 Found 78 hits with Last Name = 'janiczek' and Initial = 'n'
Found 78 hits with Last Name = 'janiczek' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726
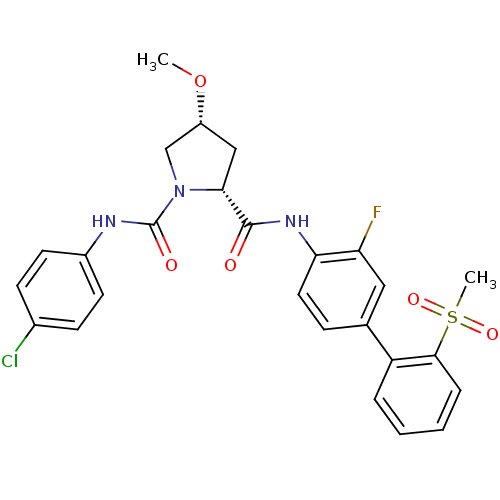
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328728
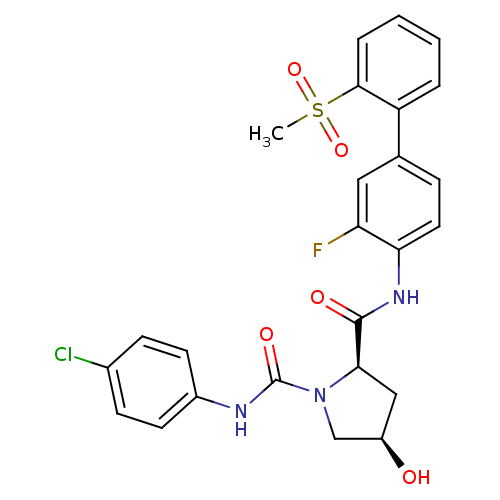
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2C[C@@H](O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81692
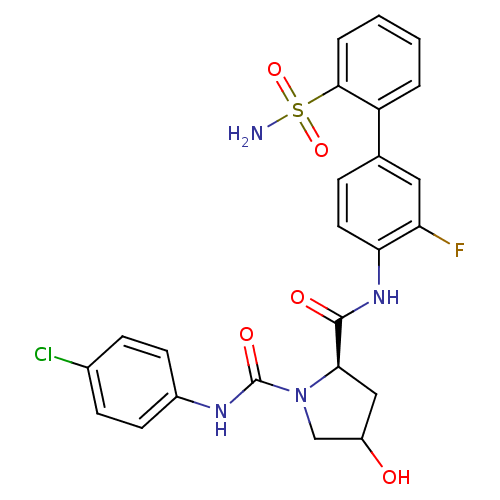
(4-Substituted Pyrrolidine Ring, 14)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O5S/c25-15-6-8-16(9-7-15)28-24(33)30-13-17(31)12-21(30)23(32)29-20-10-5-14(11-19(20)26)18-3-1-2-4-22(18)36(27,34)35/h1-11,17,21,31H,12-13H2,(H,28,33)(H,29,32)(H2,27,34,35)/t17?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81698
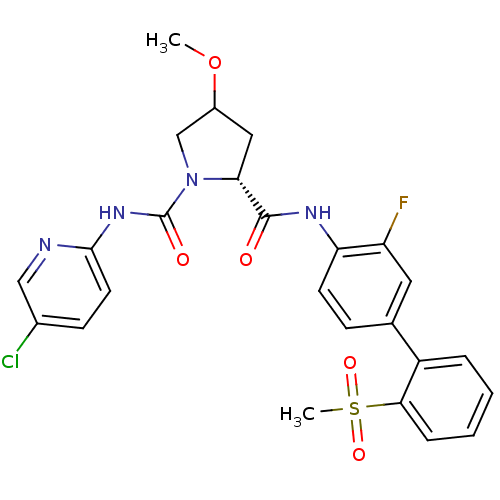
(4-Substituted Pyrrolidine Ring, 35)Show SMILES COC1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cn1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C25H24ClFN4O5S/c1-36-17-12-21(31(14-17)25(33)30-23-10-8-16(26)13-28-23)24(32)29-20-9-7-15(11-19(20)27)18-5-3-4-6-22(18)37(2,34)35/h3-11,13,17,21H,12,14H2,1-2H3,(H,29,32)(H,28,30,33)/t17?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81693
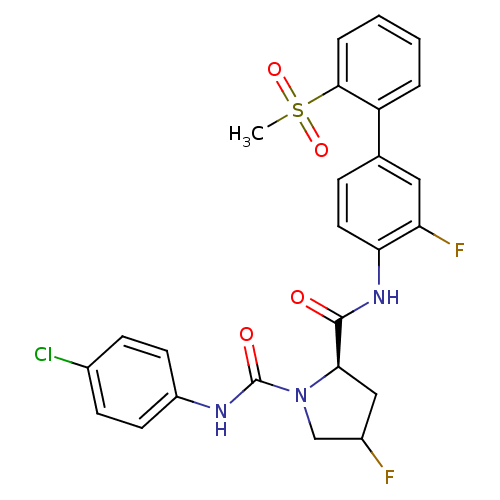
(4-Substituted Pyrrolidine Ring, 16 | 4-Substituted...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(F)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H22ClF2N3O4S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(28)12-15)30-24(32)22-13-17(27)14-31(22)25(33)29-18-9-7-16(26)8-10-18/h2-12,17,22H,13-14H2,1H3,(H,29,33)(H,30,32)/t17?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726

