 Found 164 hits with Last Name = 'liu' and Initial = 'zy'
Found 164 hits with Last Name = 'liu' and Initial = 'zy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fgr
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Fgr |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SIK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Phosphorylase b kinase gamma catalytic chain, liver/testis isoform
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PhKg2 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate incubated for 5 mins followed by NADPH addition and measured after 20 mi... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112465
BindingDB Entry DOI: 10.7270/Q23X8BCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SAPK2a |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cSrc |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 5 mins followed by NADPH addition and measured after 20 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112465
BindingDB Entry DOI: 10.7270/Q23X8BCZ |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TSSK2 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ALK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 5 mins followed by NADPH addition and measured after... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112465
BindingDB Entry DOI: 10.7270/Q23X8BCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MLK1 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLT4 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase WNK3
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of WNK3 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129663
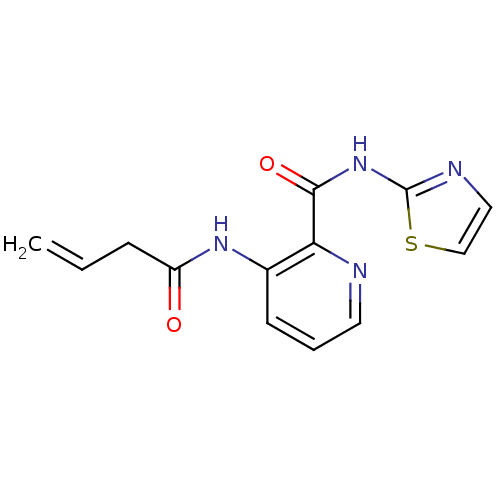
(3-But-3-enoylamino-pyridine-2-carboxylic acid thia...)Show InChI InChI=1S/C13H12N4O2S/c1-2-4-10(18)16-9-5-3-6-14-11(9)12(19)17-13-15-7-8-20-13/h2-3,5-8H,1,4H2,(H,16,18)(H,15,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129648
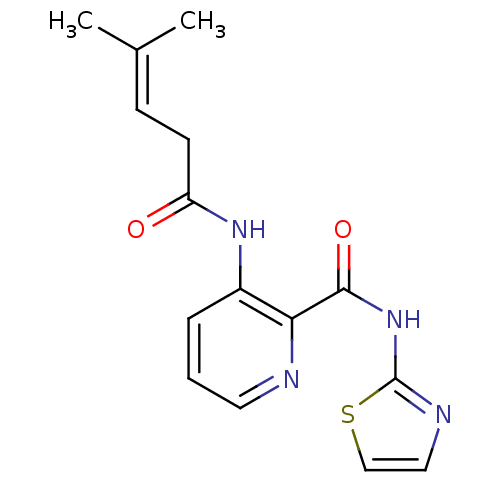
(3-(4-Methyl-pent-3-enoylamino)-pyridine-2-carboxyl...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6](=O)-[#7]-c1cccnc1-[#6](=O)-[#7]-c1nccs1 Show InChI InChI=1S/C15H16N4O2S/c1-10(2)5-6-12(20)18-11-4-3-7-16-13(11)14(21)19-15-17-8-9-22-15/h3-5,7-9H,6H2,1-2H3,(H,18,20)(H,17,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129660

