 Found 218 hits with Last Name = 'macintyre' and Initial = 't'
Found 218 hits with Last Name = 'macintyre' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fgr
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Fgr |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50364484
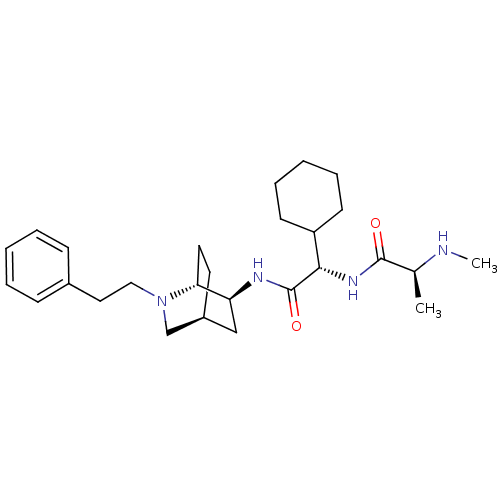
(CHEMBL1950864)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@H]1C[C@@H]2CC[C@H]1N(CCc1ccccc1)C2 |r,TLB:24:23:17.18:21.20| Show InChI InChI=1S/C27H42N4O2/c1-19(28-2)26(32)30-25(22-11-7-4-8-12-22)27(33)29-23-17-21-13-14-24(23)31(18-21)16-15-20-9-5-3-6-10-20/h3,5-6,9-10,19,21-25,28H,4,7-8,11-18H2,1-2H3,(H,29,33)(H,30,32)/t19-,21-,23-,24+,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Antagonist activity at GST-tagged BIR3 domain of cIAP1 using AbuRPFK-5FAM after 20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 1690-4 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.109
BindingDB Entry DOI: 10.7270/Q2G161BG |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044926
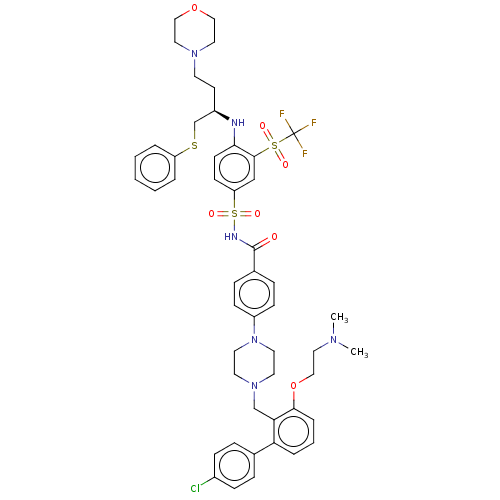
(CHEMBL3309312)Show SMILES CN(C)CCOc1cccc(c1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C49H56ClF3N6O7S3/c1-56(2)27-32-66-46-10-6-9-43(36-11-15-38(50)16-12-36)44(46)34-58-23-25-59(26-24-58)40-17-13-37(14-18-40)48(60)55-69(63,64)42-19-20-45(47(33-42)68(61,62)49(51,52)53)54-39(21-22-57-28-30-65-31-29-57)35-67-41-7-4-3-5-8-41/h3-20,33,39,54H,21-32,34-35H2,1-2H3,(H,55,60)/t39-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human BCL-2 overexpressed in mouse FDC-P1 cells assessed as cell viability after 24 hrs by Cell Titer Glo assay |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SIK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24705

(4-aminopyrazolylpyrimidine analogue, 10k | 5-bromo...)Show SMILES C[C@H](Nc1ncc(Br)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C18H18BrFN6/c1-10(11-4-6-13(20)7-5-11)22-18-21-9-14(19)17(24-18)23-16-8-15(25-26-16)12-2-3-12/h4-10,12H,2-3H2,1H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
Phosphorylase b kinase gamma catalytic chain, liver/testis isoform
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PhKg2 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044927
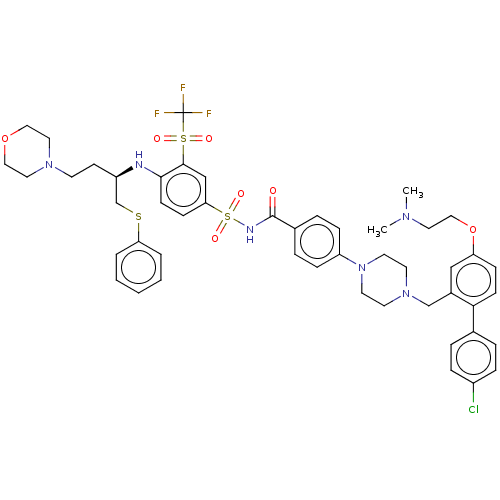
(CHEMBL3309313)Show SMILES CN(C)CCOc1ccc(c(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)c1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C49H56ClF3N6O7S3/c1-56(2)26-31-66-42-16-18-45(36-8-12-39(50)13-9-36)38(32-42)34-58-22-24-59(25-23-58)41-14-10-37(11-15-41)48(60)55-69(63,64)44-17-19-46(47(33-44)68(61,62)49(51,52)53)54-40(20-21-57-27-29-65-30-28-57)35-67-43-6-4-3-5-7-43/h3-19,32-33,40,54H,20-31,34-35H2,1-2H3,(H,55,60)/t40-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044936