((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81695
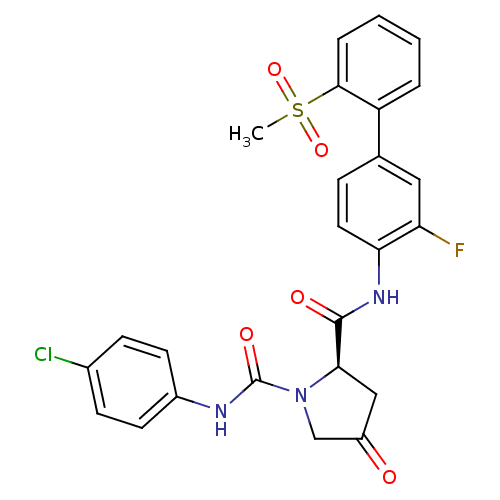
(4-Substituted Pyrrolidine Ring, 20)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(=O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H21ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,22H,13-14H2,1H3,(H,28,33)(H,29,32)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093310

(3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C27H36N4O2/c1-19-10-8-11-20(2)30(19)16-6-3-7-17-31-23-14-4-5-15-24(23)33-25(27(31)32)21-12-9-13-22(18-21)26(28)29/h4-5,9,12-15,18-20,25H,3,6-8,10-11,16-17H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093310

(3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C27H36N4O2/c1-19-10-8-11-20(2)30(19)16-6-3-7-17-31-23-14-4-5-15-24(23)33-25(27(31)32)21-12-9-13-22(18-21)26(28)29/h4-5,9,12-15,18-20,25H,3,6-8,10-11,16-17H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Compound was tested for in vitro inhibitory activity against Prothrombinase |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81697
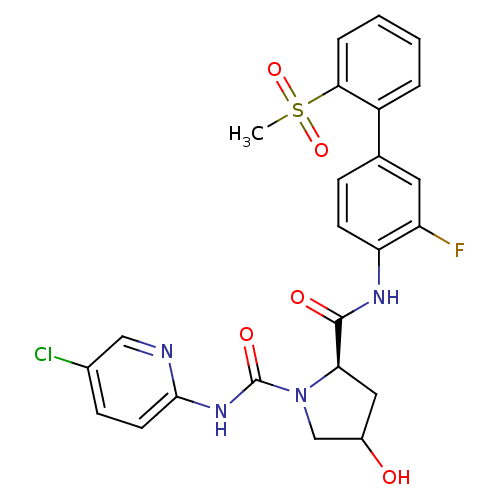
(4-Substituted Pyrrolidine Ring, 34)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(O)CN2C(=O)Nc2ccc(Cl)cn2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O5S/c1-36(34,35)21-5-3-2-4-17(21)14-6-8-19(18(26)10-14)28-23(32)20-11-16(31)13-30(20)24(33)29-22-9-7-15(25)12-27-22/h2-10,12,16,20,31H,11,13H2,1H3,(H,28,32)(H,27,29,33)/t16?,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81696

(4-Substituted Pyrrolidine Ring, 21)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(F)(F)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H21ClF3N3O4S/c1-37(35,36)22-5-3-2-4-18(22)15-6-11-20(19(27)12-15)31-23(33)21-13-25(28,29)14-32(21)24(34)30-17-9-7-16(26)8-10-17/h2-12,21H,13-14H2,1H3,(H,30,34)(H,31,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328728

((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2C[C@@H](O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81693

