

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170654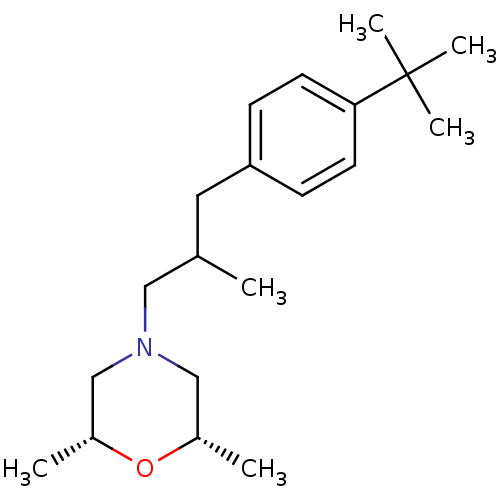 ((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230127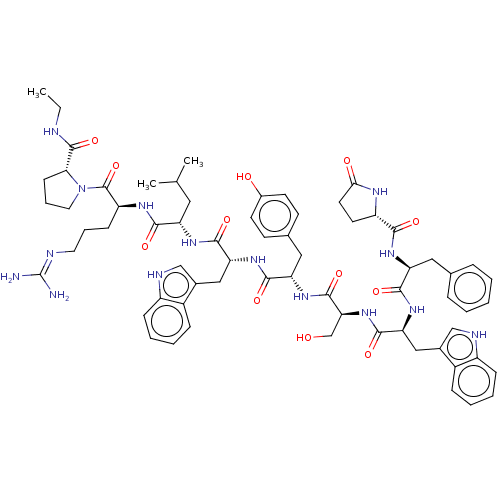 (CHEMBL407606) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170652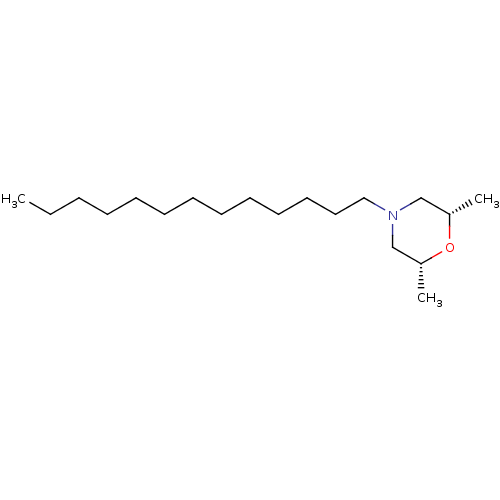 ((2R,6S)-2,6-Dimethyl-4-tridecyl-morpholine | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230129 (CHEMBL407123) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM50170654 ((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230120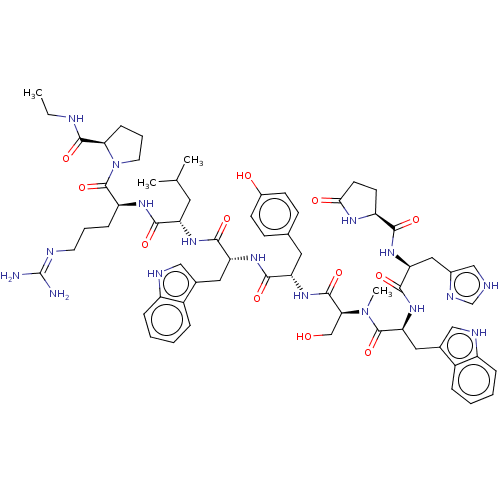 (CHEMBL405737) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.0776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM50170652 ((2R,6S)-2,6-Dimethyl-4-tridecyl-morpholine | CHEMB...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230123 (CHEMBL385042) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM50047016 (1-(4-Fluoro-phenyl)-4-[4-hydroxy-4-(3-trifluoromet...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230122 (CHEMBL415571) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230126 (CHEMBL385468) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM50170651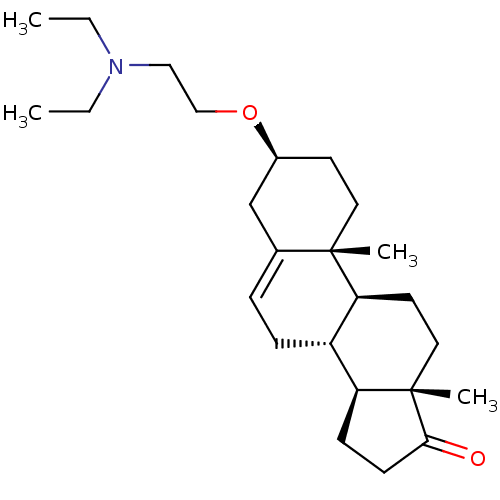 ((3S,8R,9S,10R,13S,14S)-3-(2-Diethylamino-ethoxy)-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50272695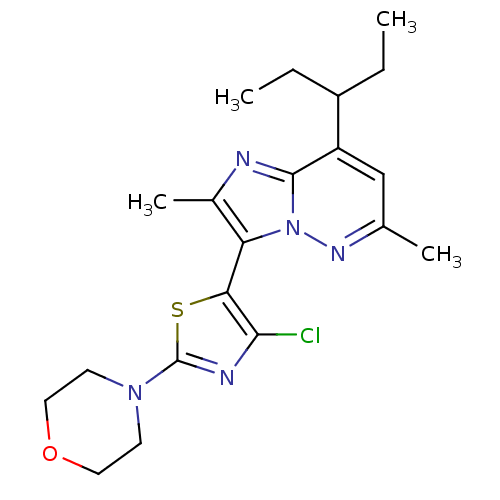 (3-(4-chloro-2-morpholinothiazol-5-yl)-2,6-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from human CRF1 receptor expressed in IMR32 cells | Bioorg Med Chem Lett 18: 4486-90 (2008) Article DOI: 10.1016/j.bmcl.2008.07.063 BindingDB Entry DOI: 10.7270/Q2NG4RJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170636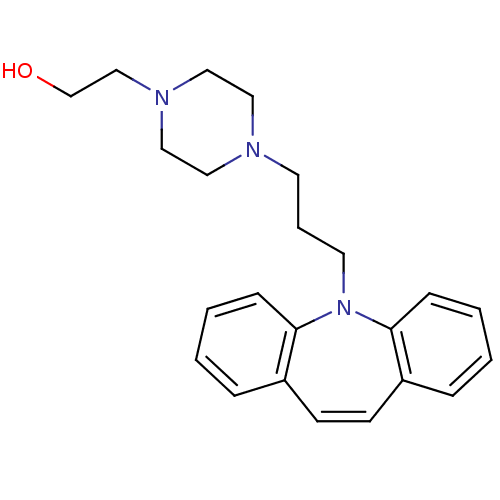 (2-[4-(3-Dibenzo[b,f]azepin-5-yl-propyl)-piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50272695 (3-(4-chloro-2-morpholinothiazol-5-yl)-2,6-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CRF-1 receptor | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230124 (CHEMBL429240) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50082040 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German National Research Center for Information Technology Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | J Med Chem 42: 4422-33 (1999) BindingDB Entry DOI: 10.7270/Q2D21WVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230128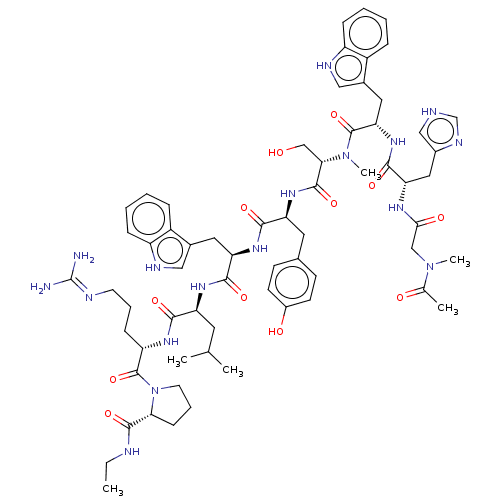 (CHEMBL437798) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM50003027 ((4aS,5S,6S,8aS)-5,8a-Dimethyl-2-(1,5,9-trimethyl-d...