 Found 324 hits with Last Name = 'mcclure' and Initial = 'ld'
Found 324 hits with Last Name = 'mcclure' and Initial = 'ld' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50118009

((6,7-Diethoxy-quinazolin-4-yl)-(3-thiazol-2-yl-phe...)Show InChI InChI=1S/C21H20N4O2S/c1-3-26-18-11-16-17(12-19(18)27-4-2)23-13-24-20(16)25-15-7-5-6-14(10-15)21-22-8-9-28-21/h5-13H,3-4H2,1-2H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of (EGFR) epidermal growth factor receptor tyrosine kinase |
J Med Chem 45: 3865-77 (2002)
BindingDB Entry DOI: 10.7270/Q2PN94Z0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50117952
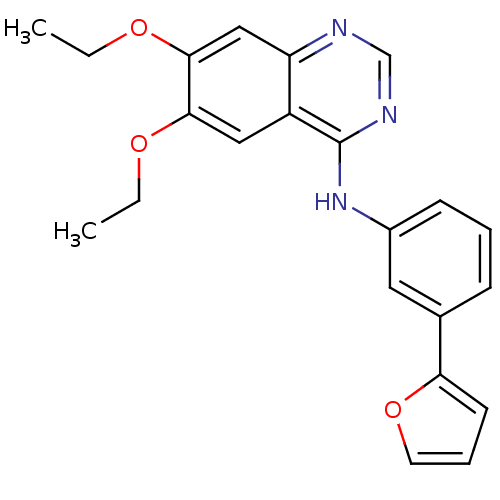
((6,7-Diethoxy-quinazolin-4-yl)-(3-furan-2-yl-pheny...)Show InChI InChI=1S/C22H21N3O3/c1-3-26-20-12-17-18(13-21(20)27-4-2)23-14-24-22(17)25-16-8-5-7-15(11-16)19-9-6-10-28-19/h5-14H,3-4H2,1-2H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of (EGFR) epidermal growth factor receptor tyrosine kinase |
J Med Chem 45: 3865-77 (2002)
BindingDB Entry DOI: 10.7270/Q2PN94Z0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50095256

((6,7-Dimethoxy-quinazolin-4-yl)-(3-ethynyl-4-fluor...)Show InChI InChI=1S/C18H14FN3O2/c1-4-11-7-12(5-6-14(11)19)22-18-13-8-16(23-2)17(24-3)9-15(13)20-10-21-18/h1,5-10H,2-3H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of (EGFR) epidermal growth factor receptor tyrosine kinase |
J Med Chem 45: 3865-77 (2002)
BindingDB Entry DOI: 10.7270/Q2PN94Z0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15494
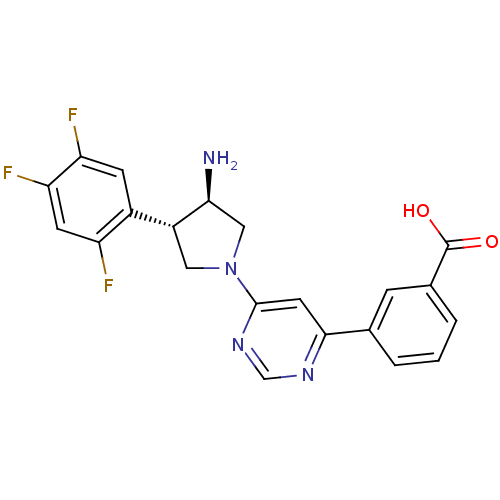