(4-Substituted Pyrrolidine Ring, 16 | 4-Substituted...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(F)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H22ClF2N3O4S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(28)12-15)30-24(32)22-13-17(27)14-31(22)25(33)29-18-9-7-16(26)8-10-18/h2-12,17,22H,13-14H2,1H3,(H,29,33)(H,30,32)/t17?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81699
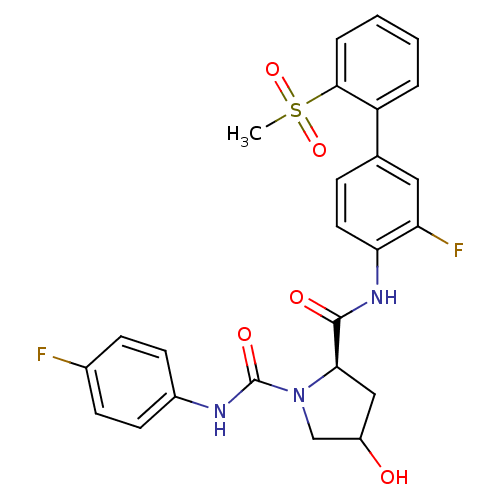
(4-Substituted Pyrrolidine Ring, 36)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(O)CN2C(=O)Nc2ccc(F)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23F2N3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093302
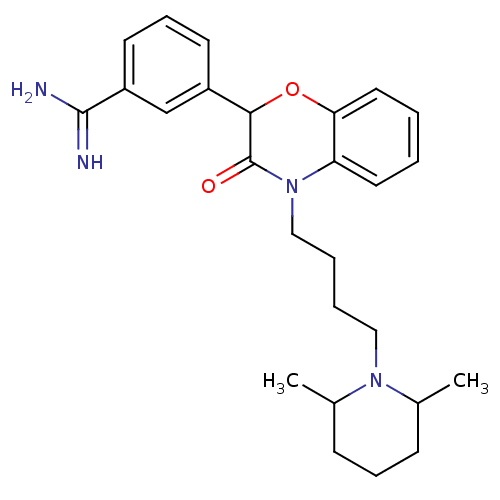
(3-{4-[4-(2,6-Dimethyl-piperidin-1-yl)-butyl]-3-oxo...)Show SMILES CC1CCCC(C)N1CCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C26H34N4O2/c1-18-9-7-10-19(2)29(18)15-5-6-16-30-22-13-3-4-14-23(22)32-24(26(30)31)20-11-8-12-21(17-20)25(27)28/h3-4,8,11-14,17-19,24H,5-7,9-10,15-16H2,1-2H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328725
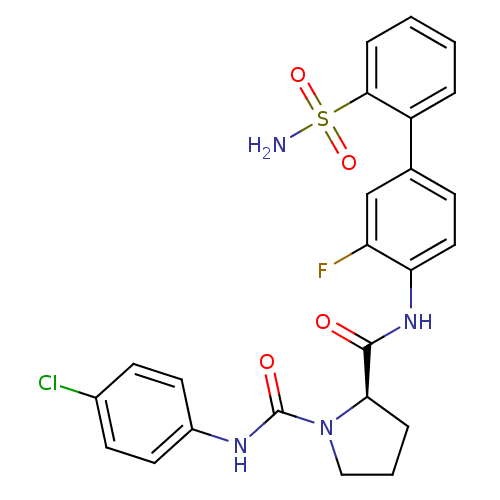
((R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-sulfamoylb...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CCCN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O4S/c25-16-8-10-17(11-9-16)28-24(32)30-13-3-5-21(30)23(31)29-20-12-7-15(14-19(20)26)18-4-1-2-6-22(18)35(27,33)34/h1-2,4,6-12,14,21H,3,5,13H2,(H,28,32)(H,29,31)(H2,27,33,34)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81681
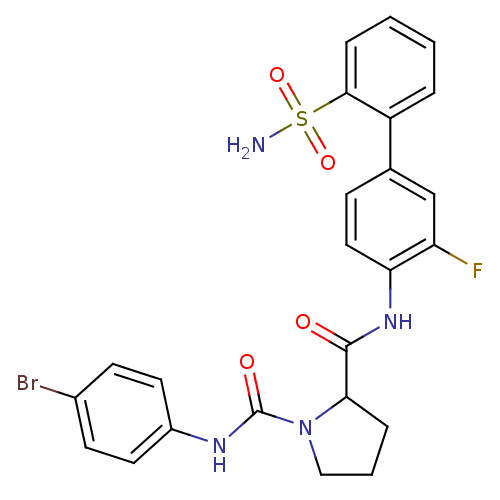
(P1 Phenyl Ring, 22)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)C2CCCN2C(=O)Nc2ccc(Br)cc2)c(F)c1 Show InChI InChI=1S/C24H22BrFN4O4S/c25-16-8-10-17(11-9-16)28-24(32)30-13-3-5-21(30)23(31)29-20-12-7-15(14-19(20)26)18-4-1-2-6-22(18)35(27,33)34/h1-2,4,6-12,14,21H,3,5,13H2,(H,28,32)(H,29,31)(H2,27,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328725

((R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-sulfamoylb...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CCCN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O4S/c25-16-8-10-17(11-9-16)28-24(32)30-13-3-5-21(30)23(31)29-20-12-7-15(14-19(20)26)18-4-1-2-6-22(18)35(27,33)34/h1-2,4,6-12,14,21H,3,5,13H2,(H,28,32)(H,29,31)(H2,27,33,34)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093306
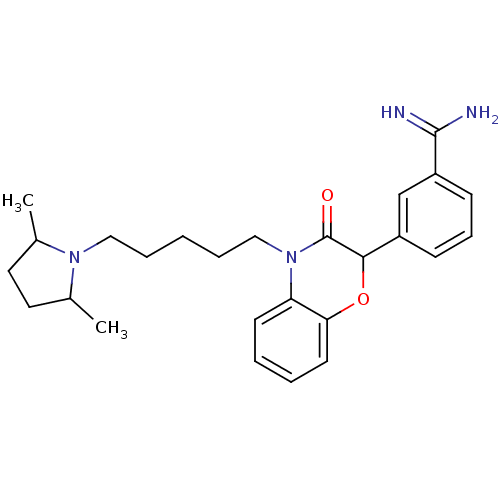
(3-{4-[5-(2,5-Dimethyl-pyrrolidin-1-yl)-pentyl]-3-o...)Show SMILES CC1CCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C26H34N4O2/c1-18-13-14-19(2)29(18)15-6-3-7-16-30-22-11-4-5-12-23(22)32-24(26(30)31)20-9-8-10-21(17-20)25(27)28/h4-5,8-12,17-19,24H,3,6-7,13-16H2,1-2H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093313
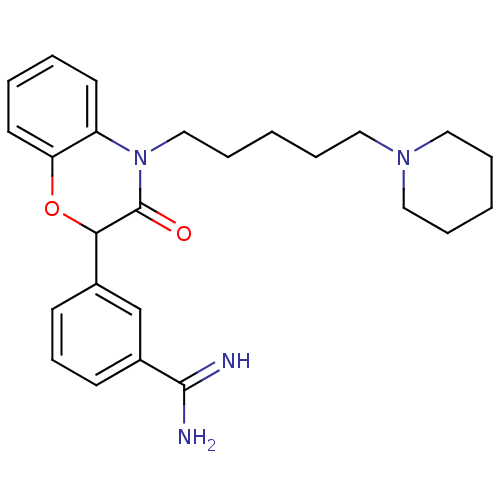
(3-[3-Oxo-4-(5-piperidin-1-yl-pentyl)-3,4-dihydro-2...)Show SMILES NC(=N)c1cccc(c1)C1Oc2ccccc2N(CCCCCN2CCCCC2)C1=O Show InChI InChI=1S/C25H32N4O2/c26-24(27)20-11-9-10-19(18-20)23-25(30)29(21-12-3-4-13-22(21)31-23)17-8-2-7-16-28-14-5-1-6-15-28/h3-4,9-13,18,23H,1-2,5-8,14-17H2,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093312