(CHEMBL3311486)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCC(CC1)C(=O)c1ccccc1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C46H46ClF3N4O7S3/c47-35-14-10-32(11-15-35)40-8-4-5-9-41(40)44(55)33-20-24-54(25-21-33)37-16-12-34(13-17-37)45(56)52-64(59,60)39-18-19-42(43(30-39)63(57,58)46(48,49)50)51-36(22-23-53-26-28-61-29-27-53)31-62-38-6-2-1-3-7-38/h1-19,30,33,36,51H,20-29,31H2,(H,52,56)/t36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044927

(CHEMBL3309313)Show SMILES CN(C)CCOc1ccc(c(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)c1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C49H56ClF3N6O7S3/c1-56(2)26-31-66-42-16-18-45(36-8-12-39(50)13-9-36)38(32-42)34-58-22-24-59(25-23-58)41-14-10-37(11-15-41)48(60)55-69(63,64)44-17-19-46(47(33-44)68(61,62)49(51,52)53)54-40(20-21-57-27-29-65-30-28-57)35-67-43-6-4-3-5-7-43/h3-19,32-33,40,54H,20-31,34-35H2,1-2H3,(H,55,60)/t40-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044926

(CHEMBL3309312)Show SMILES CN(C)CCOc1cccc(c1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C49H56ClF3N6O7S3/c1-56(2)27-32-66-46-10-6-9-43(36-11-15-38(50)16-12-36)44(46)34-58-23-25-59(26-24-58)40-17-13-37(14-18-40)48(60)55-69(63,64)42-19-20-45(47(33-42)68(61,62)49(51,52)53)54-39(21-22-57-28-30-65-31-29-57)35-67-41-7-4-3-5-8-41/h3-20,33,39,54H,21-32,34-35H2,1-2H3,(H,55,60)/t39-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044925
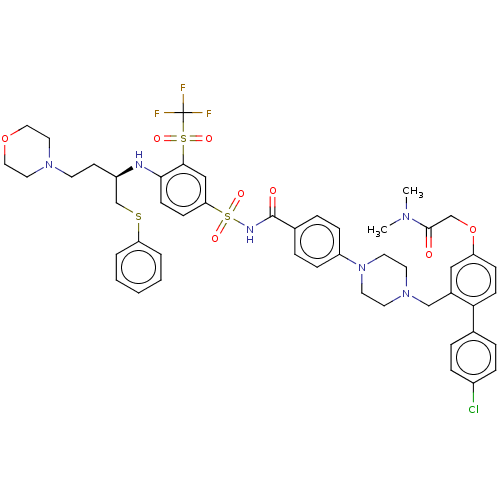
(CHEMBL3309262)Show SMILES CN(C)C(=O)COc1ccc(c(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)c1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C49H54ClF3N6O8S3/c1-56(2)47(60)33-67-41-16-18-44(35-8-12-38(50)13-9-35)37(30-41)32-58-22-24-59(25-23-58)40-14-10-36(11-15-40)48(61)55-70(64,65)43-17-19-45(46(31-43)69(62,63)49(51,52)53)54-39(20-21-57-26-28-66-29-27-57)34-68-42-6-4-3-5-7-42/h3-19,30-31,39,54H,20-29,32-34H2,1-2H3,(H,55,61)/t39-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044924