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170650 ((2-{4-[(2-Chloro-benzylamino)-methyl]-cyclohexyl}-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50003027 ((4aS,5S,6S,8aS)-5,8a-Dimethyl-2-(1,5,9-trimethyl-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50003027 ((4aS,5S,6S,8aS)-5,8a-Dimethyl-2-(1,5,9-trimethyl-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for human EMP expressed in ERG2 deficient strain of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine as radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170657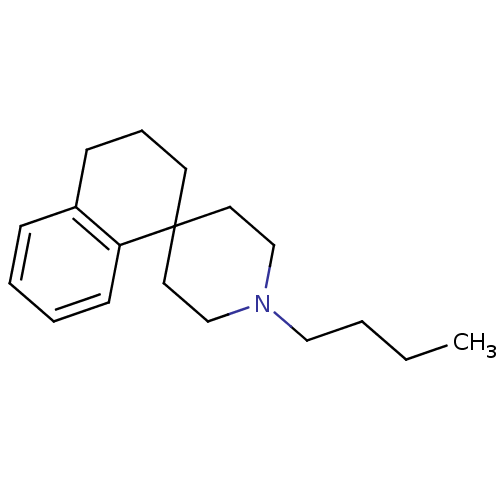 (1''-butyl-3,4-dihydro-2H-spiro[naphthalene-1,4''-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM50170656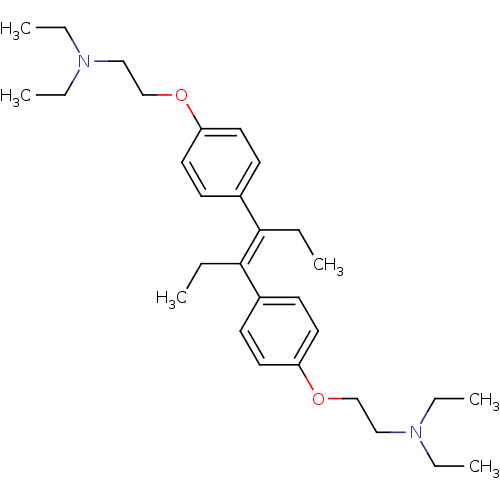 (CHEMBL190631 | [2-(4-{(E)-2-[4-(2-Diethylamino-eth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50047016 (1-(4-Fluoro-phenyl)-4-[4-hydroxy-4-(3-trifluoromet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50065941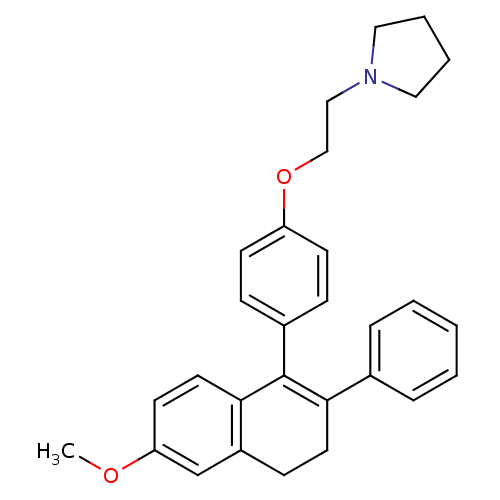 (1-{2-[4-(6-Methoxy-2-phenyl-3,4-dihydro-naphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for human EMP expressed in ERG2 deficient strain of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine as radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50132951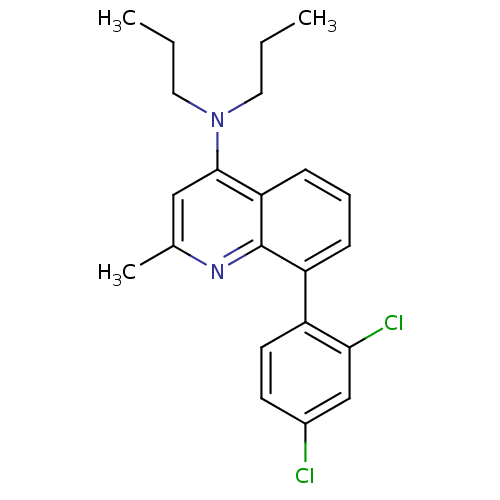 (8-(2,4-dichlorophenyl)-2-methyl-N,N-dipropylquinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CRF-1 receptor | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50132951 (8-(2,4-dichlorophenyl)-2-methyl-N,N-dipropylquinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170651 ((3S,8R,9S,10R,13S,14S)-3-(2-Diethylamino-ethoxy)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM55354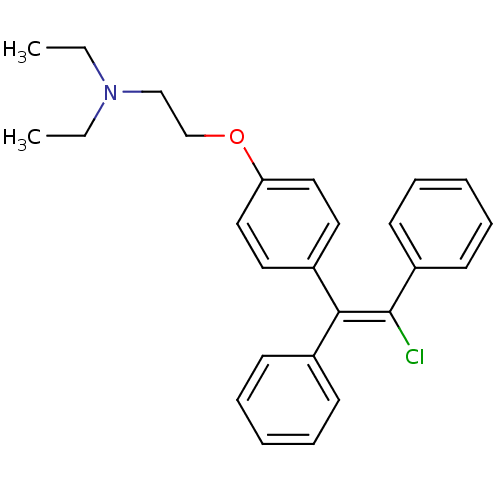 (CLOMIPHENE | CLOMIPHENE CITRATE | MLS000069760 | S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for human EMP expressed in ERG2 deficient strain of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine as radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM50080017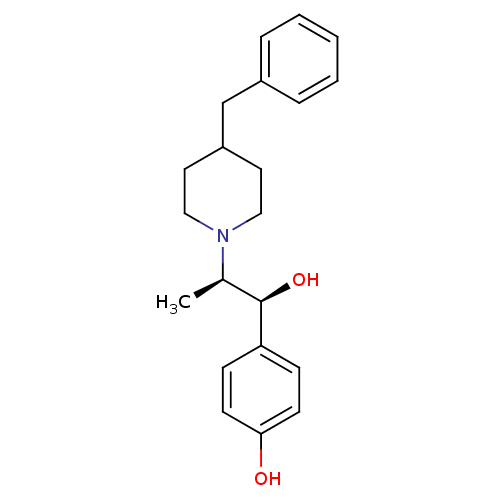 ((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50170657 (1''-butyl-3,4-dihydro-2H-spiro[naphthalene-1,4''-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for human EMP expressed in ERG2 deficient strain of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine as radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50170652 ((2R,6S)-2,6-Dimethyl-4-tridecyl-morpholine | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for human EMP expressed in ERG2 deficient strain of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine as radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for human EMP expressed in ERG2 deficient strain of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine as radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170659 (2-[3-(Methyl-phenethyl-amino)-propyl]-2-phenyl-tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170660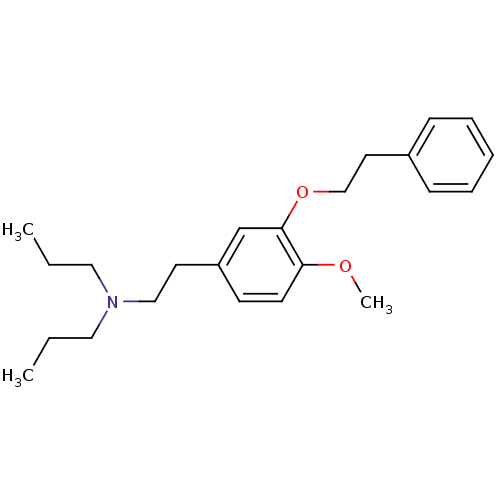 (CHEMBL190883 | CHEMBL521582 | N,N-dipropyl-2-[4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM18957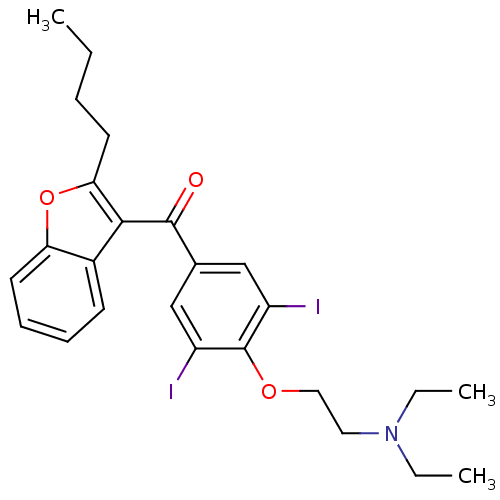 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170648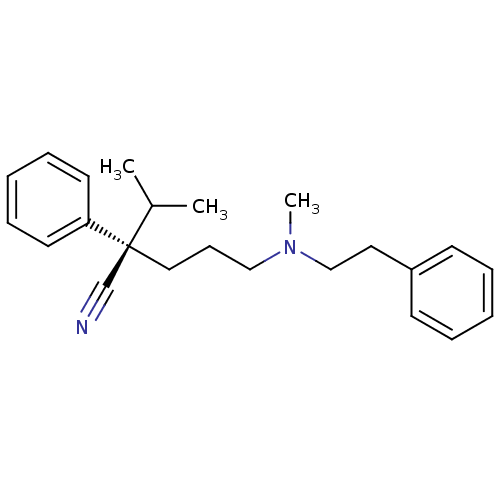 ((R)-2-Isopropyl-5-(methyl-phenethyl-amino)-2-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348782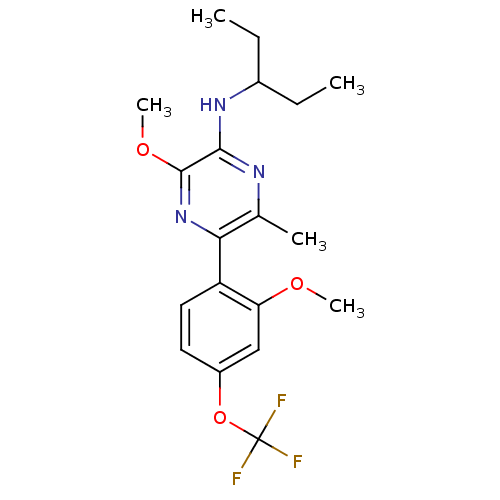 (CHEMBL1807053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Binding affinity towards Dopamine type 2 receptor was determined by displacement assays using [3H]-YM 09151 as the competitive ligand | Bioorg Med Chem Lett 12: 3111-5 (2002) BindingDB Entry DOI: 10.7270/Q2DZ07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348778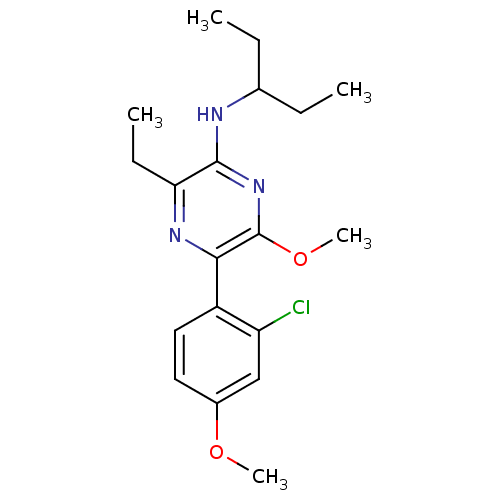 (CHEMBL1807058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50170639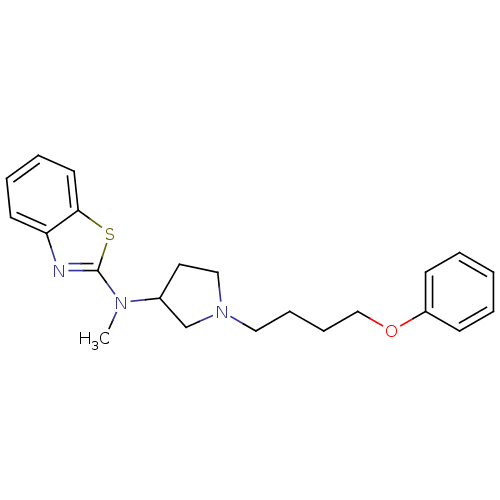 (Benzothiazol-2-yl-methyl-[1-(4-phenoxy-butyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for sigma receptor type 1 of guinea pig using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348829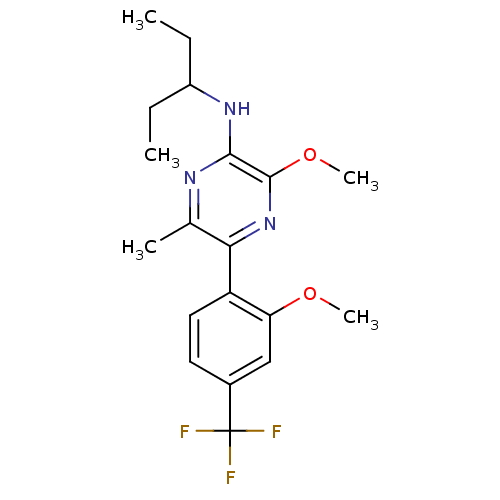 (CHEMBL1807054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Rattus norvegicus) | BDBM50230130 (CHEMBL266205) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay | J Med Chem 35: 3890-4 (1992) BindingDB Entry DOI: 10.7270/Q24Q7X6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348783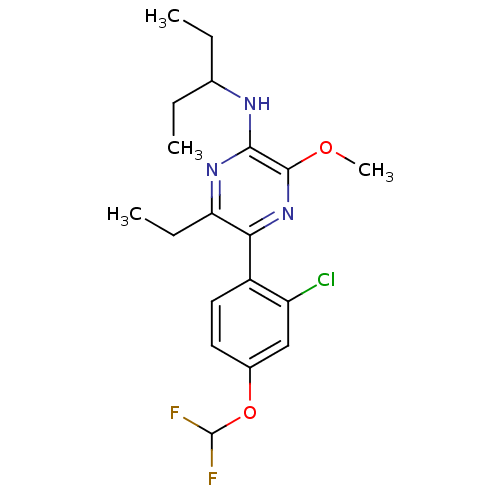 (CHEMBL1807052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348786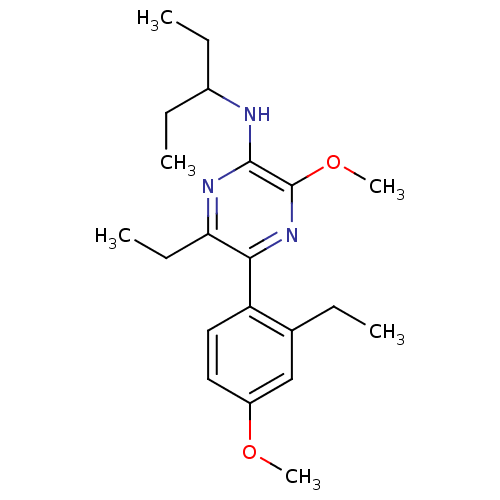 (CHEMBL1807049) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50119970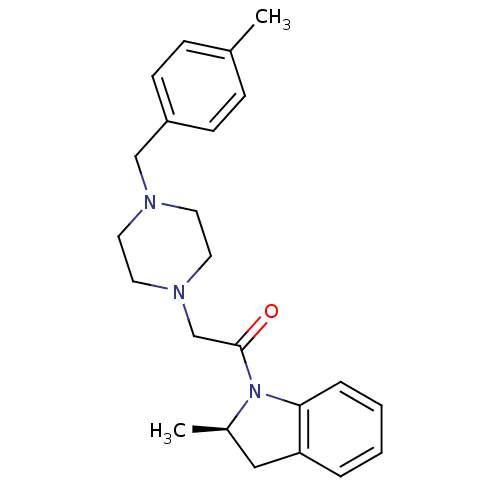 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D4 was determined via standard competitive displacement assays using [3H]-YM 09151 as the competitive liga... | Bioorg Med Chem Lett 12: 3111-5 (2002) BindingDB Entry DOI: 10.7270/Q2DZ07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-8 sterol isomerase ERG2 (Saccharomyces cerevisiae) | BDBM71545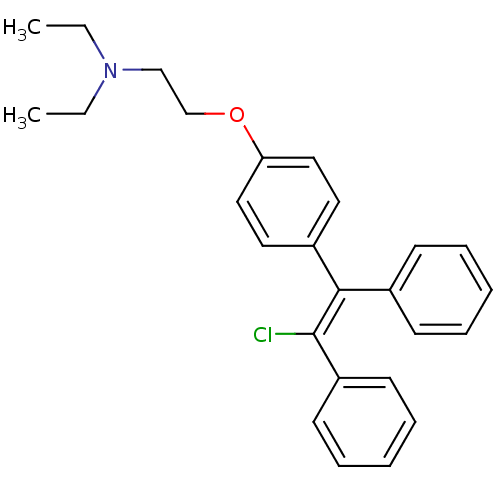 (2-[4-[(Z)-2-chloranyl-1,2-diphenyl-ethenyl]phenoxy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Affinity for ERG2 of Sacchromyces cerevisiae using [3H]ifenprodil or (+)-[3H]pentazocine radioligand | J Med Chem 48: 4754-64 (2005) Article DOI: 10.1021/jm049073+ BindingDB Entry DOI: 10.7270/Q2639QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2642 total ) | Next | Last >> |