(3-{4-[6-(2,6-Dimethyl-piperidin-1-yl)-hexyl]-3-oxo...)Show SMILES CC1CCCC(C)N1CCCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C28H38N4O2/c1-20-11-9-12-21(2)31(20)17-7-3-4-8-18-32-24-15-5-6-16-25(24)34-26(28(32)33)22-13-10-14-23(19-22)27(29)30/h5-6,10,13-16,19-21,26H,3-4,7-9,11-12,17-18H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093309
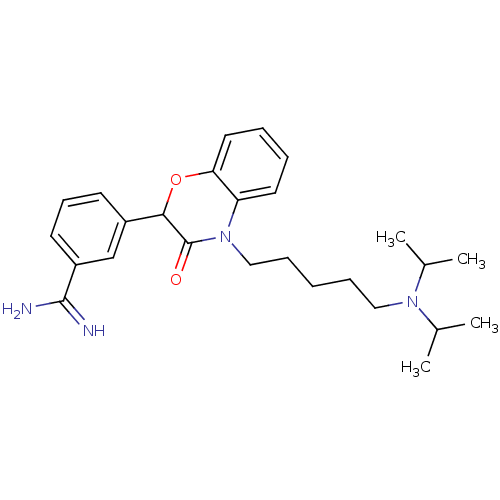
(3-[4-(5-Diisopropylamino-pentyl)-3-oxo-3,4-dihydro...)Show SMILES CC(C)N(CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N)C(C)C Show InChI InChI=1S/C26H36N4O2/c1-18(2)29(19(3)4)15-8-5-9-16-30-22-13-6-7-14-23(22)32-24(26(30)31)20-11-10-12-21(17-20)25(27)28/h6-7,10-14,17-19,24H,5,8-9,15-16H2,1-4H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093299

(3-[4-(5-Morpholin-4-yl-pentyl)-3-oxo-3,4-dihydro-2...)Show SMILES NC(=N)c1cccc(c1)C1Oc2ccccc2N(CCCCCN2CCOCC2)C1=O Show InChI InChI=1S/C24H30N4O3/c25-23(26)19-8-6-7-18(17-19)22-24(29)28(20-9-2-3-10-21(20)31-22)12-5-1-4-11-27-13-15-30-16-14-27/h2-3,6-10,17,22H,1,4-5,11-16H2,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093302

(3-{4-[4-(2,6-Dimethyl-piperidin-1-yl)-butyl]-3-oxo...)Show SMILES CC1CCCC(C)N1CCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C26H34N4O2/c1-18-9-7-10-19(2)29(18)15-5-6-16-30-22-13-3-4-14-23(22)32-24(26(30)31)20-11-8-12-21(17-20)25(27)28/h3-4,8,11-14,17-19,24H,5-7,9-10,15-16H2,1-2H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093310