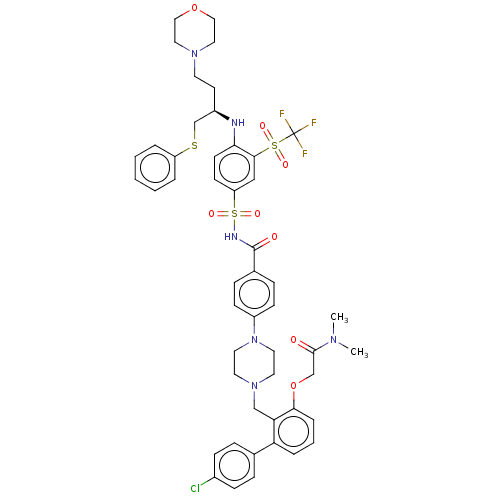
(CHEMBL3309311)Show SMILES CN(C)C(=O)COc1cccc(c1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C49H54ClF3N6O8S3/c1-56(2)47(60)33-67-45-10-6-9-42(35-11-15-37(50)16-12-35)43(45)32-58-23-25-59(26-24-58)39-17-13-36(14-18-39)48(61)55-70(64,65)41-19-20-44(46(31-41)69(62,63)49(51,52)53)54-38(21-22-57-27-29-66-30-28-57)34-68-40-7-4-3-5-8-40/h3-20,31,38,54H,21-30,32-34H2,1-2H3,(H,55,61)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044953
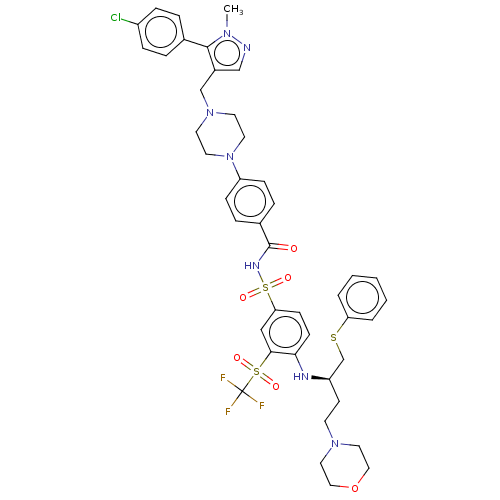
(CHEMBL3309310)Show SMILES Cn1ncc(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)c1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C43H47ClF3N7O6S3/c1-51-41(31-7-11-34(44)12-8-31)33(28-48-51)29-53-19-21-54(22-20-53)36-13-9-32(10-14-36)42(55)50-63(58,59)38-15-16-39(40(27-38)62(56,57)43(45,46)47)49-35(17-18-52-23-25-60-26-24-52)30-61-37-5-3-2-4-6-37/h2-16,27-28,35,49H,17-26,29-30H2,1H3,(H,50,55)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044949
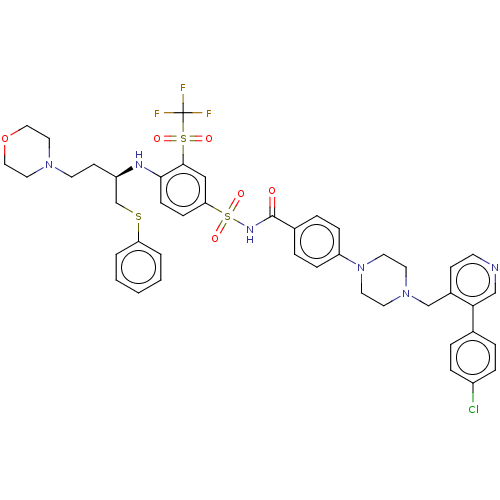
(CHEMBL3309307)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccncc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H46ClF3N6O6S3/c45-35-10-6-32(7-11-35)40-29-49-18-16-34(40)30-53-20-22-54(23-21-53)37-12-8-33(9-13-37)43(55)51-63(58,59)39-14-15-41(42(28-39)62(56,57)44(46,47)48)50-36(17-19-52-24-26-60-27-25-52)31-61-38-4-2-1-3-5-38/h1-16,18,28-29,36,50H,17,19-27,30-31H2,(H,51,55)/t36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044923
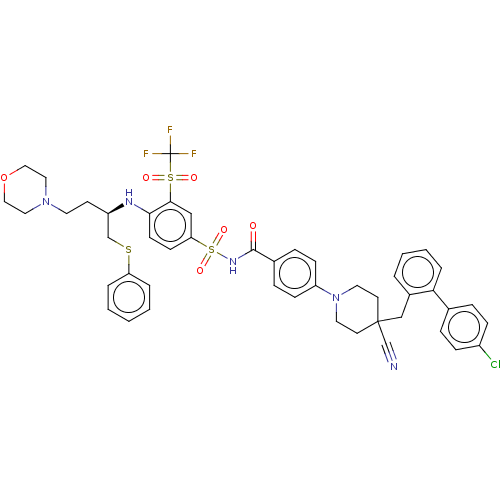
(CHEMBL3311490)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCC(Cc2ccccc2-c2ccc(Cl)cc2)(CC1)C#N |r| Show InChI InChI=1S/C47H47ClF3N5O6S3/c48-37-14-10-34(11-15-37)42-9-5-4-6-36(42)31-46(33-52)21-24-56(25-22-46)39-16-12-35(13-17-39)45(57)54-65(60,61)41-18-19-43(44(30-41)64(58,59)47(49,50)51)53-38(20-23-55-26-28-62-29-27-55)32-63-40-7-2-1-3-8-40/h1-19,30,38,53H,20-29,31-32H2,(H,54,57)/t38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044940