(3-{6-[(3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)pyr...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C21H17F3N4O2/c22-15-6-17(24)16(23)5-13(15)14-8-28(9-18(14)25)20-7-19(26-10-27-20)11-2-1-3-12(4-11)21(29)30/h1-7,10,14,18H,8-9,25H2,(H,29,30)/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50117986
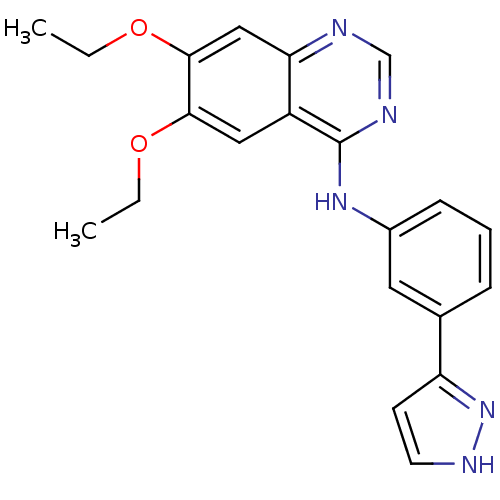
((6,7-Diethoxy-quinazolin-4-yl)-[3-(1H-pyrazol-3-yl...)Show SMILES CCOc1cc2ncnc(Nc3cccc(c3)-c3cc[nH]n3)c2cc1OCC Show InChI InChI=1S/C21H21N5O2/c1-3-27-19-11-16-18(12-20(19)28-4-2)22-13-23-21(16)25-15-7-5-6-14(10-15)17-8-9-24-26-17/h5-13H,3-4H2,1-2H3,(H,24,26)(H,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of (EGFR) epidermal growth factor receptor tyrosine kinase |
J Med Chem 45: 3865-77 (2002)
BindingDB Entry DOI: 10.7270/Q2PN94Z0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50117983

((6,7-Diethoxy-quinazolin-4-yl)-(3-thiazol-5-yl-phe...)Show InChI InChI=1S/C21H20N4O2S/c1-3-26-18-9-16-17(10-19(18)27-4-2)23-12-24-21(16)25-15-7-5-6-14(8-15)20-11-22-13-28-20/h5-13H,3-4H2,1-2H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of (EGFR) epidermal growth factor receptor tyrosine kinase |
J Med Chem 45: 3865-77 (2002)
BindingDB Entry DOI: 10.7270/Q2PN94Z0 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158278
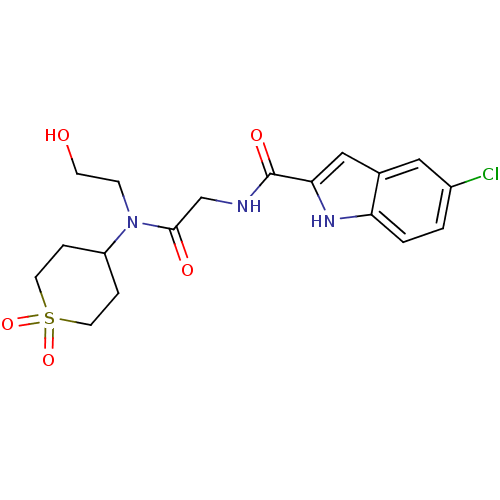
(5-Chloro-1H-indole-2-carboxylic acid {[(1,1-dioxo-...)Show SMILES OCCN(C1CCS(=O)(=O)CC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C18H22ClN3O5S/c19-13-1-2-15-12(9-13)10-16(21-15)18(25)20-11-17(24)22(5-6-23)14-3-7-28(26,27)8-4-14/h1-2,9-10,14,21,23H,3-8,11H2,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221102