(3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C27H36N4O2/c1-19-10-8-11-20(2)30(19)16-6-3-7-17-31-23-14-4-5-15-24(23)33-25(27(31)32)21-12-9-13-22(18-21)26(28)29/h4-5,9,12-15,18-20,25H,3,6-8,10-11,16-17H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81682
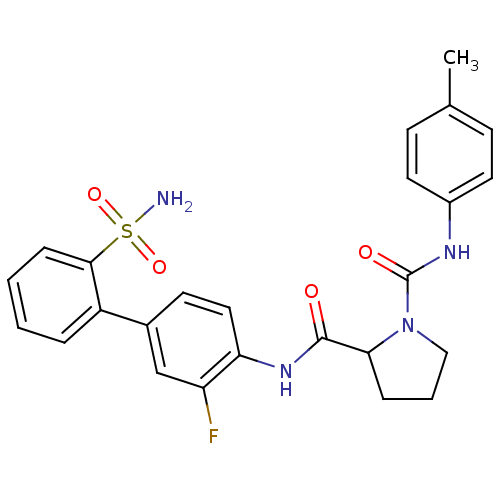
(P1 Phenyl Ring, 24)Show SMILES Cc1ccc(NC(=O)N2CCCC2C(=O)Nc2ccc(cc2F)-c2ccccc2S(N)(=O)=O)cc1 Show InChI InChI=1S/C25H25FN4O4S/c1-16-8-11-18(12-9-16)28-25(32)30-14-4-6-22(30)24(31)29-21-13-10-17(15-20(21)26)19-5-2-3-7-23(19)35(27,33)34/h2-3,5,7-13,15,22H,4,6,14H2,1H3,(H,28,32)(H,29,31)(H2,27,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81683
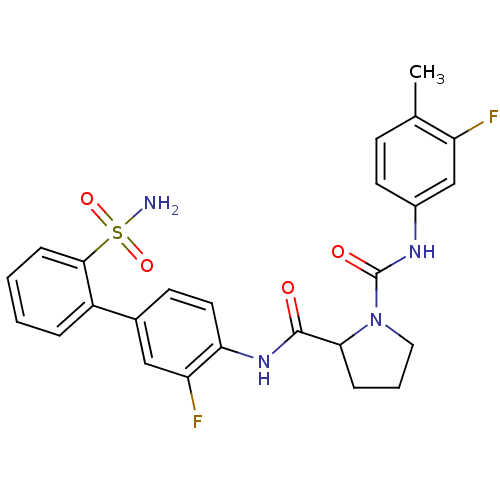
(P1 Phenyl Ring, 25)Show SMILES Cc1ccc(NC(=O)N2CCCC2C(=O)Nc2ccc(cc2F)-c2ccccc2S(N)(=O)=O)cc1F Show InChI InChI=1S/C25H24F2N4O4S/c1-15-8-10-17(14-19(15)26)29-25(33)31-12-4-6-22(31)24(32)30-21-11-9-16(13-20(21)27)18-5-2-3-7-23(18)36(28,34)35/h2-3,5,7-11,13-14,22H,4,6,12H2,1H3,(H,29,33)(H,30,32)(H2,28,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 516 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093306

(3-{4-[5-(2,5-Dimethyl-pyrrolidin-1-yl)-pentyl]-3-o...)Show SMILES CC1CCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C26H34N4O2/c1-18-13-14-19(2)29(18)15-6-3-7-16-30-22-11-4-5-12-23(22)32-24(26(30)31)20-9-8-10-21(17-20)25(27)28/h4-5,8-12,17-19,24H,3,6-7,13-16H2,1-2H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093308
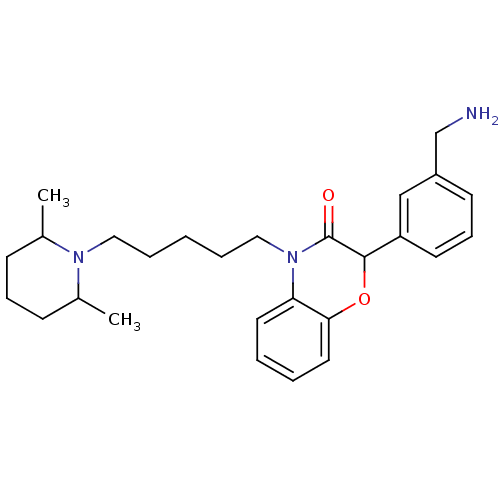
(2-(3-Aminomethyl-phenyl)-4-[5-(2,6-dimethyl-piperi...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(CN)c1 Show InChI InChI=1S/C27H37N3O2/c1-20-10-8-11-21(2)29(20)16-6-3-7-17-30-24-14-4-5-15-25(24)32-26(27(30)31)23-13-9-12-22(18-23)19-28/h4-5,9,12-15,18,20-21,26H,3,6-8,10-11,16-17,19,28H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81684
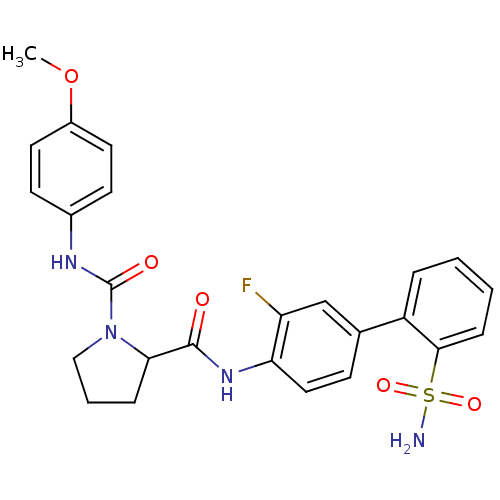
(P1 Phenyl Ring, 26)Show SMILES COc1ccc(NC(=O)N2CCCC2C(=O)Nc2ccc(cc2F)-c2ccccc2S(N)(=O)=O)cc1 Show InChI InChI=1S/C25H25FN4O5S/c1-35-18-11-9-17(10-12-18)28-25(32)30-14-4-6-22(30)24(31)29-21-13-8-16(15-20(21)26)19-5-2-3-7-23(19)36(27,33)34/h2-3,5,7-13,15,22H,4,6,14H2,1H3,(H,28,32)(H,29,31)(H2,27,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 873 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093310

