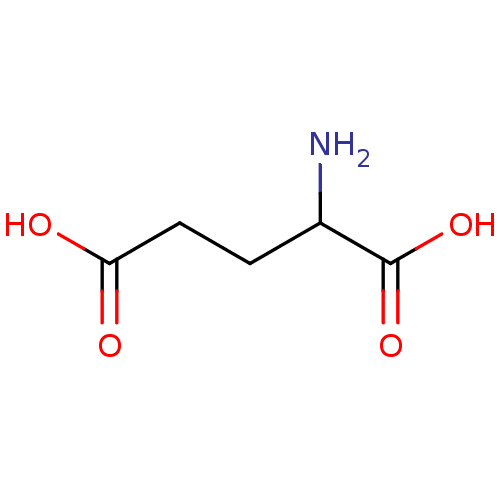 (2-aminopentanedioic acidglutamic acid | CHEMBL2763...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21279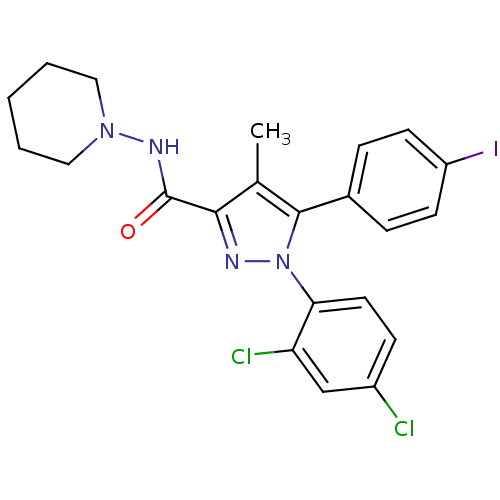 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50171313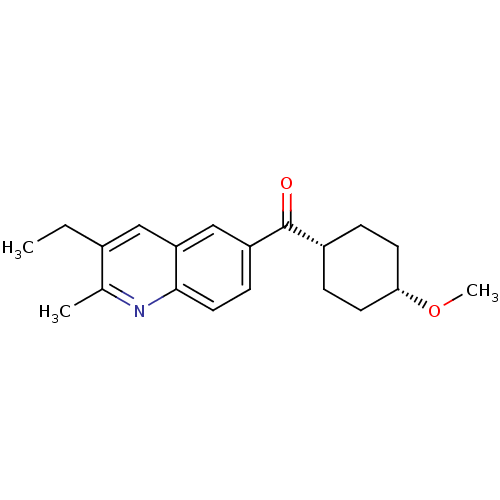 ((3-Ethyl-2-methyl-quinolin-6-yl)-(4-methoxy-cycloh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro affinity for cloned rat metabotropic glutamate 1 receptors stably expressed on CHO cells determined using [3H]-R214127 as radioligand | J Med Chem 48: 5096-9 (2005) Article DOI: 10.1021/jm050263+ BindingDB Entry DOI: 10.7270/Q29S1QJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50089896 (4-(Amino-carboxy-methyl)-3-methyl-benzoic acid((+)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50163606 (1-(3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-2-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50231744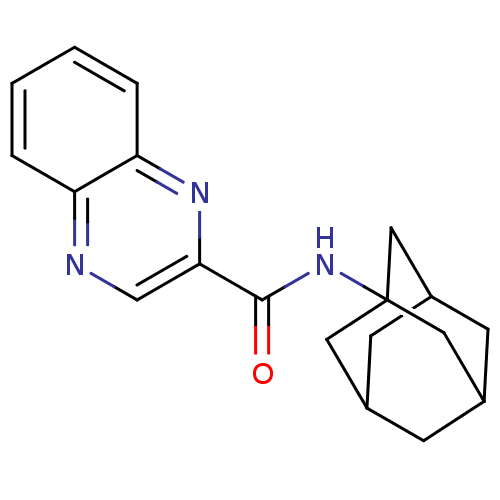 (CHEMBL399160 | NPS 2390 | quinoxaline-2-carboxylic...