(CHEMBL236719 | N-(3-(6-((3R,4S)-3-amino-4-(2,4,5-t...)Show SMILES CC(=O)Nc1cccc(c1)-c1cc(ncn1)N1C[C@H](N)[C@H](C1)c1cc(F)c(F)cc1F Show InChI InChI=1S/C22H20F3N5O/c1-12(31)29-14-4-2-3-13(5-14)21-8-22(28-11-27-21)30-9-16(20(26)10-30)15-6-18(24)19(25)7-17(15)23/h2-8,11,16,20H,9-10,26H2,1H3,(H,29,31)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158249
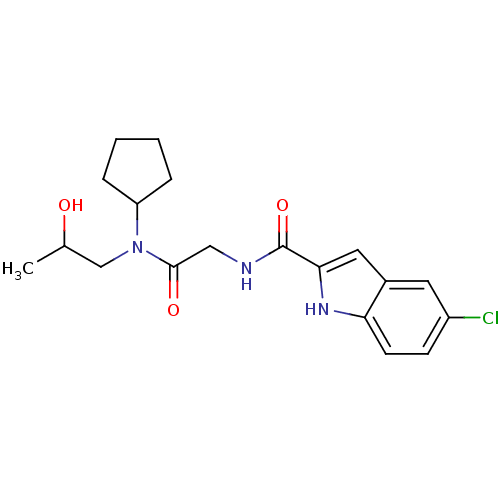
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES CC(O)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H24ClN3O3/c1-12(24)11-23(15-4-2-3-5-15)18(25)10-21-19(26)17-9-13-8-14(20)6-7-16(13)22-17/h6-9,12,15,22,24H,2-5,10-11H2,1H3,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50117970
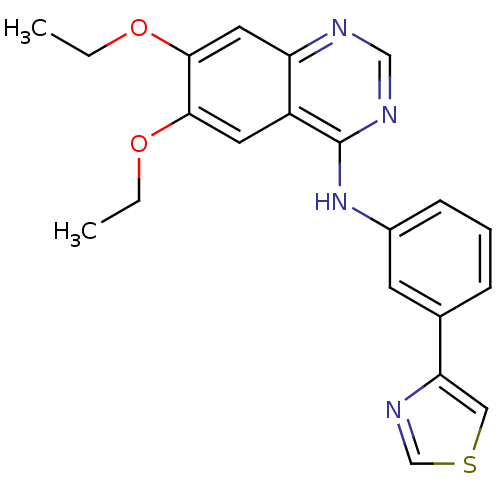
((6,7-Diethoxy-quinazolin-4-yl)-(3-thiazol-4-yl-phe...)Show InChI InChI=1S/C21H20N4O2S/c1-3-26-19-9-16-17(10-20(19)27-4-2)22-12-23-21(16)25-15-7-5-6-14(8-15)18-11-28-13-24-18/h5-13H,3-4H2,1-2H3,(H,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of (EGFR) epidermal growth factor receptor tyrosine kinase |
J Med Chem 45: 3865-77 (2002)
BindingDB Entry DOI: 10.7270/Q2PN94Z0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221075
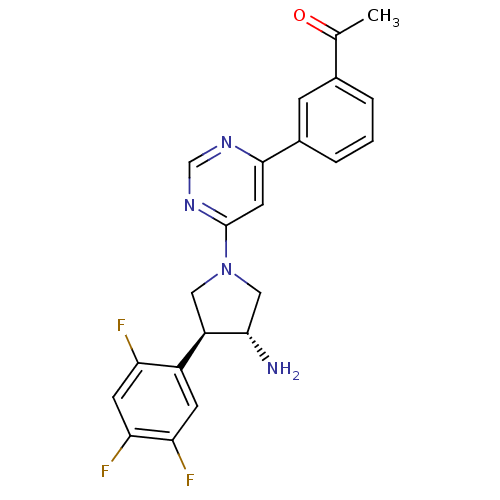
(1-(3-(6-((3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)...)Show SMILES CC(=O)c1cccc(c1)-c1cc(ncn1)N1C[C@H](N)[C@H](C1)c1cc(F)c(F)cc1F Show InChI InChI=1S/C22H19F3N4O/c1-12(30)13-3-2-4-14(5-13)21-8-22(28-11-27-21)29-9-16(20(26)10-29)15-6-18(24)19(25)7-17(15)23/h2-8,11,16,20H,9-10,26H2,1H3/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15492