(CHEMBL3311491)Show SMILES NCC1(Cc2ccccc2-c2ccc(Cl)cc2)CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F |r| Show InChI InChI=1S/C47H51ClF3N5O6S3/c48-37-14-10-34(11-15-37)42-9-5-4-6-36(42)31-46(33-52)21-24-56(25-22-46)39-16-12-35(13-17-39)45(57)54-65(60,61)41-18-19-43(44(30-41)64(58,59)47(49,50)51)53-38(20-23-55-26-28-62-29-27-55)32-63-40-7-2-1-3-8-40/h1-19,30,38,53H,20-29,31-33,52H2,(H,54,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044923

(CHEMBL3311490)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCC(Cc2ccccc2-c2ccc(Cl)cc2)(CC1)C#N |r| Show InChI InChI=1S/C47H47ClF3N5O6S3/c48-37-14-10-34(11-15-37)42-9-5-4-6-36(42)31-46(33-52)21-24-56(25-22-46)39-16-12-35(13-17-39)45(57)54-65(60,61)41-18-19-43(44(30-41)64(58,59)47(49,50)51)53-38(20-23-55-26-28-62-29-27-55)32-63-40-7-2-1-3-8-40/h1-19,30,38,53H,20-29,31-32H2,(H,54,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044940

(CHEMBL3311491)Show SMILES NCC1(Cc2ccccc2-c2ccc(Cl)cc2)CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F |r| Show InChI InChI=1S/C47H51ClF3N5O6S3/c48-37-14-10-34(11-15-37)42-9-5-4-6-36(42)31-46(33-52)21-24-56(25-22-46)39-16-12-35(13-17-39)45(57)54-65(60,61)41-18-19-43(44(30-41)64(58,59)47(49,50)51)53-38(20-23-55-26-28-62-29-27-55)32-63-40-7-2-1-3-8-40/h1-19,30,38,53H,20-29,31-33,52H2,(H,54,57)/t38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044944

(CHEMBL3311493)Show SMILES OCC1(Cc2ccccc2-c2ccc(Cl)cc2)CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F |r| Show InChI InChI=1S/C47H50ClF3N4O7S3/c48-37-14-10-34(11-15-37)42-9-5-4-6-36(42)31-46(33-56)21-24-55(25-22-46)39-16-12-35(13-17-39)45(57)53-65(60,61)41-18-19-43(44(30-41)64(58,59)47(49,50)51)52-38(20-23-54-26-28-62-29-27-54)32-63-40-7-2-1-3-8-40/h1-19,30,38,52,56H,20-29,31-33H2,(H,53,57)/t38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24706
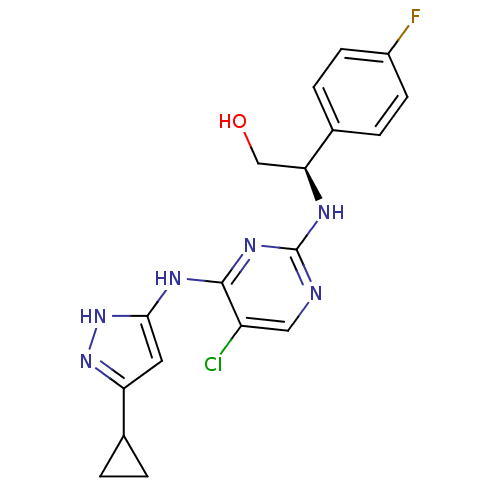
((2R)-2-({5-chloro-4-[(5-cyclopropyl-1H-pyrazol-3-y...)Show SMILES OC[C@H](Nc1ncc(Cl)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C18H18ClFN6O/c19-13-8-21-18(22-15(9-27)11-3-5-12(20)6-4-11)24-17(13)23-16-7-14(25-26-16)10-1-2-10/h3-8,10,15,27H,1-2,9H2,(H3,21,22,23,24,25,26)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50495454

(CHEMBL3108923)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H]1[C@@H](CCc2ccccc12)OCC#C)C(C)(C)C |r| Show InChI InChI=1S/C28H40N4O4/c1-7-17-36-22-15-14-19-11-8-9-12-20(19)23(22)30-26(34)21-13-10-16-32(21)27(35)24(28(3,4)5)31-25(33)18(2)29-6/h1,8-9,11-12,18,21-24,29H,10,13-17H2,2-6H3,(H,30,34)(H,31,33)/t18-,21-,22+,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of AbuRPFK-5FAM from GST-tagged cIAP1 BIR3 domain (L250 to G350) (unknown origin) after 20 mins by fluorescence polarization assay |
J Med Chem 56: 9897-919 (2013)
Article DOI: 10.1021/jm401075x
BindingDB Entry DOI: 10.7270/Q23T9M6C |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044925

(CHEMBL3309262)Show SMILES CN(C)C(=O)COc1ccc(c(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)c1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C49H54ClF3N6O8S3/c1-56(2)47(60)33-67-41-16-18-44(35-8-12-38(50)13-9-35)37(30-41)32-58-22-24-59(25-23-58)40-14-10-36(11-15-40)48(61)55-70(64,65)43-17-19-45(46(31-43)69(62,63)49(51,52)53)54-39(20-21-57-26-28-66-29-27-57)34-68-42-6-4-3-5-7-42/h3-19,30-31,39,54H,20-29,32-34H2,1-2H3,(H,55,61)/t39-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044924

(CHEMBL3309311)Show SMILES CN(C)C(=O)COc1cccc(c1CN1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C49H54ClF3N6O8S3/c1-56(2)47(60)33-67-45-10-6-9-42(35-11-15-37(50)16-12-35)43(45)32-58-23-25-59(26-24-58)39-17-13-36(14-18-39)48(61)55-70(64,65)41-19-20-44(46(31-41)69(62,63)49(51,52)53)54-38(21-22-57-27-29-66-30-28-57)34-68-40-7-4-3-5-8-40/h3-20,31,38,54H,21-30,32-34H2,1-2H3,(H,55,61)/t38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044953

(CHEMBL3309310)Show SMILES Cn1ncc(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)c1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C43H47ClF3N7O6S3/c1-51-41(31-7-11-34(44)12-8-31)33(28-48-51)29-53-19-21-54(22-20-53)36-13-9-32(10-14-36)42(55)50-63(58,59)38-15-16-39(40(27-38)62(56,57)43(45,46)47)49-35(17-18-52-23-25-60-26-24-52)30-61-37-5-3-2-4-6-37/h2-16,27-28,35,49H,17-26,29-30H2,1H3,(H,50,55)/t35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044920