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123685 (4-Bromo-5-(4-chloro-phenyl)-1-(2,4-dichloro-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50030628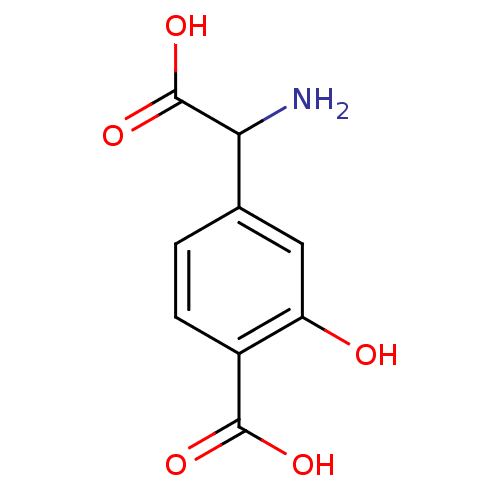 ((S)-4C3HPG | 4-(Amino-carboxy-methyl)-2-hydroxy-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM86212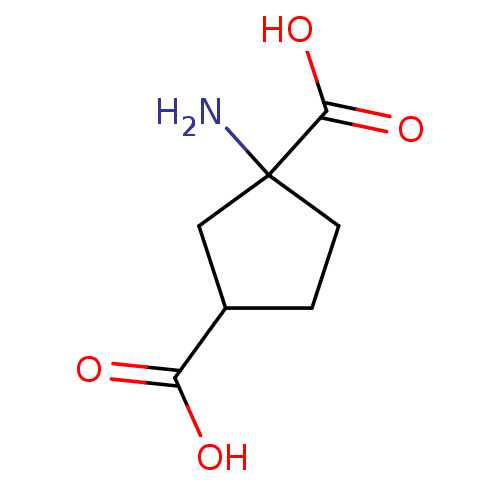 (1S, 3R-ACPD | CAS_104766 | NSC_104766) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266832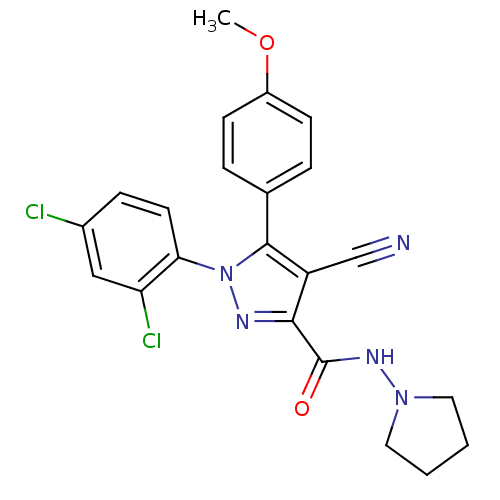 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-methoxyphenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123690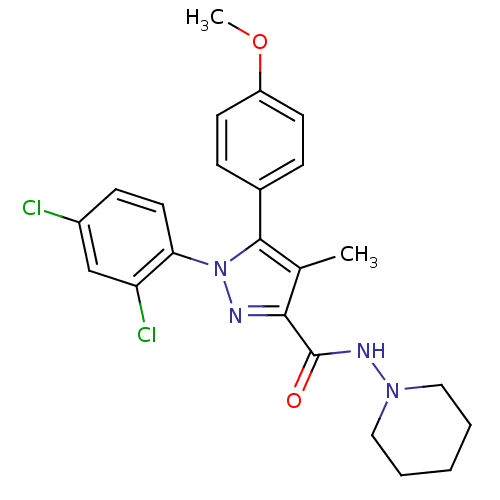 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50030629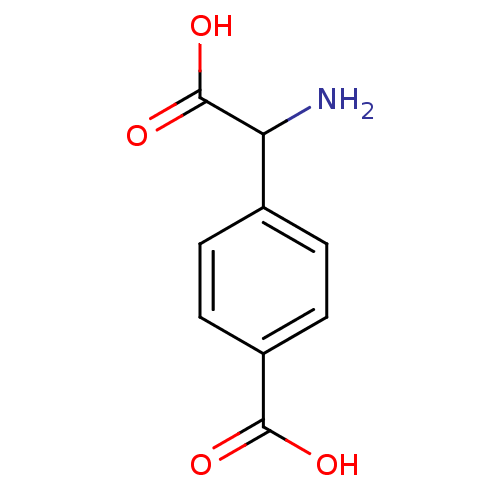 ((R,S)-4-Carboxy-3-hydroxyphenylglycine | (S)-4CPG ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267374 (1-(2-bromophenyl)-4-cyano-5-(4-methoxyphenyl)-N-(p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123688 (4-Bromo-1-(2,4-dichloro-phenyl)-5-(4-methoxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123693 (4-Bromo-1-(2-chloro-phenyl)-5-(4-methoxy-phenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123689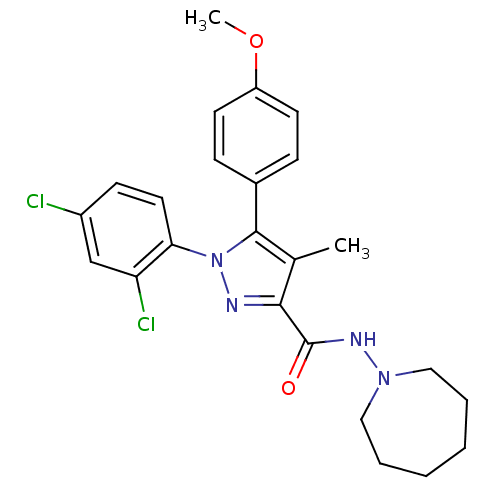 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123691 (5-Benzo[1,3]dioxol-5-yl-1-(2,4-dichloro-phenyl)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123684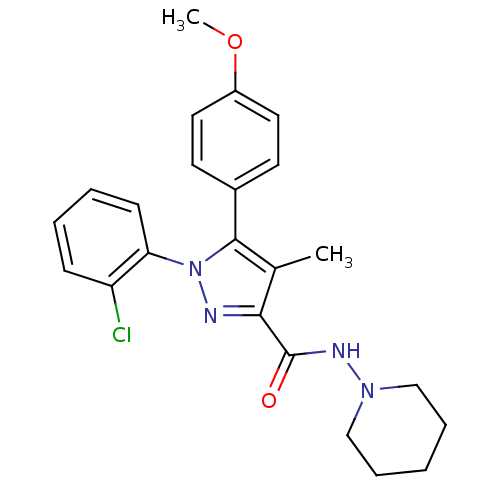 (1-(2-Chloro-phenyl)-5-(4-methoxy-phenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123692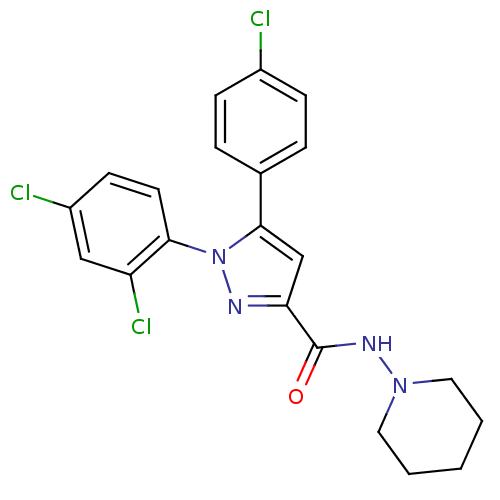 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Mus musculus (mouse)) | BDBM50442299 (CHEMBL2442750) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from mouse CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266833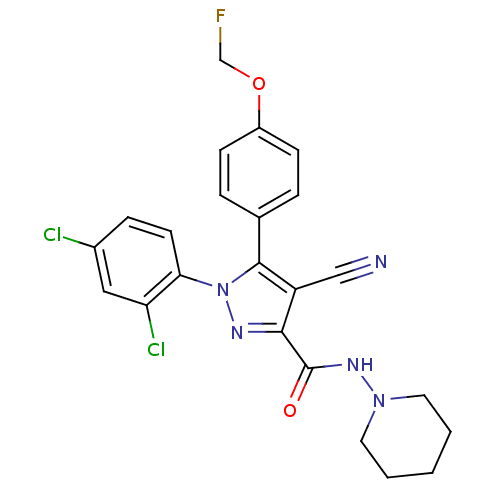 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-(fluoromethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM17786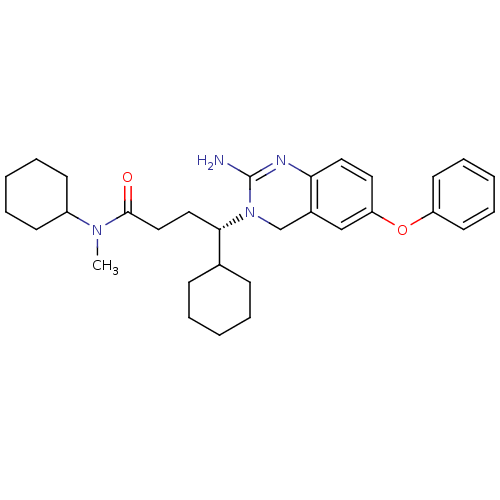 ((4S)-4-(2-amino-6-phenoxy-3,4-dihydroquinazolin-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267373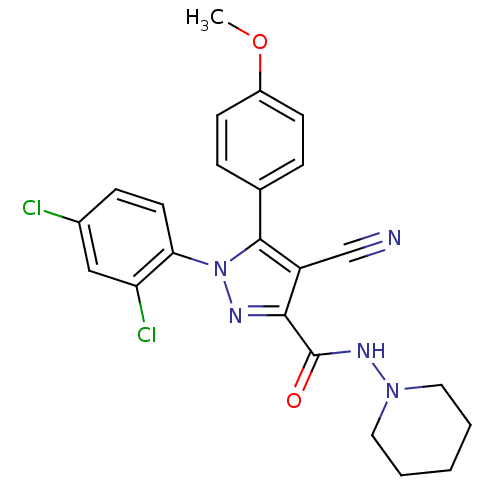 (4-cyano-1-(2,4-dichlorophenyl)-5-(4-methoxyphenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50212323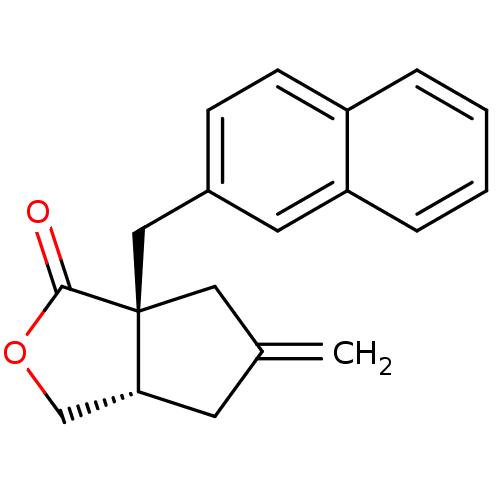 ((3aS,6aS)-5-methylene-6a-(naphthalen-2-ylmethyl)-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267375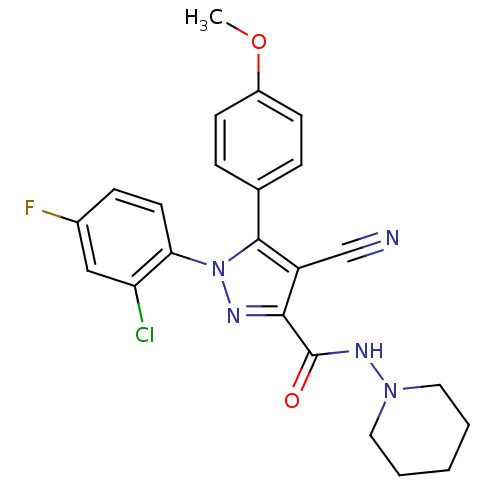 (1-(2-Chloro-4-fluorophenyl)-4-cyano-5-(4-methoxyph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266809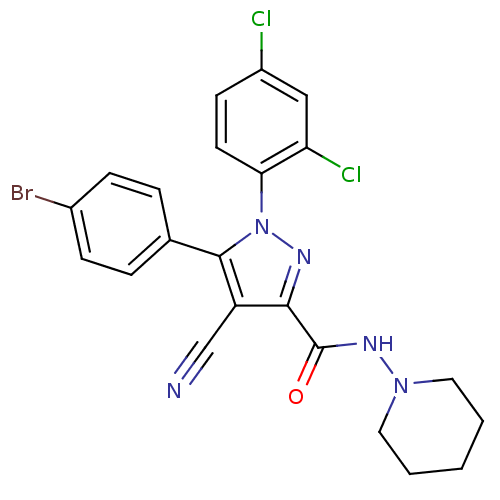 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-bromophenyl)-N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123683 (1-(2-Chloro-phenyl)-4-fluoro-5-(4-methoxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266864 (5-(4-chlorophenyl)-4-cyano-1-(2,4-dichlorophenyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM17785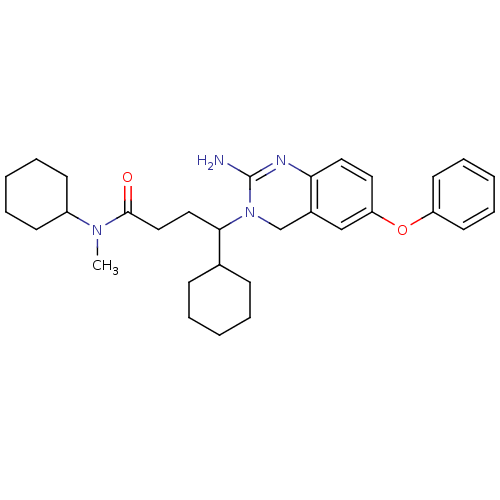 (2-aminoquinazoline, 3 | 4-(2-amino-6-phenoxy-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50296848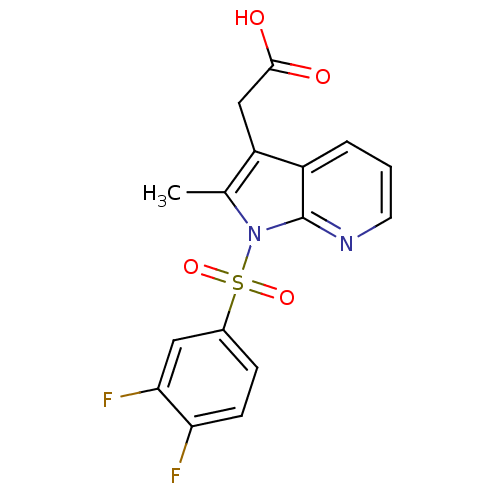 (2-(1-(3,4-difluorophenylsulfonyl)-2-methyl-1H-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266807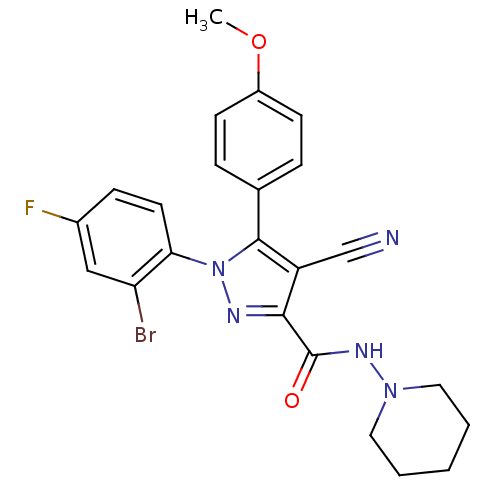 (1-(2-Bromo-4-fluorophenyl)-4-cyano-5-(4-methoxyphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266808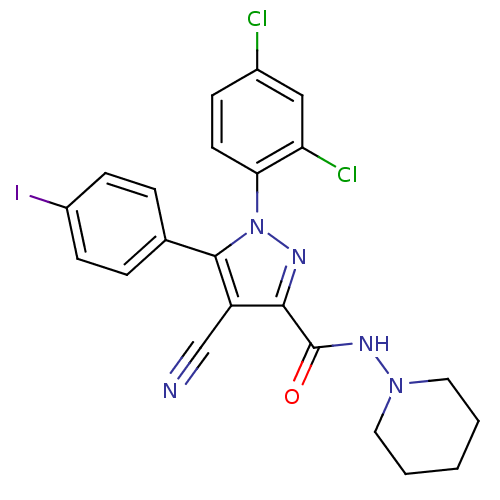 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-iodophenyl)-N-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50442299 (CHEMBL2442750) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123687 (1-(2-Fluoro-phenyl)-5-(4-methoxy-phenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50030630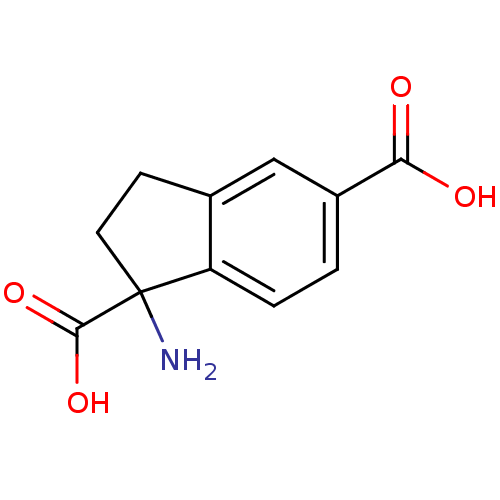 ((RS)-1-aminoindan-1,5-dicarboxylic acid | 1-Amino-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 98.