

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bombesin receptor subtype-3 (RAT) | BDBM50336889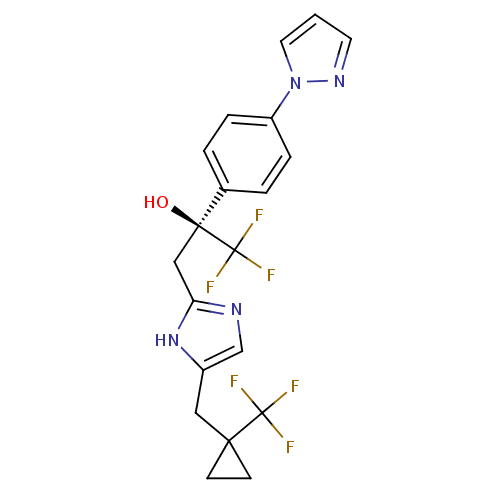 ((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]Bag-3 from rat BRS-3 | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (RAT) | BDBM50336889 ((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]Bag-3 from rat BRS-3 | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Mus musculus) | BDBM50336889 ((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]Bag-3 from mouse BRS-3 | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50336889 ((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin (6-14) from human BRS-3 | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50336886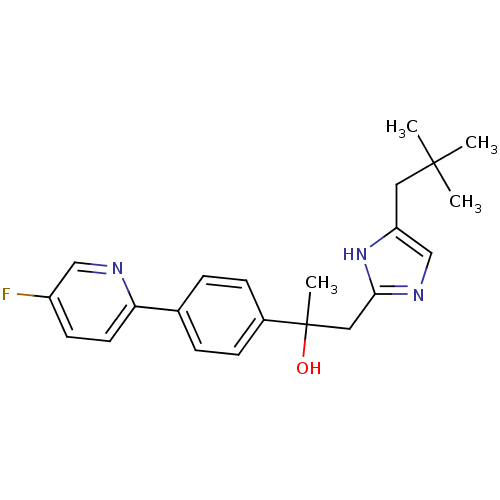 (1-[4-(2,2-dimethylpropyl)-1H-imidazol-2-yl]-2-[4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50336885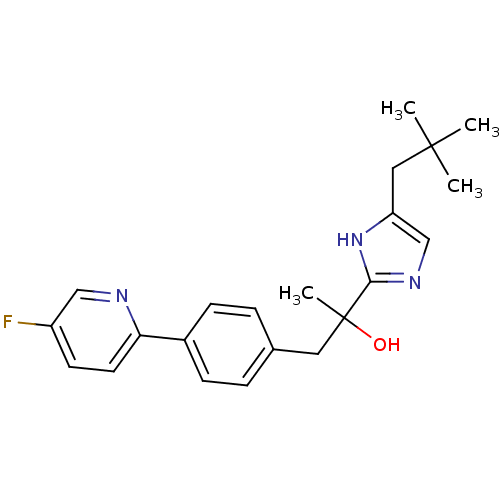 (2-[4-(2,2-dimethylpropyl)-1H-imidazol-2-yl]-1-[4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50336889 ((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 2: 43-47 (2011) Article DOI: 10.1021/ml100196d BindingDB Entry DOI: 10.7270/Q26D5T81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075813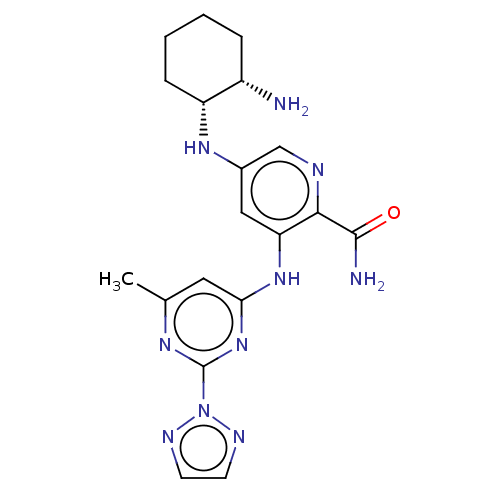 (CHEMBL3415598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075735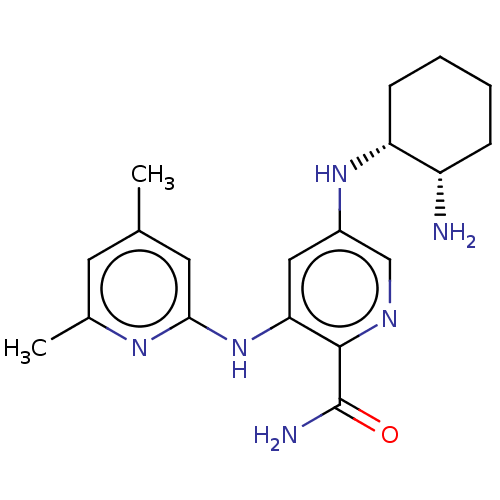 (CHEMBL3415583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50128319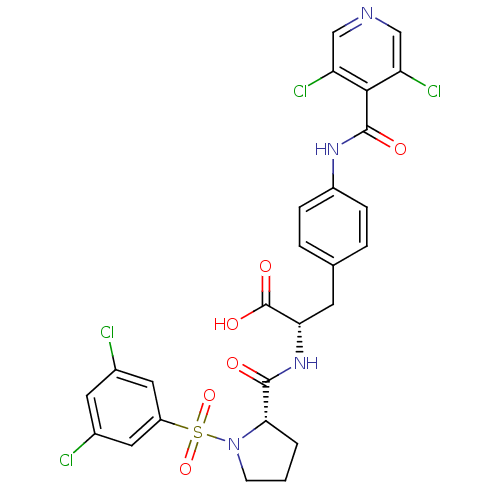 ((S)-2-{[(S)-1-(3,5-Dichloro-benzenesulfonyl)-pyrro...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258532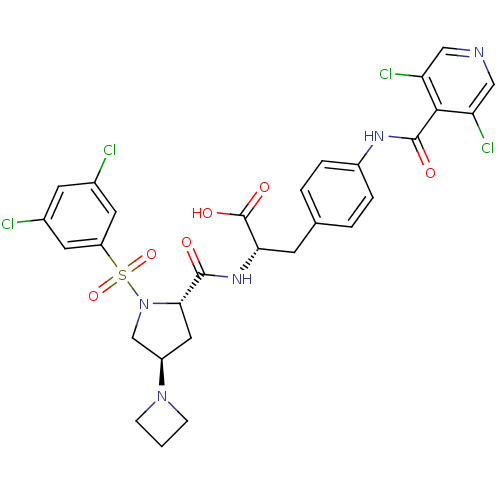 (CHEMBL502641 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258535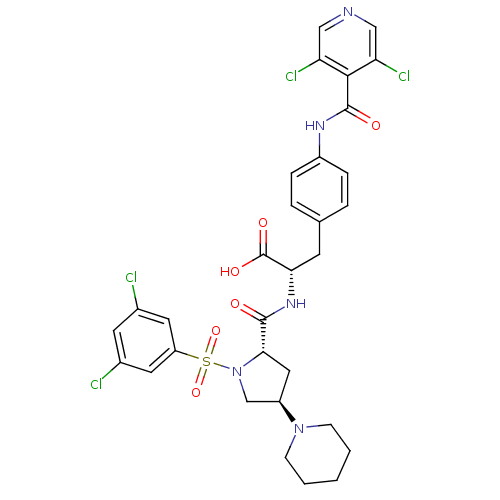 (CHEMBL448863 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258531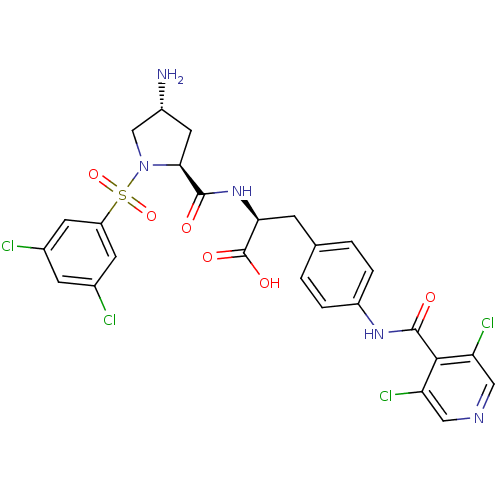 (CHEMBL507665 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075744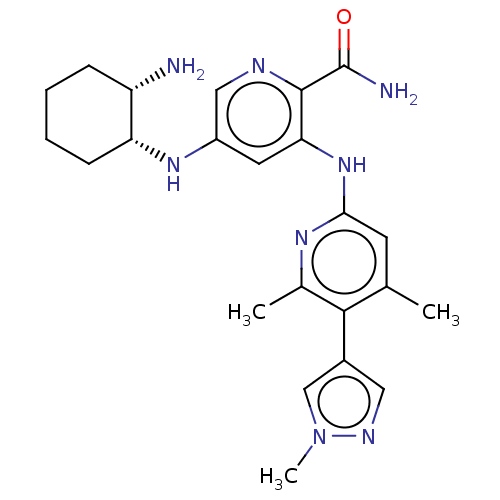 (CHEMBL3415594) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258536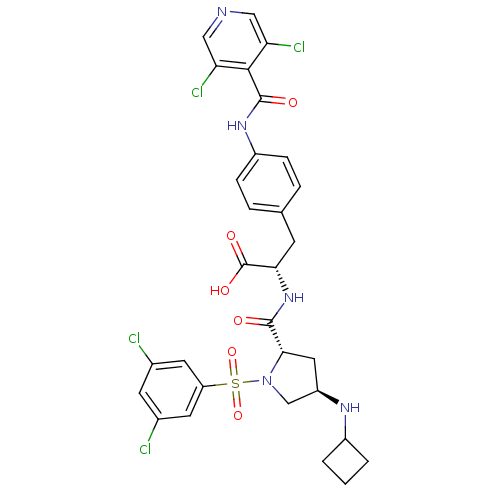 (CHEMBL445303 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075740 (CHEMBL3415589) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258534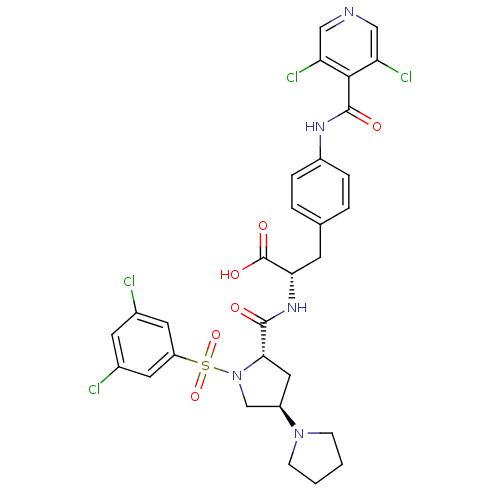 (CHEMBL505992 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258536 (CHEMBL445303 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075922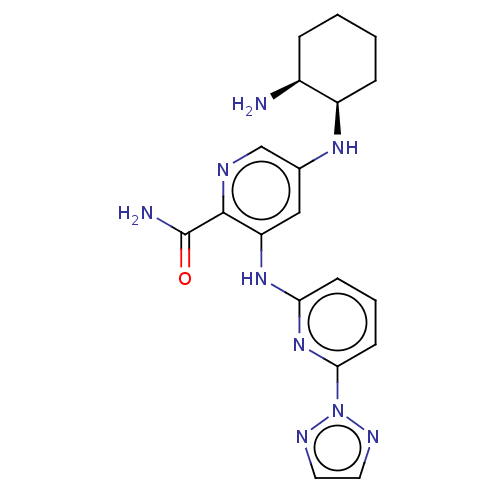 (CHEMBL3415606) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258530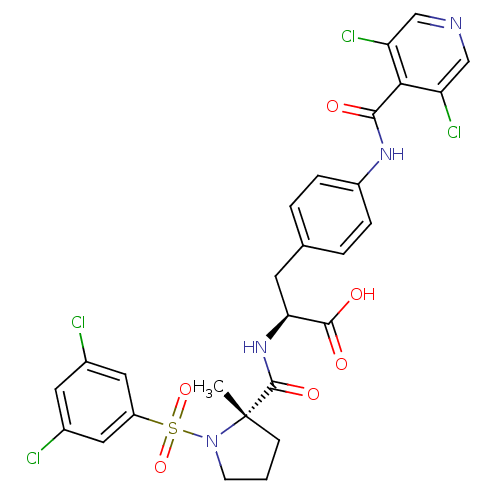 ((S)-3-(4-(3,5-dichloroisonicotinamido)phenyl)-2-((...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258533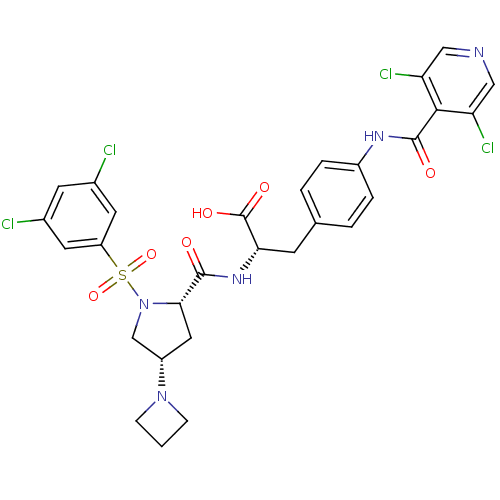 (CHEMBL444822 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075747 (CHEMBL3415597) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258532 (CHEMBL502641 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50347672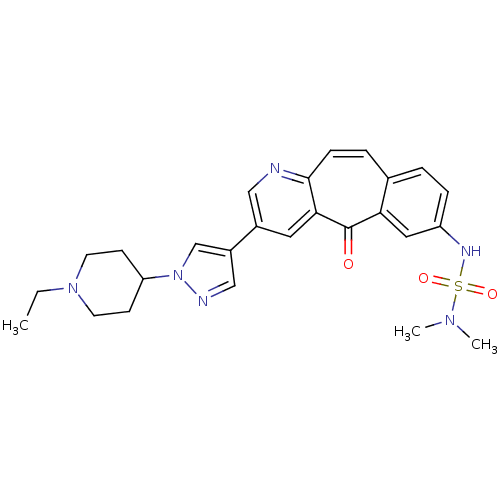 (CHEMBL1802904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant c-Met-catalyzed phosphorylation of N-biotinylated peptide (EQEDEPEGDYFEWLE-CONH2) by time-resolved fluorescence reson... | J Med Chem 54: 4092-108 (2011) Article DOI: 10.1021/jm200112k BindingDB Entry DOI: 10.7270/Q2X34ZFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258534 (CHEMBL505992 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 5195-9 (2009) Article DOI: 10.1016/j.bmcl.2009.07.046 BindingDB Entry DOI: 10.7270/Q2028RMH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells | J Med Chem 49: 7584-7 (2006) Article DOI: 10.1021/jm060996+ BindingDB Entry DOI: 10.7270/Q2QN67KG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258535 (CHEMBL448863 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200841 (CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 50: 3427-30 (2007) Article DOI: 10.1021/jm070131b BindingDB Entry DOI: 10.7270/Q2Z037VJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50205166 (CHEMBL231636 | N-((2S,3S)-4-(4-chlorophenyl)-3-(3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 2184-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.087 BindingDB Entry DOI: 10.7270/Q2HM584G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075819 (CHEMBL3415604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50258531 (CHEMBL507665 | N-{N-[(3,5-Dichlorophenyl)sulfonyl]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-(S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-iodophenylsulfonyl)pyrrolidine-2-carboxamido)-3-(4-(3,5-dichloroisonicotinamido)phenyl)p... | J Med Chem 52: 3449-52 (2009) Article DOI: 10.1021/jm900257b BindingDB Entry DOI: 10.7270/Q2TT4QT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50217220 (CHEMBL226590 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 50: 3427-30 (2007) Article DOI: 10.1021/jm070131b BindingDB Entry DOI: 10.7270/Q2Z037VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200833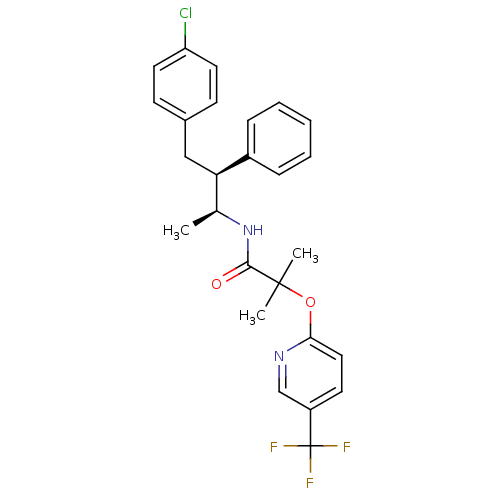 (CHEMBL373626 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells | J Med Chem 49: 7584-7 (2006) Article DOI: 10.1021/jm060996+ BindingDB Entry DOI: 10.7270/Q2QN67KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182065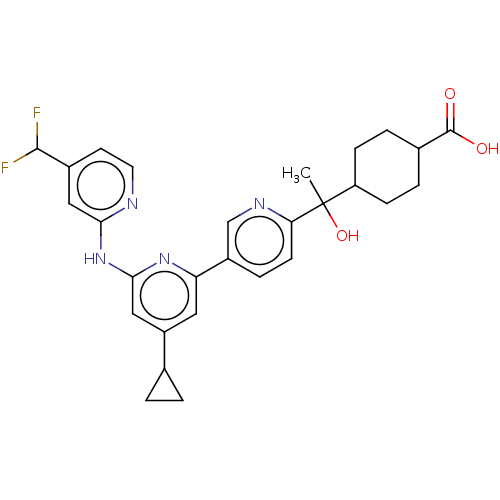 (US9145391, 1-33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182067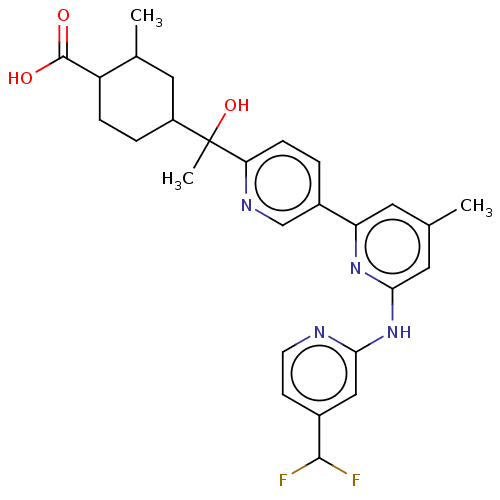 (US9145391, 1-35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182063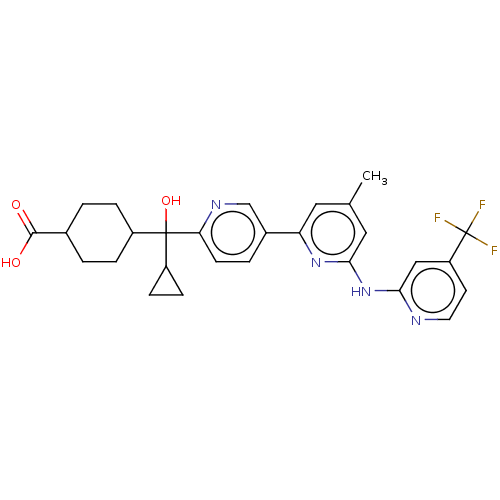 (US9145391, 1-31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182062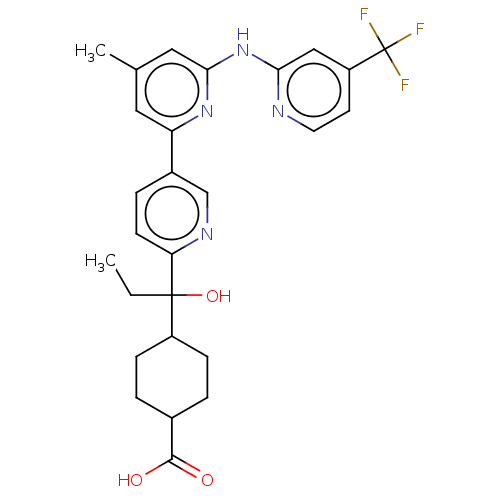 (US9145391, 1-30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182052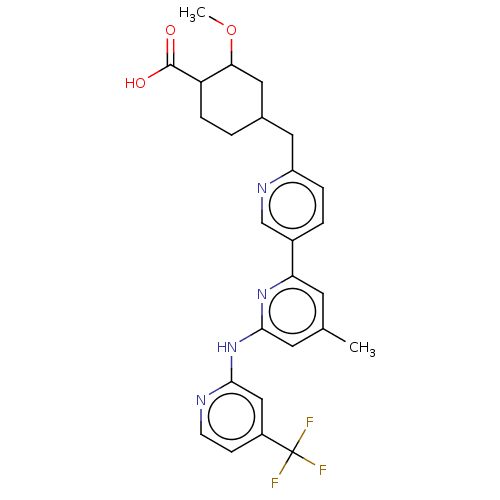 (US9145391, 1-20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182038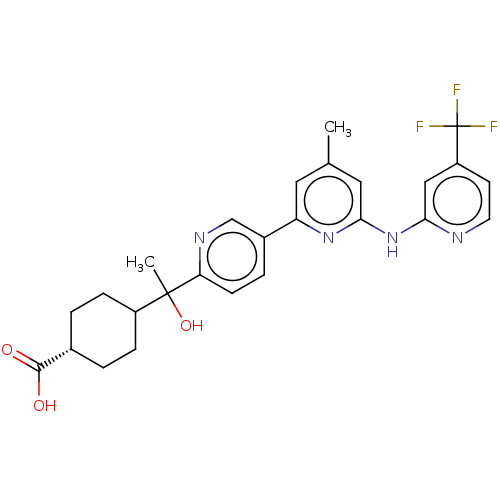 (US9145391, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182147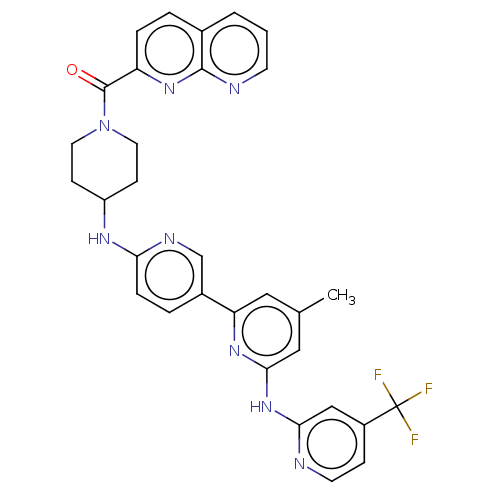 (US9145391, 4-44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182100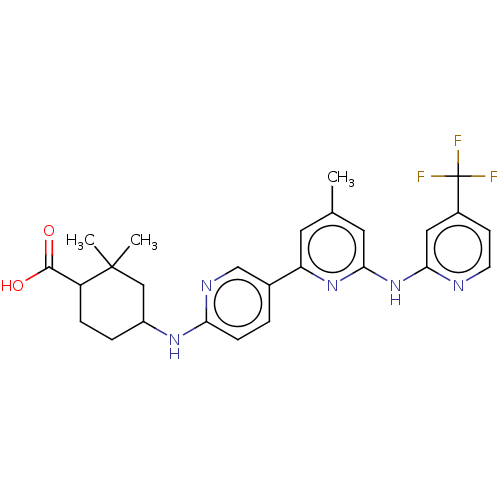 (US9145391, 3-4 | US9145391, 3-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182098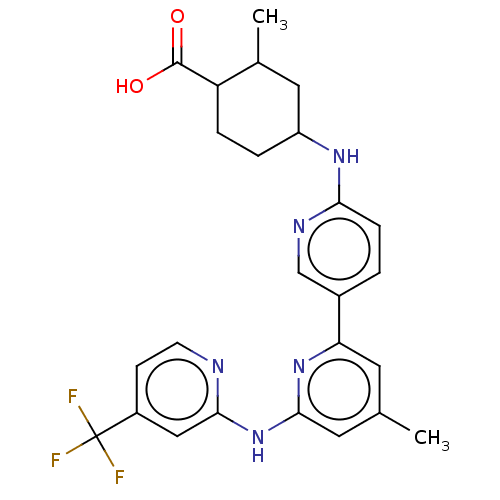 (US9145391, 3-2 | US9145391, 3-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182244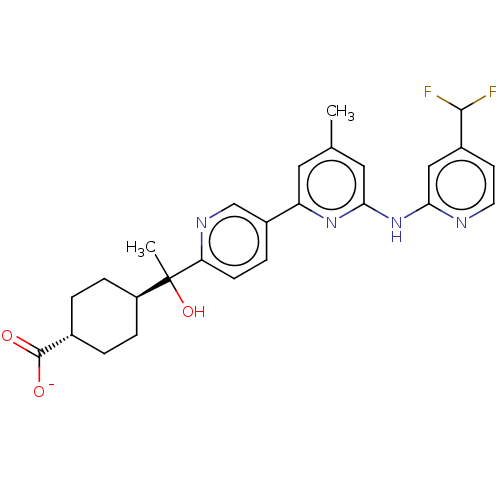 (US9145391, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182242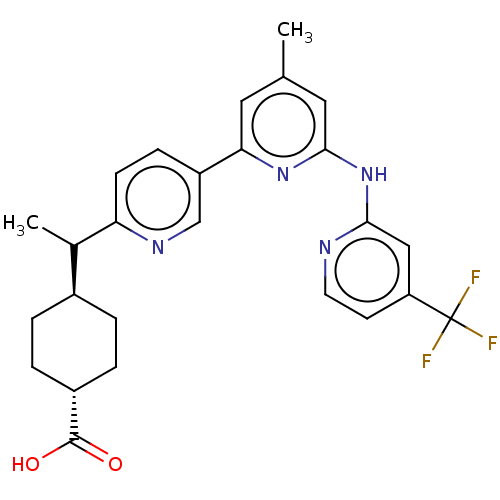 (US9145391, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182241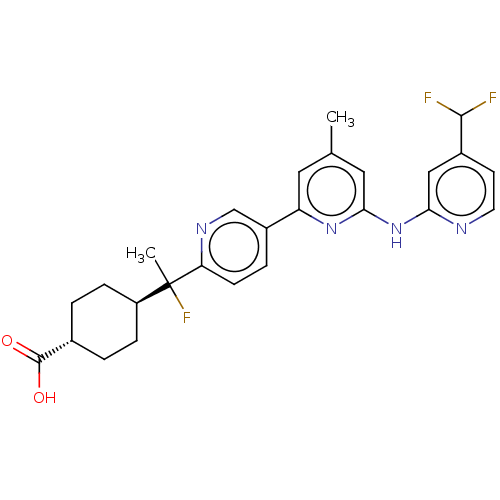 (US9145391, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182298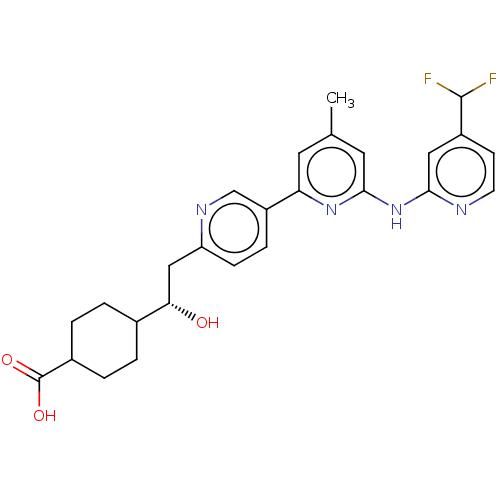 (US9145391, 12-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM182240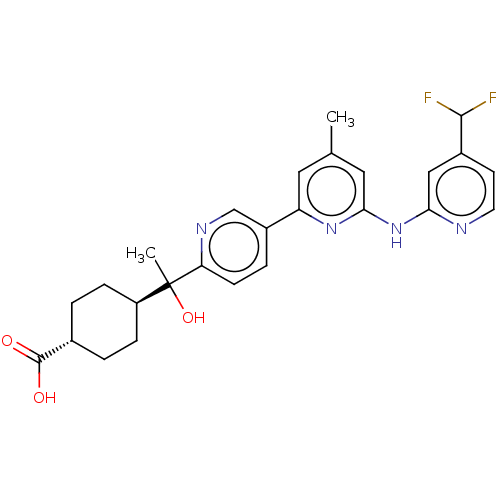 (US9145391, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.; Merck Canada Inc. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Carna Bi... | US Patent US9145391 (2015) BindingDB Entry DOI: 10.7270/Q2SQ8Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075732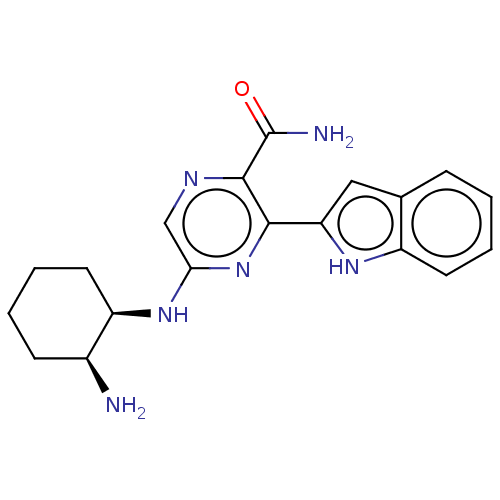 (CHEMBL3414584 | US9775839, 2.1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50075742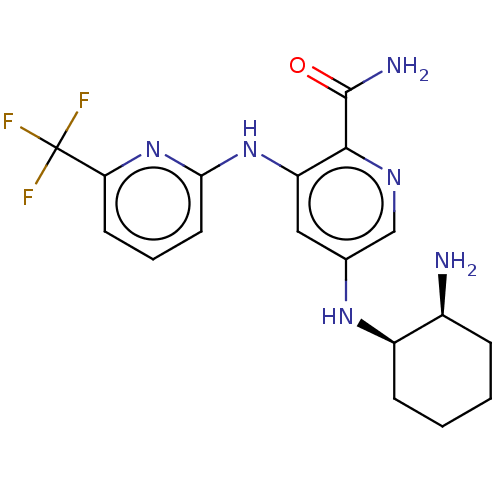 (CHEMBL3415592) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... | J Med Chem 58: 1929-39 (2015) Article DOI: 10.1021/jm5018169 BindingDB Entry DOI: 10.7270/Q2028T7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1097 total ) | Next | Last >> |