(CHEMBL3311329)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C45H47ClF3N5O6S3/c46-36-14-10-33(11-15-36)41-9-5-4-6-35(41)31-53-22-24-54(25-23-53)38-16-12-34(13-17-38)44(55)51-63(58,59)40-18-19-42(43(30-40)62(56,57)45(47,48)49)50-37(20-21-52-26-28-60-29-27-52)32-61-39-7-2-1-3-8-39/h1-19,30,37,50H,20-29,31-32H2,(H,51,55)/t37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044922
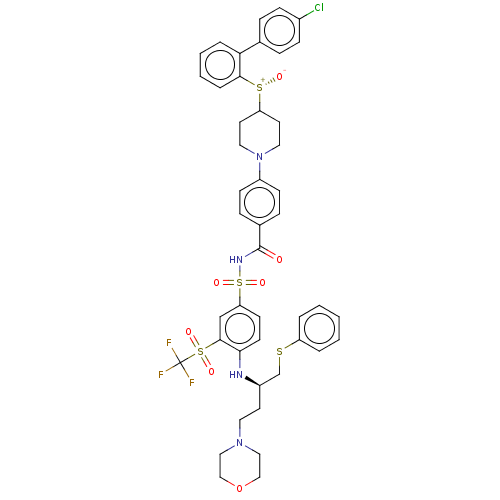
(CHEMBL3311488)Show SMILES [O-][S@+](C1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)c1ccccc1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C45H46ClF3N4O7S4/c46-34-14-10-32(11-15-34)40-8-4-5-9-42(40)62(55)38-21-24-53(25-22-38)36-16-12-33(13-17-36)44(54)51-64(58,59)39-18-19-41(43(30-39)63(56,57)45(47,48)49)50-35(20-23-52-26-28-60-29-27-52)31-61-37-6-2-1-3-7-37/h1-19,30,35,38,50H,20-29,31H2,(H,51,54)/t35-,62-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24707

((3S)-3-({5-chloro-4-[(5-cyclopropyl-1H-pyrazol-3-y...)Show SMILES CN(C)C(=O)C[C@H](Nc1ncc(Cl)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C21H23ClFN7O/c1-30(2)19(31)10-16(12-5-7-14(23)8-6-12)25-21-24-11-15(22)20(27-21)26-18-9-17(28-29-18)13-3-4-13/h5-9,11,13,16H,3-4,10H2,1-2H3,(H3,24,25,26,27,28,29)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SAPK2a |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044920

(CHEMBL3311329)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C45H47ClF3N5O6S3/c46-36-14-10-33(11-15-36)41-9-5-4-6-35(41)31-53-22-24-54(25-23-53)38-16-12-34(13-17-38)44(55)51-63(58,59)40-18-19-42(43(30-40)62(56,57)45(47,48)49)50-37(20-21-52-26-28-60-29-27-52)32-61-39-7-2-1-3-8-39/h1-19,30,37,50H,20-29,31-32H2,(H,51,55)/t37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human BCL-2 overexpressed in mouse FDC-P1 cells assessed as cell viability after 24 hrs by Cell Titer Glo assay |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50495447
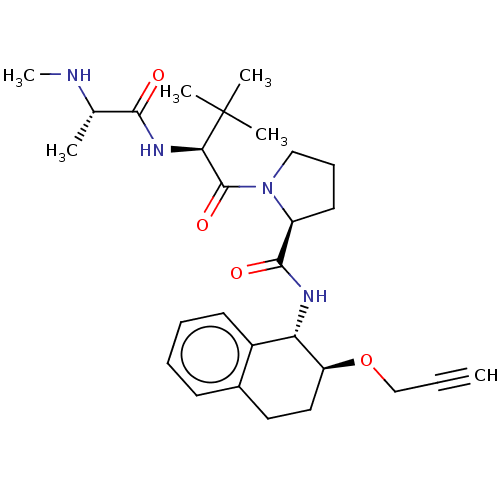
(CHEMBL3108925)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H]1[C@H](CCc2ccccc12)OCC#C)C(C)(C)C |r| Show InChI InChI=1S/C28H40N4O4/c1-7-17-36-22-15-14-19-11-8-9-12-20(19)23(22)30-26(34)21-13-10-16-32(21)27(35)24(28(3,4)5)31-25(33)18(2)29-6/h1,8-9,11-12,18,21-24,29H,10,13-17H2,2-6H3,(H,30,34)(H,31,33)/t18-,21-,22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of AbuRPFK-5FAM from GST-tagged cIAP1 BIR3 domain (L250 to G350) (unknown origin) after 20 mins by fluorescence polarization assay |
J Med Chem 56: 9897-919 (2013)
Article DOI: 10.1021/jm401075x
BindingDB Entry DOI: 10.7270/Q23T9M6C |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24708
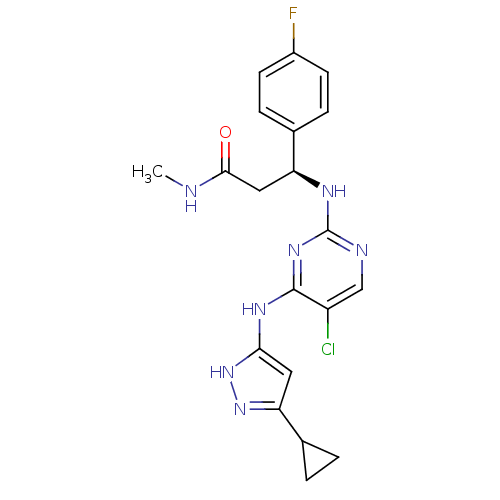
((3S)-3-({5-chloro-4-[(5-cyclopropyl-1H-pyrazol-3-y...)Show SMILES CNC(=O)C[C@H](Nc1ncc(Cl)c(Nc2cc(n[nH]2)C2CC2)n1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H21ClFN7O/c1-23-18(30)9-15(11-4-6-13(22)7-5-11)25-20-24-10-14(21)19(27-20)26-17-8-16(28-29-17)12-2-3-12/h4-8,10,12,15H,2-3,9H2,1H3,(H,23,30)(H3,24,25,26,27,28,29)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
TrkA kinase activity was determined by measuring the kinase ability to phosphorylate synthetic tyrosine residues within a generic polypeptide substra... |
J Med Chem 51: 4672-84 (2008)
Article DOI: 10.1021/jm800343j
BindingDB Entry DOI: 10.7270/Q2RN365V |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044949