(3-(3-Methyl-but-3-enoylamino)-pyridine-2-carboxyli...)Show InChI InChI=1S/C14H14N4O2S/c1-9(2)8-11(19)17-10-4-3-5-15-12(10)13(20)18-14-16-6-7-21-14/h3-7H,1,8H2,2H3,(H,17,19)(H,16,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM17847
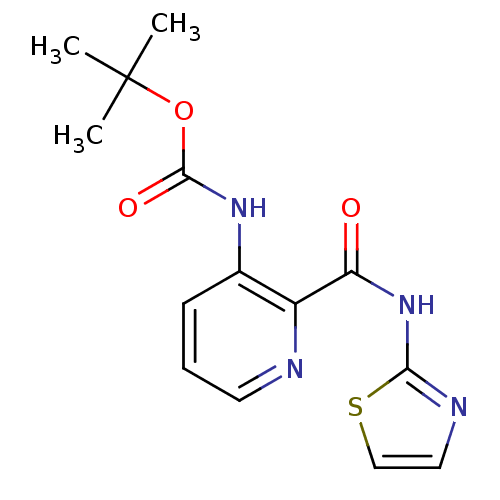
(CHEMBL327579 | pyridine-2-carboxylic acid inhibito...)Show InChI InChI=1S/C14H16N4O3S/c1-14(2,3)21-13(20)17-9-5-4-6-15-10(9)11(19)18-12-16-7-8-22-12/h4-8H,1-3H3,(H,17,20)(H,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129642
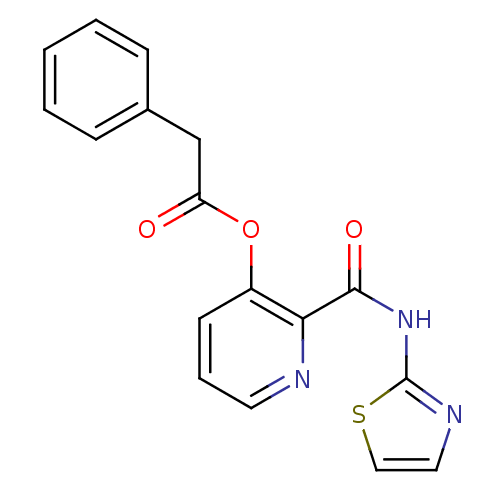
(CHEMBL86427 | Phenyl-acetic acid 2-(thiazol-2-ylca...)Show InChI InChI=1S/C17H13N3O3S/c21-14(11-12-5-2-1-3-6-12)23-13-7-4-8-18-15(13)16(22)20-17-19-9-10-24-17/h1-10H,11H2,(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129640
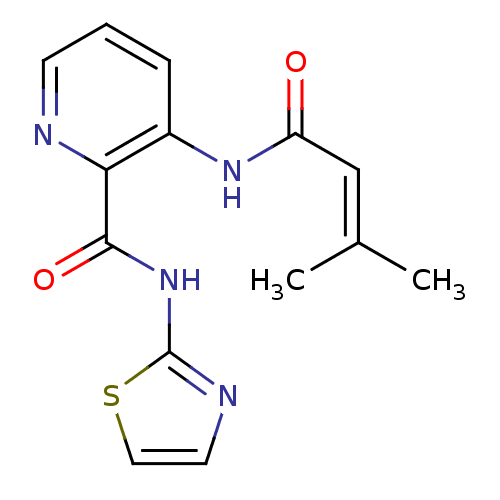
(3-(3-Methyl-but-2-enoylamino)-pyridine-2-carboxyli...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#7]-c1cccnc1-[#6](=O)-[#7]-c1nccs1 Show InChI InChI=1S/C14H14N4O2S/c1-9(2)8-11(19)17-10-4-3-5-15-12(10)13(20)18-14-16-6-7-21-14/h3-8H,1-2H3,(H,17,19)(H,16,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129648

(3-(4-Methyl-pent-3-enoylamino)-pyridine-2-carboxyl...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6](=O)-[#7]-c1cccnc1-[#6](=O)-[#7]-c1nccs1 Show InChI InChI=1S/C15H16N4O2S/c1-10(2)5-6-12(20)18-11-4-3-7-16-13(11)14(21)19-15-17-8-9-22-15/h3-5,7-9H,6H2,1-2H3,(H,18,20)(H,17,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129672
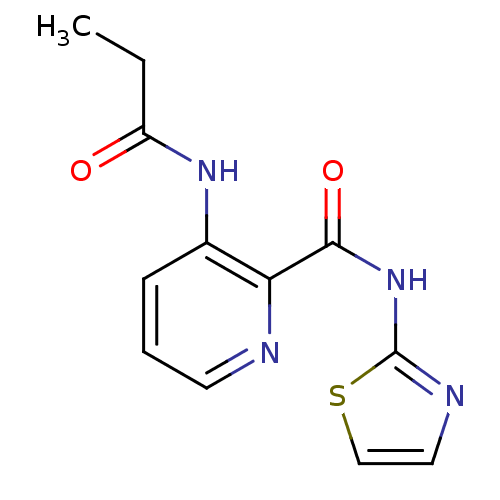
(3-Propionylamino-pyridine-2-carboxylic acid thiazo...)Show InChI InChI=1S/C12H12N4O2S/c1-2-9(17)15-8-4-3-5-13-10(8)11(18)16-12-14-6-7-19-12/h3-7H,2H2,1H3,(H,15,17)(H,14,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129672