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50296851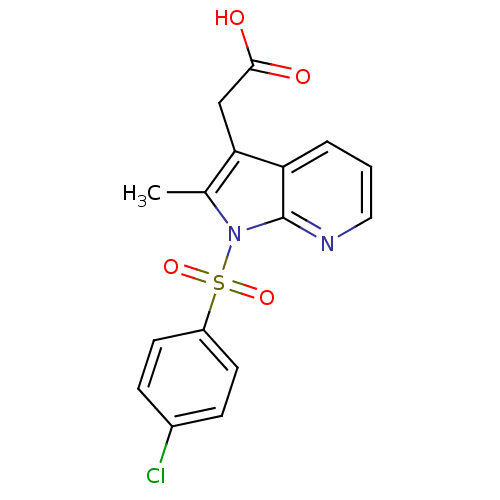 (2-(1-(4-chlorophenylsulfonyl)-2-methyl-1H-pyrrolo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123694 (1-(2,4-Dichloro-phenyl)-5-(4-hydroxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50442309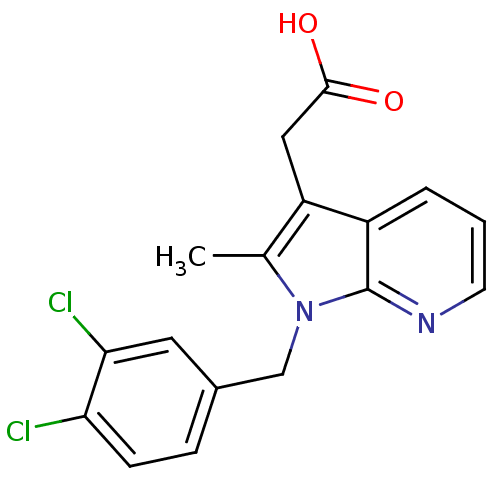 (CHEMBL2442743) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50266830 (1-(2,4-Dichlorophenyl)-4-cyano-5-(4-iodophenyl)-N-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 form human recombinant CB1 receptor expressed in HEK293 cells by liquid scintillation counting | Eur J Med Chem 44: 593-608 (2009) Article DOI: 10.1016/j.ejmech.2008.03.040 BindingDB Entry DOI: 10.7270/Q2K35TFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50442310 (CHEMBL2442742) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50442300 (CHEMBL2442751) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50442302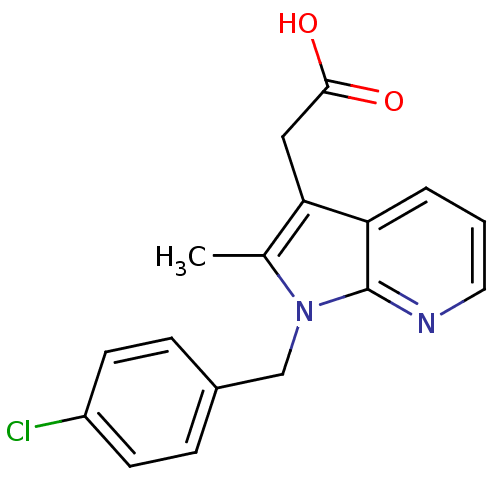 (CHEMBL2442736) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM17784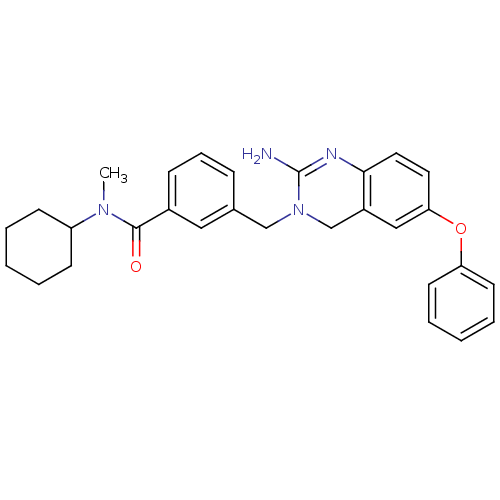 (2-aminoquinazoline, 2 | 3-[(2-amino-6-phenoxy-3,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 158 | -38.