(CHEMBL3309307)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccncc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C44H46ClF3N6O6S3/c45-35-10-6-32(7-11-35)40-29-49-18-16-34(40)30-53-20-22-54(23-21-53)37-12-8-33(9-13-37)43(55)51-63(58,59)39-14-15-41(42(28-39)62(56,57)44(46,47)48)50-36(17-19-52-24-26-60-27-25-52)31-61-38-4-2-1-3-5-38/h1-16,18,28-29,36,50H,17,19-27,30-31H2,(H,51,55)/t36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50495451
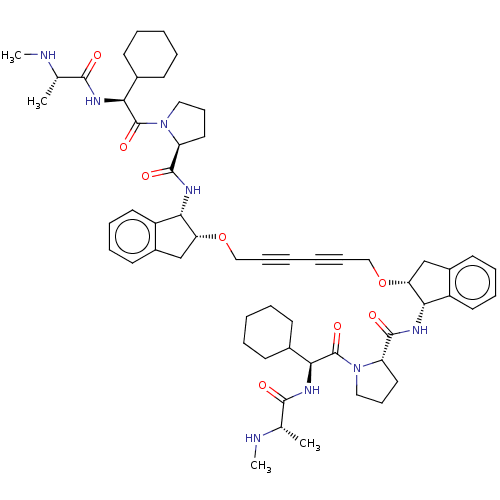
(CHEMBL3108827)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H]1[C@@H](Cc2ccccc12)OCC#CC#CCO[C@@H]1Cc2ccccc2[C@@H]1NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC)C1CCCCC1 |r| Show InChI InChI=1S/C58H78N8O8/c1-37(59-3)53(67)61-49(39-21-9-7-10-22-39)57(71)65-31-19-29-45(65)55(69)63-51-43-27-15-13-25-41(43)35-47(51)73-33-17-5-6-18-34-74-48-36-42-26-14-16-28-44(42)52(48)64-56(70)46-30-20-32-66(46)58(72)50(40-23-11-8-12-24-40)62-54(68)38(2)60-4/h13-16,25-28,37-40,45-52,59-60H,7-12,19-24,29-36H2,1-4H3,(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t37-,38-,45-,46-,47+,48+,49-,50-,51-,52-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of AbuRPFK-5FAM from GST-tagged cIAP1 BIR3 domain (L250 to G350) (unknown origin) after 20 mins by fluorescence polarization assay |
J Med Chem 56: 9897-919 (2013)
Article DOI: 10.1021/jm401075x
BindingDB Entry DOI: 10.7270/Q23T9M6C |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50270877
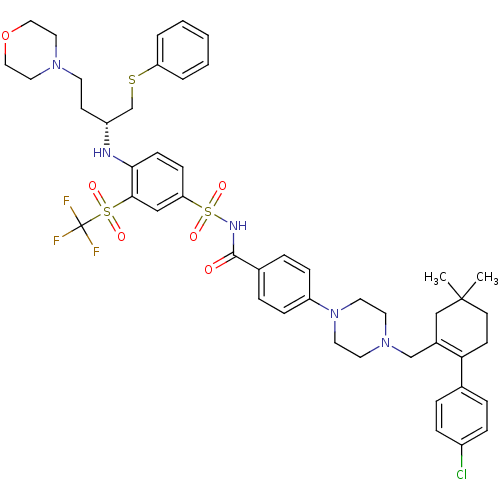
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human BCL-2 overexpressed in mouse FDC-P1 cells assessed as cell viability after 24 hrs by Cell Titer Glo assay |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50495451