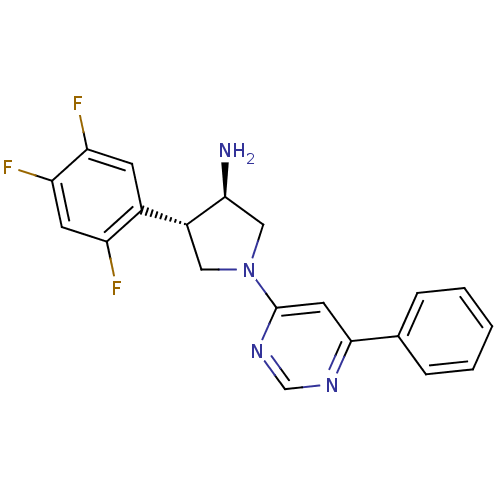
((3R,4S)-1-(6-phenylpyrimidin-4-yl)-4-(2,4,5-triflu...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1ccccc1 |r| Show InChI InChI=1S/C20H17F3N4/c21-15-7-17(23)16(22)6-13(15)14-9-27(10-18(14)24)20-8-19(25-11-26-20)12-4-2-1-3-5-12/h1-8,11,14,18H,9-10,24H2/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM15492

((3R,4S)-1-(6-phenylpyrimidin-4-yl)-4-(2,4,5-triflu...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1ccccc1 |r| Show InChI InChI=1S/C20H17F3N4/c21-15-7-17(23)16(22)6-13(15)14-9-27(10-18(14)24)20-8-19(25-11-26-20)12-4-2-1-3-5-12/h1-8,11,14,18H,9-10,24H2/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158254
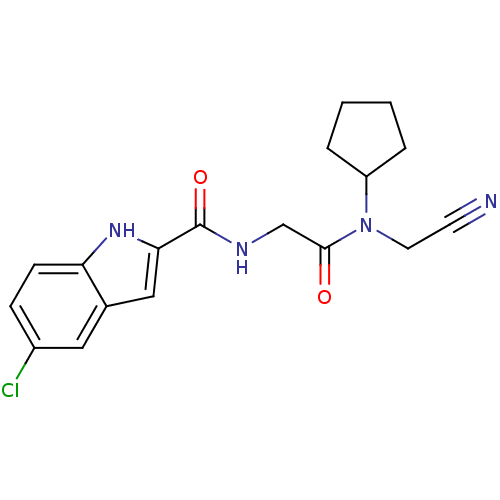
(5-Chloro-1H-indole-2-carboxylic acid [(cyanomethyl...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)NCC(=O)N(CC#N)C1CCCC1 Show InChI InChI=1S/C18H19ClN4O2/c19-13-5-6-15-12(9-13)10-16(22-15)18(25)21-11-17(24)23(8-7-20)14-3-1-2-4-14/h5-6,9-10,14,22H,1-4,8,11H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158255
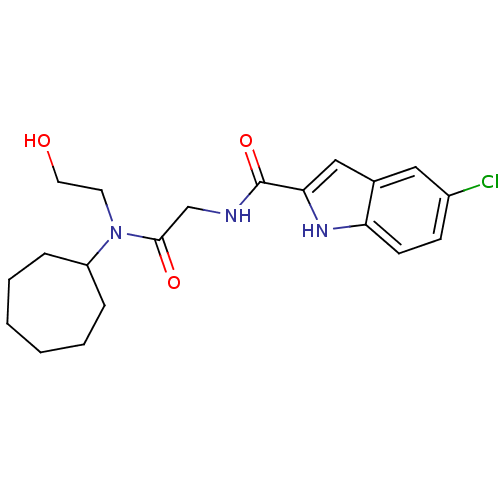
(5-Chloro-1H-indole-2-carboxylic acid {[cycloheptyl...)Show SMILES OCCN(C1CCCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O3/c21-15-7-8-17-14(11-15)12-18(23-17)20(27)22-13-19(26)24(9-10-25)16-5-3-1-2-4-6-16/h7-8,11-12,16,23,25H,1-6,9-10,13H2,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221083
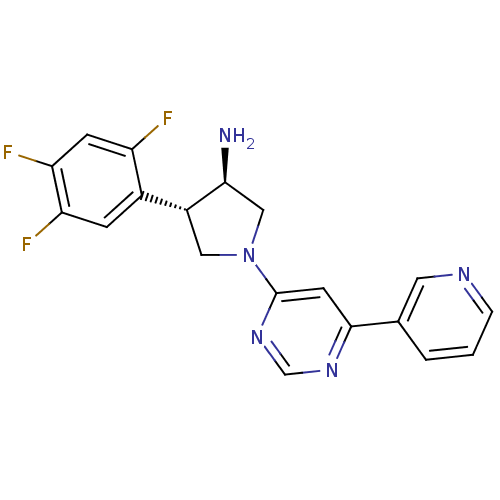