(3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C27H36N4O2/c1-19-10-8-11-20(2)30(19)16-6-3-7-17-31-23-14-4-5-15-24(23)33-25(27(31)32)21-12-9-13-22(18-21)26(28)29/h4-5,9,12-15,18-20,25H,3,6-8,10-11,16-17H2,1-2H3,(H3,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Compound was tested for in vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093306

(3-{4-[5-(2,5-Dimethyl-pyrrolidin-1-yl)-pentyl]-3-o...)Show SMILES CC1CCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C26H34N4O2/c1-18-13-14-19(2)29(18)15-6-3-7-16-30-22-11-4-5-12-23(22)32-24(26(30)31)20-9-8-10-21(17-20)25(27)28/h4-5,8-12,17-19,24H,3,6-7,13-16H2,1-2H3,(H3,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81685
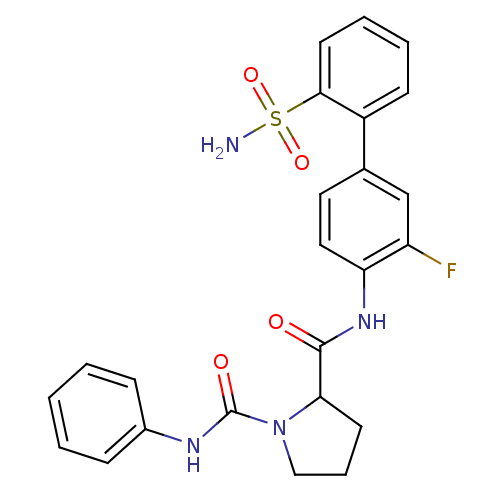
(P1 Phenyl Ring, 27)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)C2CCCN2C(=O)Nc2ccccc2)c(F)c1 Show InChI InChI=1S/C24H23FN4O4S/c25-19-15-16(18-9-4-5-11-22(18)34(26,32)33)12-13-20(19)28-23(30)21-10-6-14-29(21)24(31)27-17-7-2-1-3-8-17/h1-5,7-9,11-13,15,21H,6,10,14H2,(H,27,31)(H,28,30)(H2,26,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 951 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093313

(3-[3-Oxo-4-(5-piperidin-1-yl-pentyl)-3,4-dihydro-2...)Show SMILES NC(=N)c1cccc(c1)C1Oc2ccccc2N(CCCCCN2CCCCC2)C1=O Show InChI InChI=1S/C25H32N4O2/c26-24(27)20-11-9-10-19(18-20)23-25(30)29(21-12-3-4-13-22(21)31-23)17-8-2-7-16-28-14-5-1-6-15-28/h3-4,9-13,18,23H,1-2,5-8,14-17H2,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093302

(3-{4-[4-(2,6-Dimethyl-piperidin-1-yl)-butyl]-3-oxo...)Show SMILES CC1CCCC(C)N1CCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C26H34N4O2/c1-18-9-7-10-19(2)29(18)15-5-6-16-30-22-13-3-4-14-23(22)32-24(26(30)31)20-11-8-12-21(17-20)25(27)28/h3-4,8,11-14,17-19,24H,5-7,9-10,15-16H2,1-2H3,(H3,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093299

(3-[4-(5-Morpholin-4-yl-pentyl)-3-oxo-3,4-dihydro-2...)Show SMILES NC(=N)c1cccc(c1)C1Oc2ccccc2N(CCCCCN2CCOCC2)C1=O Show InChI InChI=1S/C24H30N4O3/c25-23(26)19-8-6-7-18(17-19)22-24(29)28(20-9-2-3-10-21(20)31-22)12-5-1-4-11-27-13-15-30-16-14-27/h2-3,6-10,17,22H,1,4-5,11-16H2,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093309

(3-[4-(5-Diisopropylamino-pentyl)-3-oxo-3,4-dihydro...)Show SMILES CC(C)N(CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N)C(C)C Show InChI InChI=1S/C26H36N4O2/c1-18(2)29(19(3)4)15-8-5-9-16-30-22-13-6-7-14-23(22)32-24(26(30)31)20-11-10-12-21(17-20)25(27)28/h6-7,10-14,17-19,24H,5,8-9,15-16H2,1-4H3,(H3,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093309

(3-[4-(5-Diisopropylamino-pentyl)-3-oxo-3,4-dihydro...)Show SMILES CC(C)N(CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N)C(C)C Show InChI InChI=1S/C26H36N4O2/c1-18(2)29(19(3)4)15-8-5-9-16-30-22-13-6-7-14-23(22)32-24(26(30)31)20-11-10-12-21(17-20)25(27)28/h6-7,10-14,17-19,24H,5,8-9,15-16H2,1-4H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093320