(CHEMBL3108827)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H]1[C@@H](Cc2ccccc12)OCC#CC#CCO[C@@H]1Cc2ccccc2[C@@H]1NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC)C1CCCCC1 |r| Show InChI InChI=1S/C58H78N8O8/c1-37(59-3)53(67)61-49(39-21-9-7-10-22-39)57(71)65-31-19-29-45(65)55(69)63-51-43-27-15-13-25-41(43)35-47(51)73-33-17-5-6-18-34-74-48-36-42-26-14-16-28-44(42)52(48)64-56(70)46-30-20-32-66(46)58(72)50(40-23-11-8-12-24-40)62-54(68)38(2)60-4/h13-16,25-28,37-40,45-52,59-60H,7-12,19-24,29-36H2,1-4H3,(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t37-,38-,45-,46-,47+,48+,49-,50-,51-,52-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of AbuRPFK-5FAM from GST-tagged XIAP BIR3 domain (N252 to E350) (unknown origin) after 20 mins by fluorescence polarization assay |
J Med Chem 56: 9897-919 (2013)
Article DOI: 10.1021/jm401075x
BindingDB Entry DOI: 10.7270/Q23T9M6C |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044920

(CHEMBL3311329)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C45H47ClF3N5O6S3/c46-36-14-10-33(11-15-36)41-9-5-4-6-35(41)31-53-22-24-54(25-23-53)38-16-12-34(13-17-38)44(55)51-63(58,59)40-18-19-42(43(30-40)62(56,57)45(47,48)49)50-37(20-21-52-26-28-60-29-27-52)32-61-39-7-2-1-3-8-39/h1-19,30,37,50H,20-29,31-32H2,(H,51,55)/t37-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044942
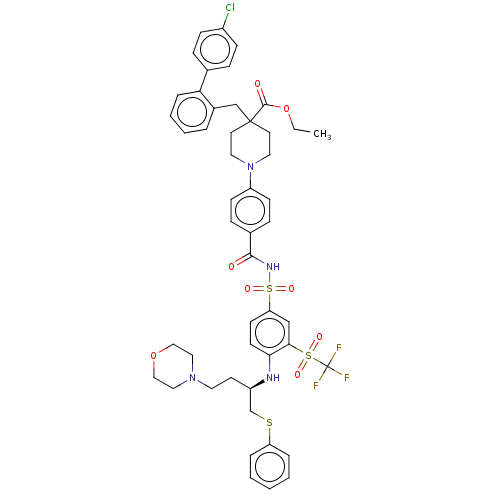
(CHEMBL3311492)Show SMILES CCOC(=O)C1(Cc2ccccc2-c2ccc(Cl)cc2)CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F |r| Show InChI InChI=1S/C49H52ClF3N4O8S3/c1-2-65-47(59)48(33-37-8-6-7-11-43(37)35-12-16-38(50)17-13-35)23-26-57(27-24-48)40-18-14-36(15-19-40)46(58)55-68(62,63)42-20-21-44(45(32-42)67(60,61)49(51,52)53)54-39(22-25-56-28-30-64-31-29-56)34-66-41-9-4-3-5-10-41/h3-21,32,39,54H,2,22-31,33-34H2,1H3,(H,55,58)/t39-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50044934

(CHEMBL3311484)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCP(=O)(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C45H47ClF3N4O7PS3/c46-36-14-10-33(11-15-36)41-9-5-4-6-35(41)31-61(55)28-24-53(25-29-61)38-16-12-34(13-17-38)44(54)51-64(58,59)40-18-19-42(43(30-40)63(56,57)45(47,48)49)50-37(20-21-52-22-26-60-27-23-52)32-62-39-7-2-1-3-8-39/h1-19,30,37,50H,20-29,31-32H2,(H,51,54)/t37-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044921
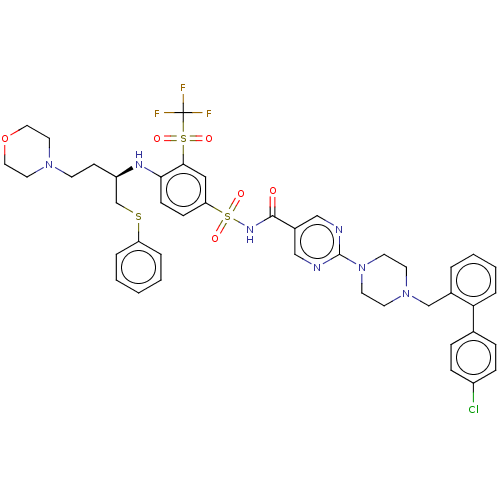
(CHEMBL3311480)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=O)c1cnc(nc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C43H45ClF3N7O6S3/c44-34-12-10-31(11-13-34)38-9-5-4-6-32(38)29-53-18-20-54(21-19-53)42-48-27-33(28-49-42)41(55)51-63(58,59)37-14-15-39(40(26-37)62(56,57)43(45,46)47)50-35(16-17-52-22-24-60-25-23-52)30-61-36-7-2-1-3-8-36/h1-15,26-28,35,50H,16-25,29-30H2,(H,51,55)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50364500
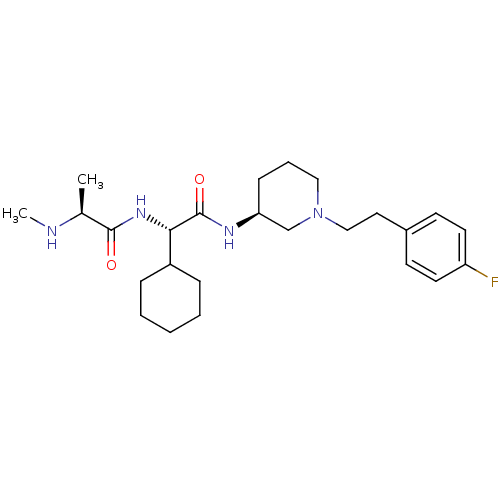
(CHEMBL1950850)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N[C@H]1CCCN(CCc2ccc(F)cc2)C1 |r| Show InChI InChI=1S/C25H39FN4O2/c1-18(27-2)24(31)29-23(20-7-4-3-5-8-20)25(32)28-22-9-6-15-30(17-22)16-14-19-10-12-21(26)13-11-19/h10-13,18,20,22-23,27H,3-9,14-17H2,1-2H3,(H,28,32)(H,29,31)/t18-,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Antagonist activity at GST-tagged BIR3 domain of cIAP1 using AbuRPFK-5FAM after 20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 1690-4 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.109
BindingDB Entry DOI: 10.7270/Q2G161BG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50392791