((3R,4S)-1-(6-(pyridin-3-yl)pyrimidin-4-yl)-4-(2,4,...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1cccnc1 Show InChI InChI=1S/C19H16F3N5/c20-14-5-16(22)15(21)4-12(14)13-8-27(9-17(13)23)19-6-18(25-10-26-19)11-2-1-3-24-7-11/h1-7,10,13,17H,8-9,23H2/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221094
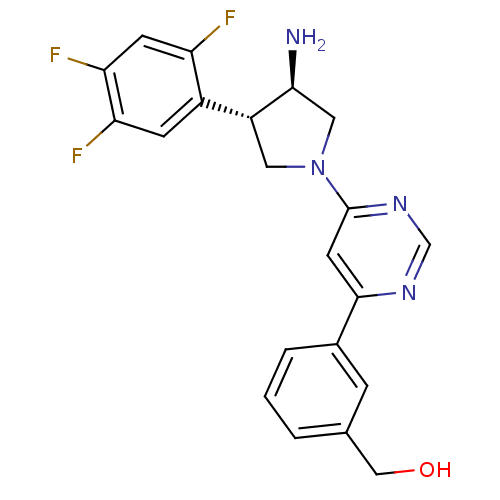
((3-(6-((3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)py...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1cccc(CO)c1 Show InChI InChI=1S/C21H19F3N4O/c22-16-6-18(24)17(23)5-14(16)15-8-28(9-19(15)25)21-7-20(26-11-27-21)13-3-1-2-12(4-13)10-29/h1-7,11,15,19,29H,8-10,25H2/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158283
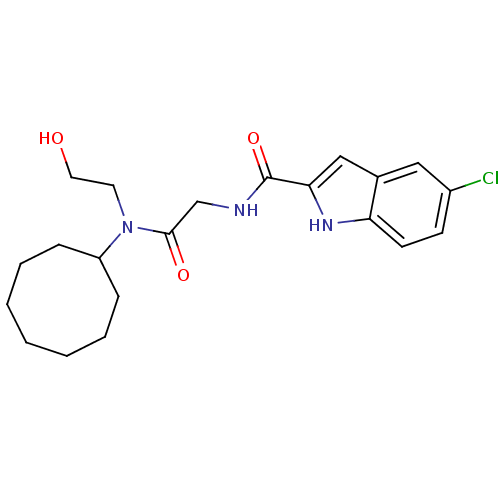
(5-Chloro-1H-indole-2-carboxylic acid {[cyclooctyl-...)Show SMILES OCCN(C1CCCCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C21H28ClN3O3/c22-16-8-9-18-15(12-16)13-19(24-18)21(28)23-14-20(27)25(10-11-26)17-6-4-2-1-3-5-7-17/h8-9,12-13,17,24,26H,1-7,10-11,14H2,(H,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221073
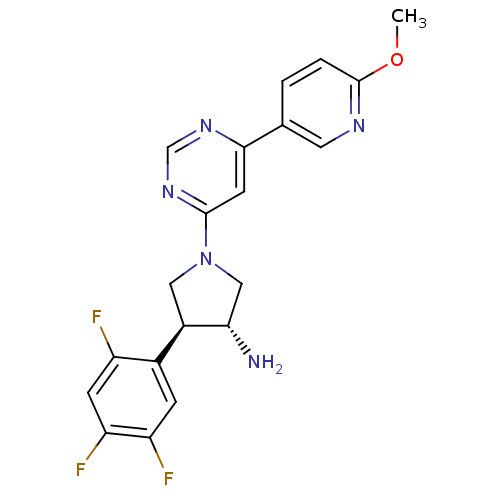
((3R,4S)-1-(6-(6-methoxypyridin-3-yl)pyrimidin-4-yl...)Show SMILES COc1ccc(cn1)-c1cc(ncn1)N1C[C@H](N)[C@H](C1)c1cc(F)c(F)cc1F Show InChI InChI=1S/C20H18F3N5O/c1-29-20-3-2-11(7-25-20)18-6-19(27-10-26-18)28-8-13(17(24)9-28)12-4-15(22)16(23)5-14(12)21/h2-7,10,13,17H,8-9,24H2,1H3/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221073