(3-Propionylamino-pyridine-2-carboxylic acid thiazo...)Show InChI InChI=1S/C12H12N4O2S/c1-2-9(17)15-8-4-3-5-13-10(8)11(18)16-12-14-6-7-19-12/h3-7H,2H2,1H3,(H,15,17)(H,14,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Escherichia coli methionine aminopeptidase 1 |
Bioorg Med Chem Lett 15: 635-8 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.034
BindingDB Entry DOI: 10.7270/Q2222T7R |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129657
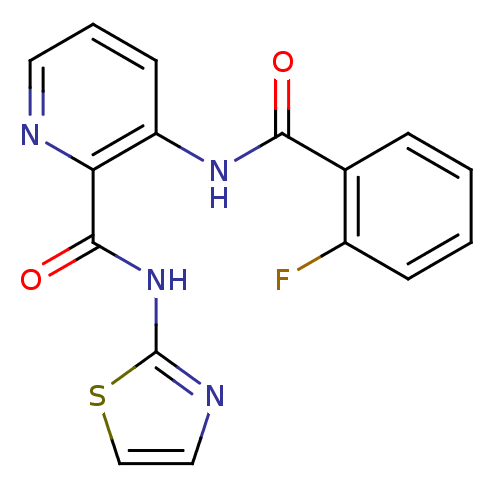
(3-(2-Fluoro-benzoylamino)-pyridine-2-carboxylic ac...)Show InChI InChI=1S/C16H11FN4O2S/c17-11-5-2-1-4-10(11)14(22)20-12-6-3-7-18-13(12)15(23)21-16-19-8-9-24-16/h1-9H,(H,20,22)(H,19,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129674