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50030627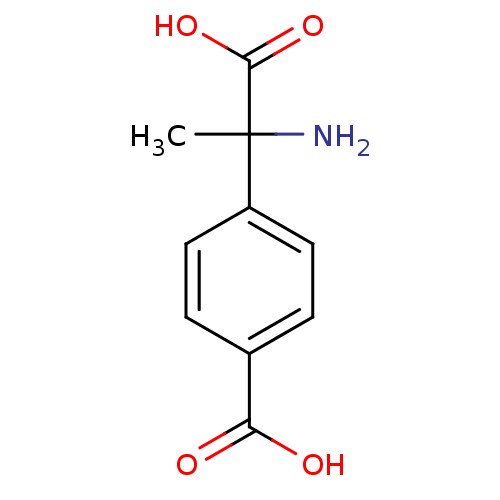 ((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development, Beerse Curated by PDSP Ki Database | Mol Pharmacol 63: 1082-93 (2003) Article DOI: 10.1124/mol.63.5.1082 BindingDB Entry DOI: 10.7270/Q2BV7F62 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50442303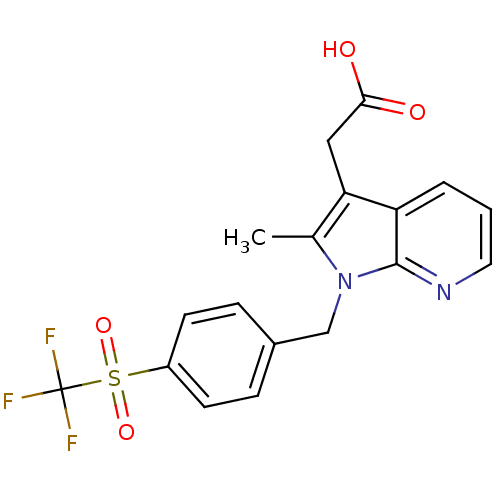 (CHEMBL2442752) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50442301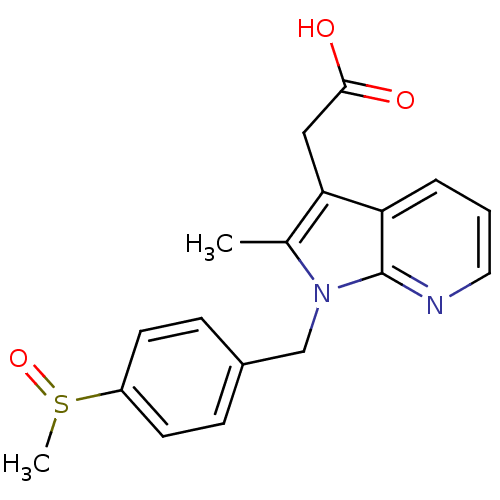 (CHEMBL2442748) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-prostaglandin D2 from human CRTh2 receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem 21: 6582-91 (2013) Article DOI: 10.1016/j.bmc.2013.08.025 BindingDB Entry DOI: 10.7270/Q2QR4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1603 total ) | Next | Last >> |