(3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=NN1CCOCC1 |w:32.36| Show InChI InChI=1S/C31H43N5O3/c1-23-10-8-11-24(2)35(23)16-6-3-7-17-36-27-14-4-5-15-28(27)39-29(31(36)37)25-12-9-13-26(22-25)30(32)33-34-18-20-38-21-19-34/h4-5,9,12-15,22-24,29H,3,6-8,10-11,16-21H2,1-2H3,(H2,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093301
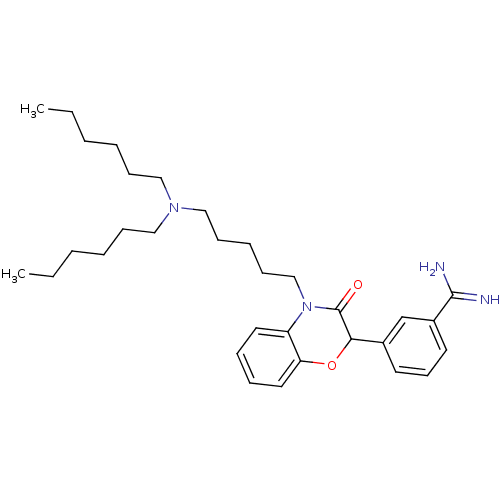
(3-[4-(5-Dihexylamino-pentyl)-3-oxo-3,4-dihydro-2H-...)Show SMILES CCCCCCN(CCCCCC)CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C32H48N4O2/c1-3-5-7-12-21-35(22-13-8-6-4-2)23-14-9-15-24-36-28-19-10-11-20-29(28)38-30(32(36)37)26-17-16-18-27(25-26)31(33)34/h10-11,16-20,25,30H,3-9,12-15,21-24H2,1-2H3,(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093318
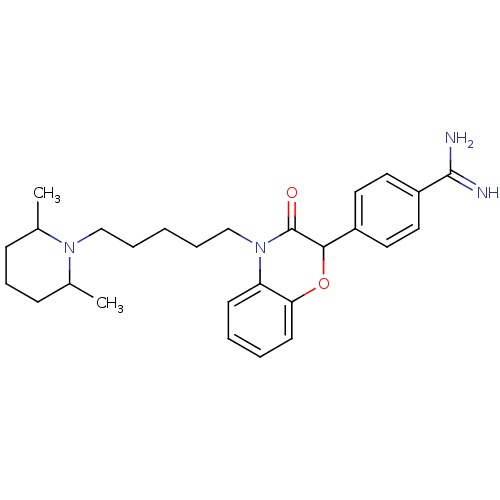
(4-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CC1CCCC(C)N1CCCCCN1C(=O)C(Oc2ccccc12)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C27H36N4O2/c1-19-9-8-10-20(2)30(19)17-6-3-7-18-31-23-11-4-5-12-24(23)33-25(27(31)32)21-13-15-22(16-14-21)26(28)29/h4-5,11-16,19-20,25H,3,6-10,17-18H2,1-2H3,(H3,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093301

(3-[4-(5-Dihexylamino-pentyl)-3-oxo-3,4-dihydro-2H-...)Show SMILES CCCCCCN(CCCCCC)CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C32H48N4O2/c1-3-5-7-12-21-35(22-13-8-6-4-2)23-14-9-15-24-36-28-19-10-11-20-29(28)38-30(32(36)37)26-17-16-18-27(25-26)31(33)34/h10-11,16-20,25,30H,3-9,12-15,21-24H2,1-2H3,(H3,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093312

(3-{4-[6-(2,6-Dimethyl-piperidin-1-yl)-hexyl]-3-oxo...)Show SMILES CC1CCCC(C)N1CCCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C28H38N4O2/c1-20-11-9-12-21(2)31(20)17-7-3-4-8-18-32-24-15-5-6-16-25(24)34-26(28(32)33)22-13-10-14-23(19-22)27(29)30/h5-6,10,13-16,19-21,26H,3-4,7-9,11-12,17-18H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093301

(3-[4-(5-Dihexylamino-pentyl)-3-oxo-3,4-dihydro-2H-...)Show SMILES CCCCCCN(CCCCCC)CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C32H48N4O2/c1-3-5-7-12-21-35(22-13-8-6-4-2)23-14-9-15-24-36-28-19-10-11-20-29(28)38-30(32(36)37)26-17-16-18-27(25-26)31(33)34/h10-11,16-20,25,30H,3-9,12-15,21-24H2,1-2H3,(H3,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Compound was tested for in vitro inhibitory activity against plasmin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093299

(3-[4-(5-Morpholin-4-yl-pentyl)-3-oxo-3,4-dihydro-2...)Show SMILES NC(=N)c1cccc(c1)C1Oc2ccccc2N(CCCCCN2CCOCC2)C1=O Show InChI InChI=1S/C24H30N4O3/c25-23(26)19-8-6-7-18(17-19)22-24(29)28(20-9-2-3-10-21(20)31-22)12-5-1-4-11-27-13-15-30-16-14-27/h2-3,6-10,17,22H,1,4-5,11-16H2,(H3,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093301