(3-(2-Methyl-but-2-enoylamino)-pyridine-2-carboxyli...)Show InChI InChI=1S/C14H14N4O2S/c1-3-9(2)12(19)17-10-5-4-6-15-11(10)13(20)18-14-16-7-8-21-14/h3-8H,1-2H3,(H,17,19)(H,16,18,20)/b9-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129669
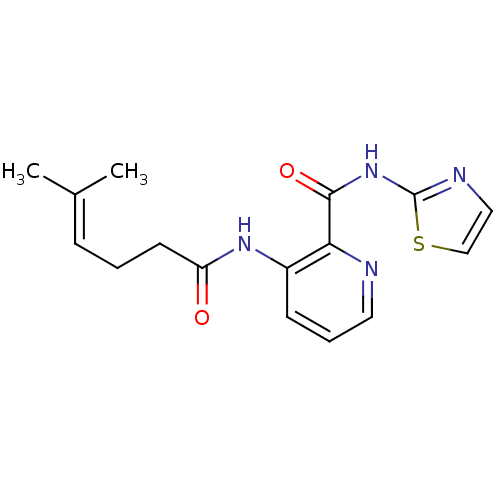
(3-(5-Methyl-hex-4-enoylamino)-pyridine-2-carboxyli...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6](=O)-[#7]-c1cccnc1-[#6](=O)-[#7]-c1nccs1 Show InChI InChI=1S/C16H18N4O2S/c1-11(2)5-3-7-13(21)19-12-6-4-8-17-14(12)15(22)20-16-18-9-10-23-16/h4-6,8-10H,3,7H2,1-2H3,(H,19,21)(H,18,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129649
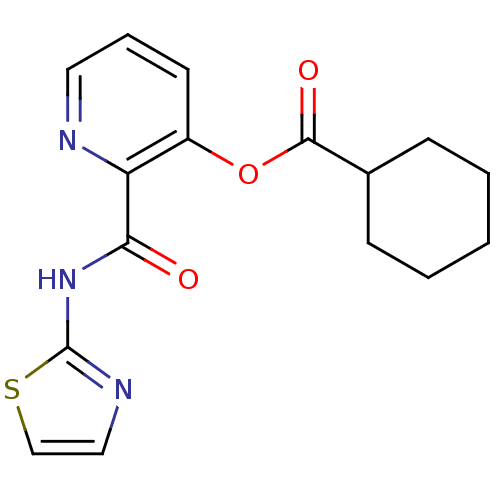
(CHEMBL315098 | Cyclohexanecarboxylic acid 2-(thiaz...)Show InChI InChI=1S/C16H17N3O3S/c20-14(19-16-18-9-10-23-16)13-12(7-4-8-17-13)22-15(21)11-5-2-1-3-6-11/h4,7-11H,1-3,5-6H2,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129635
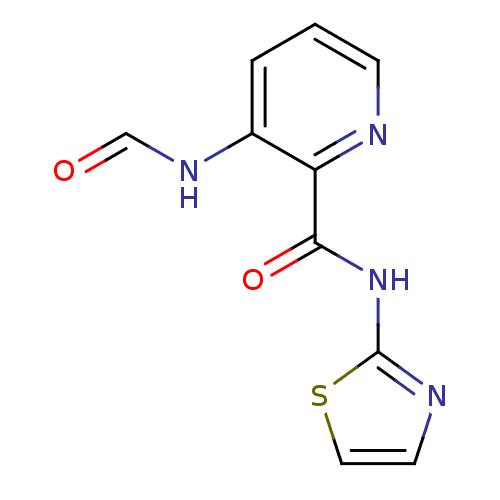
(3-Formylamino-pyridine-2-carboxylic acid thiazol-2...)Show InChI InChI=1S/C10H8N4O2S/c15-6-13-7-2-1-3-11-8(7)9(16)14-10-12-4-5-17-10/h1-6H,(H,13,15)(H,12,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129635

(3-Formylamino-pyridine-2-carboxylic acid thiazol-2...)Show InChI InChI=1S/C10H8N4O2S/c15-6-13-7-2-1-3-11-8(7)9(16)14-10-12-4-5-17-10/h1-6H,(H,13,15)(H,12,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM17849
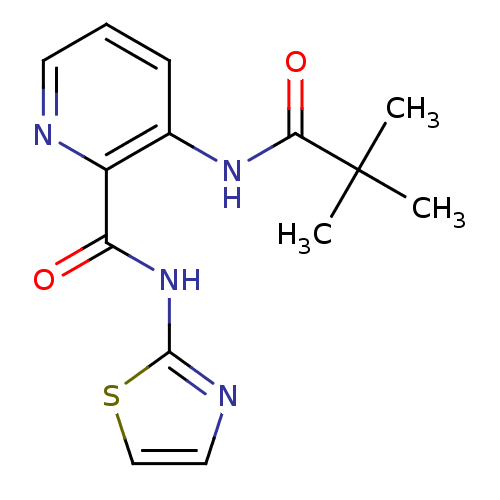
(3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...)Show InChI InChI=1S/C14H16N4O2S/c1-14(2,3)12(20)17-9-5-4-6-15-10(9)11(19)18-13-16-7-8-21-13/h4-8H,1-3H3,(H,17,20)(H,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129670
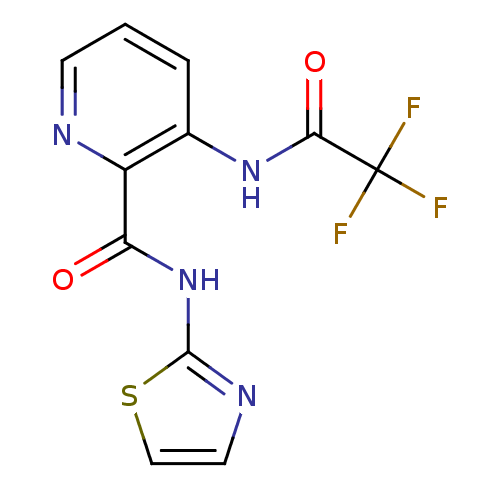
(3-(2,2,2-Trifluoro-acetylamino)-pyridine-2-carboxy...)Show InChI InChI=1S/C11H7F3N4O2S/c12-11(13,14)9(20)17-6-2-1-3-15-7(6)8(19)18-10-16-4-5-21-10/h1-5H,(H,17,20)(H,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129672