(CHEMBL2151321 | US8486966, 1)Show SMILES CC(C)Oc1cc(n[nH]1)-n1cnc2ccc(N[C@@H](C)c3ccc(F)cn3)nc12 |r| Show InChI InChI=1S/C19H20FN7O/c1-11(2)28-18-8-17(25-26-18)27-10-22-15-6-7-16(24-19(15)27)23-12(3)14-5-4-13(20)9-21-14/h4-12H,1-3H3,(H,23,24)(H,25,26)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of cSrc |
ACS Med Chem Lett 3: 705-709 (2012)
Article DOI: 10.1021/ml300074j
BindingDB Entry DOI: 10.7270/Q2GH9K2G |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044928
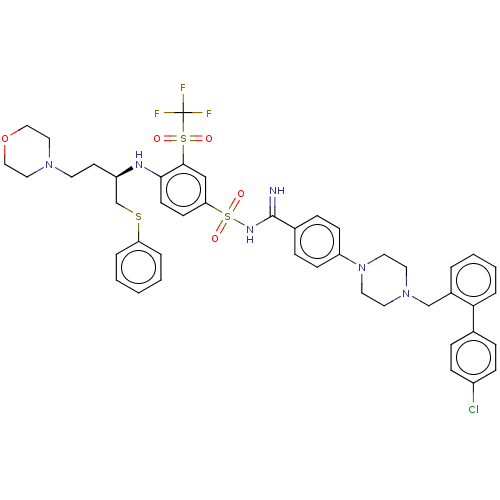
(CHEMBL3311330)Show SMILES FC(F)(F)S(=O)(=O)c1cc(ccc1N[C@H](CCN1CCOCC1)CSc1ccccc1)S(=O)(=O)NC(=N)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C45H48ClF3N6O5S3/c46-36-14-10-33(11-15-36)41-9-5-4-6-35(41)31-54-22-24-55(25-23-54)38-16-12-34(13-17-38)44(50)52-63(58,59)40-18-19-42(43(30-40)62(56,57)45(47,48)49)51-37(20-21-53-26-28-60-29-27-53)32-61-39-7-2-1-3-8-39/h1-19,30,37,51H,20-29,31-32H2,(H2,50,52)/t37-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50044922

(CHEMBL3311488)Show SMILES [O-][S@+](C1CCN(CC1)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(N[C@H](CCN2CCOCC2)CSc2ccccc2)c(c1)S(=O)(=O)C(F)(F)F)c1ccccc1-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C45H46ClF3N4O7S4/c46-34-14-10-32(11-15-34)40-8-4-5-9-42(40)62(55)38-21-24-53(25-22-38)36-16-12-33(13-17-36)44(54)51-64(58,59)39-18-19-41(43(30-39)63(56,57)45(47,48)49)50-35(20-23-52-26-28-60-29-27-52)31-61-37-6-2-1-3-7-37/h1-19,30,35,38,50H,20-29,31H2,(H,51,54)/t35-,62-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after ... |
Bioorg Med Chem Lett 24: 3026-33 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.036
BindingDB Entry DOI: 10.7270/Q27M09JW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50495454

(CHEMBL3108923)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H]1[C@@H](CCc2ccccc12)OCC#C)C(C)(C)C |r| Show InChI InChI=1S/C28H40N4O4/c1-7-17-36-22-15-14-19-11-8-9-12-20(19)23(22)30-26(34)21-13-10-16-32(21)27(35)24(28(3,4)5)31-25(33)18(2)29-6/h1,8-9,11-12,18,21-24,29H,10,13-17H2,2-6H3,(H,30,34)(H,31,33)/t18-,21-,22+,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of AbuRPFK-5FAM from GST-tagged XIAP BIR3 domain (N252 to E350) (unknown origin) after 20 mins by fluorescence polarization assay |
J Med Chem 56: 9897-919 (2013)
Article DOI: 10.1021/jm401075x
BindingDB Entry DOI: 10.7270/Q23T9M6C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data