(3-[4-(5-Dihexylamino-pentyl)-3-oxo-3,4-dihydro-2H-...)Show SMILES CCCCCCN(CCCCCC)CCCCCN1C(=O)C(Oc2ccccc12)c1cccc(c1)C(N)=N Show InChI InChI=1S/C32H48N4O2/c1-3-5-7-12-21-35(22-13-8-6-4-2)23-14-9-15-24-36-28-19-10-11-20-29(28)38-30(32(36)37)26-17-16-18-27(25-26)31(33)34/h10-11,16-20,25,30H,3-9,12-15,21-24H2,1-2H3,(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against trypsin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50093311
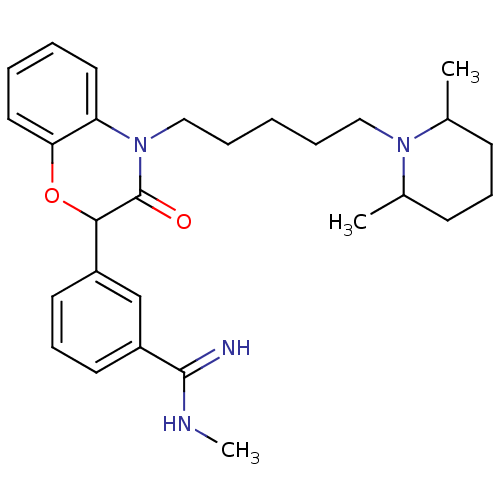
(3-{4-[5-(2,6-Dimethyl-piperidin-1-yl)-pentyl]-3-ox...)Show SMILES CNC(=N)c1cccc(c1)C1Oc2ccccc2N(CCCCCN2C(C)CCCC2C)C1=O Show InChI InChI=1S/C28H38N4O2/c1-20-11-9-12-21(2)31(20)17-7-4-8-18-32-24-15-5-6-16-25(24)34-26(28(32)33)22-13-10-14-23(19-22)27(29)30-3/h5-6,10,13-16,19-21,26H,4,7-9,11-12,17-18H2,1-3H3,(H2,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093313

(3-[3-Oxo-4-(5-piperidin-1-yl-pentyl)-3,4-dihydro-2...)Show SMILES NC(=N)c1cccc(c1)C1Oc2ccccc2N(CCCCCN2CCCCC2)C1=O Show InChI InChI=1S/C25H32N4O2/c26-24(27)20-11-9-10-19(18-20)23-25(30)29(21-12-3-4-13-22(21)31-23)17-8-2-7-16-28-14-5-1-6-15-28/h3-4,9-13,18,23H,1-2,5-8,14-17H2,(H3,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
J Med Chem 43: 4063-70 (2000)
BindingDB Entry DOI: 10.7270/Q2W66K1H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81687
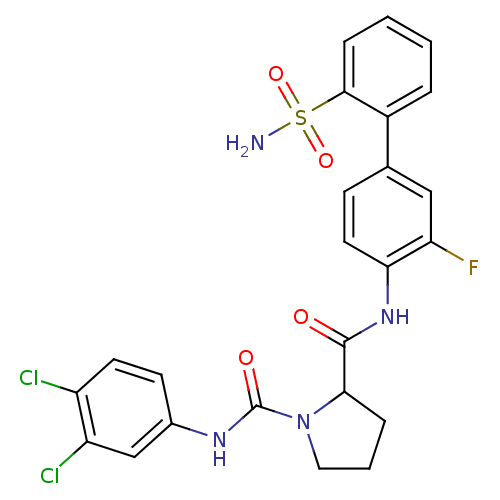
(P1 Phenyl Ring, 29)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)C2CCCN2C(=O)Nc2ccc(Cl)c(Cl)c2)c(F)c1 Show InChI InChI=1S/C24H21Cl2FN4O4S/c25-17-9-8-15(13-18(17)26)29-24(33)31-11-3-5-21(31)23(32)30-20-10-7-14(12-19(20)27)16-4-1-2-6-22(16)36(28,34)35/h1-2,4,6-10,12-13,21H,3,5,11H2,(H,29,33)(H,30,32)(H2,28,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81690
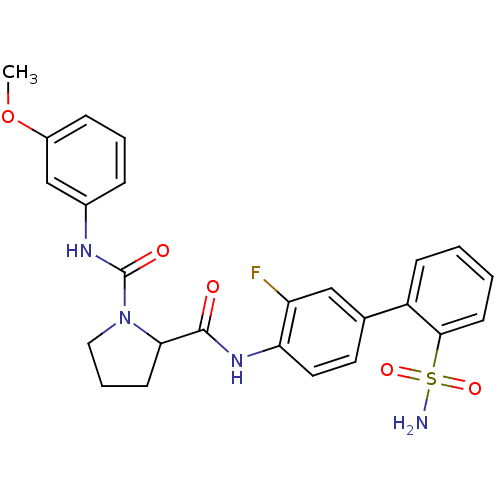
(P1 Phenyl Ring, 32)Show SMILES COc1cccc(NC(=O)N2CCCC2C(=O)Nc2ccc(cc2F)-c2ccccc2S(N)(=O)=O)c1 Show InChI InChI=1S/C25H25FN4O5S/c1-35-18-7-4-6-17(15-18)28-25(32)30-13-5-9-22(30)24(31)29-21-12-11-16(14-20(21)26)19-8-2-3-10-23(19)36(27,33)34/h2-4,6-8,10-12,14-15,22H,5,9,13H2,1H3,(H,28,32)(H,29,31)(H2,27,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data