(3-Propionylamino-pyridine-2-carboxylic acid thiazo...)Show InChI InChI=1S/C12H12N4O2S/c1-2-9(17)15-8-4-3-5-13-10(8)11(18)16-12-14-6-7-19-12/h3-7H,2H2,1H3,(H,15,17)(H,14,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129672

(3-Propionylamino-pyridine-2-carboxylic acid thiazo...)Show InChI InChI=1S/C12H12N4O2S/c1-2-9(17)15-8-4-3-5-13-10(8)11(18)16-12-14-6-7-19-12/h3-7H,2H2,1H3,(H,15,17)(H,14,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Saccharomyces cerevisiae methionine aminopeptidase 1 |
Bioorg Med Chem Lett 15: 635-8 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.034
BindingDB Entry DOI: 10.7270/Q2222T7R |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129656
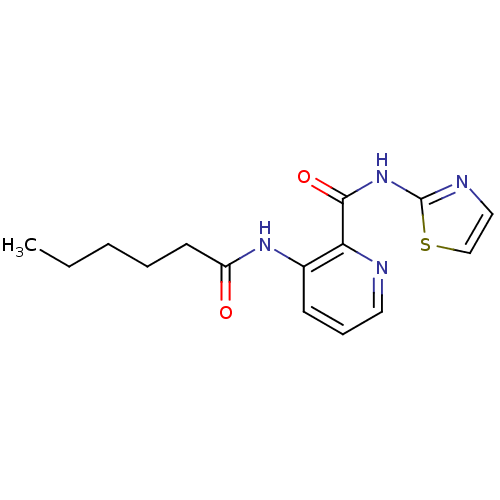
(3-Hexanoylamino-pyridine-2-carboxylic acid thiazol...)Show InChI InChI=1S/C15H18N4O2S/c1-2-3-4-7-12(20)18-11-6-5-8-16-13(11)14(21)19-15-17-9-10-22-15/h5-6,8-10H,2-4,7H2,1H3,(H,18,20)(H,17,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Escherichia coli methionine aminopeptidase 1 |
Bioorg Med Chem Lett 15: 635-8 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.034
BindingDB Entry DOI: 10.7270/Q2222T7R |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129656

(3-Hexanoylamino-pyridine-2-carboxylic acid thiazol...)Show InChI InChI=1S/C15H18N4O2S/c1-2-3-4-7-12(20)18-11-6-5-8-16-13(11)14(21)19-15-17-9-10-22-15/h5-6,8-10H,2-4,7H2,1H3,(H,18,20)(H,17,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129650

(CHEMBL314448 | Propionic acid 2-(thiazol-2-ylcarba...)Show InChI InChI=1S/C12H11N3O3S/c1-2-9(16)18-8-4-3-5-13-10(8)11(17)15-12-14-6-7-19-12/h3-7H,2H2,1H3,(H,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129663

(3-But-3-enoylamino-pyridine-2-carboxylic acid thia...)Show InChI InChI=1S/C13H12N4O2S/c1-2-4-10(18)16-9-5-3-6-14-11(9)12(19)17-13-15-7-8-20-13/h2-3,5-8H,1,4H2,(H,16,18)(H,15,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129641
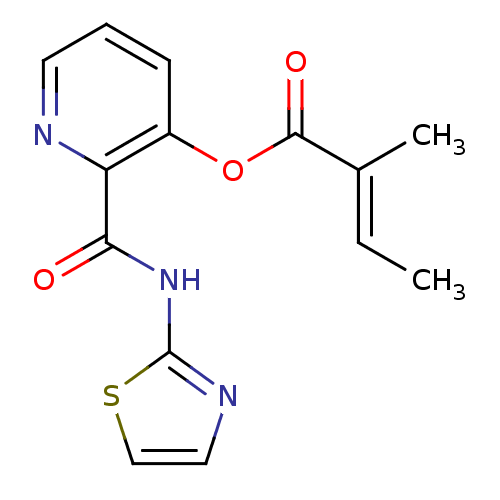
(2-Methyl-but-2-enoic acid 2-(thiazol-2-ylcarbamoyl...)Show InChI InChI=1S/C14H13N3O3S/c1-3-9(2)13(19)20-10-5-4-6-15-11(10)12(18)17-14-16-7-8-21-14/h3-8H,1-2H3,(H,16,17,18)/b9-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129666

(3-Phenylacetylamino-pyridine-2-carboxylic acid thi...)Show InChI InChI=1S/C17H14N4O2S/c22-14(11-12-5-2-1-3-6-12)20-13-7-4-8-18-15(13)16(23)21-17-19-9-10-24-17/h1-10H,11H2,(H,20,22)(H,19,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129646
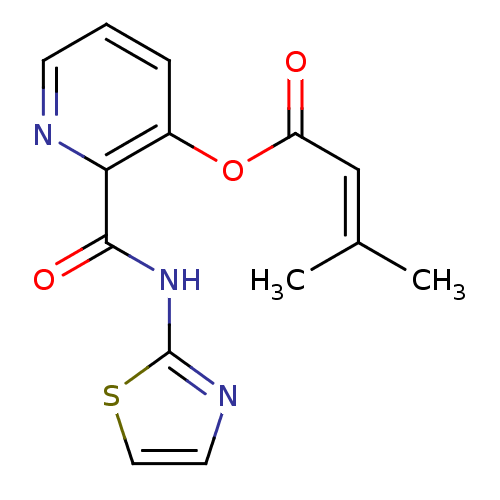
(3-Methyl-but-2-enoic acid 2-(thiazol-2-ylcarbamoyl...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#8]-c1cccnc1-[#6](=O)-[#7]-c1nccs1 Show InChI InChI=1S/C14H13N3O3S/c1-9(2)8-11(18)20-10-4-3-5-15-12(10)13(19)17-14-16-6-7-21-14/h3-8H,1-2H3,(H,16,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM17849

(3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...)Show InChI InChI=1S/C14H16N4O2S/c1-14(2,3)12(20)17-9-5-4-6-15-10(9)11(19)18-13-16-7-8-21-13/h4-8H,1-3H3,(H,17,20)(H,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase
(Escherichia coli (strain K12)) | BDBM50129642

(CHEMBL86427 | Phenyl-acetic acid 2-(thiazol-2-ylca...)Show InChI InChI=1S/C17H13N3O3S/c21-14(11-12-5-2-1-3-6-12)23-13-7-4-8-18-15(13)16(22)20-17-19-9-10-24-17/h1-10H,11H2,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Saccharomyces cerevisiae) | BDBM50129675

(2-Methoxy-benzoic acid 2-(thiazol-2-ylcarbamoyl)-p...)Show InChI InChI=1S/C17H13N3O4S/c1-23-12-6-3-2-5-11(12)16(22)24-13-7-4-8-18-14(13)15(21)20-17-19-9-10-25-17/h2-10H,1H3,(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae |
J Med Chem 46: 2631-40 (2003)
Article DOI: 10.1021/jm0300532
BindingDB Entry DOI: 10.7270/Q2CF9QTC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data