((3R,4S)-1-(6-(6-methoxypyridin-3-yl)pyrimidin-4-yl...)Show SMILES COc1ccc(cn1)-c1cc(ncn1)N1C[C@H](N)[C@H](C1)c1cc(F)c(F)cc1F Show InChI InChI=1S/C20H18F3N5O/c1-29-20-3-2-11(7-25-20)18-6-19(27-10-26-18)28-8-13(17(24)9-28)12-4-15(22)16(23)5-14(12)21/h2-7,10,13,17H,8-9,24H2,1H3/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221080

(3-(6-((3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)pyr...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1cccc(c1)C#N Show InChI InChI=1S/C21H16F3N5/c22-16-6-18(24)17(23)5-14(16)15-9-29(10-19(15)26)21-7-20(27-11-28-21)13-3-1-2-12(4-13)8-25/h1-7,11,15,19H,9-10,26H2/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50187266
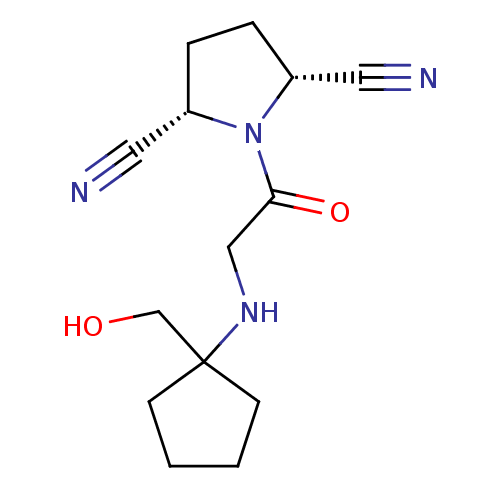
((2R,5S)-1-(2-(1-(hydroxymethyl)cyclopentylamino)ac...)Show SMILES OCC1(CCCC1)NCC(=O)N1[C@@H](CC[C@@H]1C#N)C#N Show InChI InChI=1S/C14H20N4O2/c15-7-11-3-4-12(8-16)18(11)13(20)9-17-14(10-19)5-1-2-6-14/h11-12,17,19H,1-6,9-10H2/t11-,12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in Sprague-Dawley rat plasma |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158308
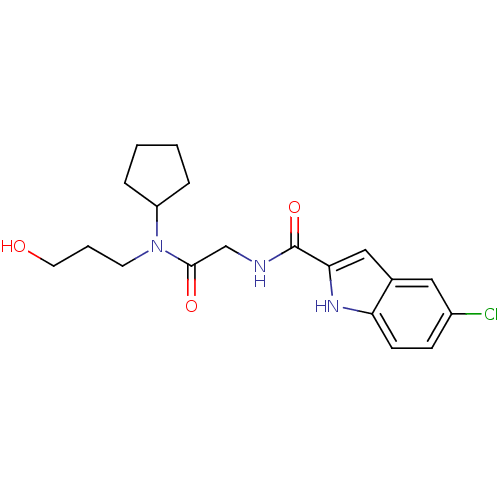
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES OCCCN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H24ClN3O3/c20-14-6-7-16-13(10-14)11-17(22-16)19(26)21-12-18(25)23(8-3-9-24)15-4-1-2-5-15/h6-7,10-11,15,22,24H,1-5,8-9,12H2,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221104

((3R,4S)-1-(6-(3-methoxyphenyl)pyrimidin-4-yl)-4-(2...)Show SMILES COc1cccc(c1)-c1cc(ncn1)N1C[C@H](N)[C@H](C1)c1cc(F)c(F)cc1F Show InChI InChI=1S/C21H19F3N4O/c1-29-13-4-2-3-12(5-13)20-8-21(27-11-26-20)28-9-15(19(25)10-28)14-6-17(23)18(24)7-16(14)22/h2-8,11,15,19H,9-10,25H2,1H3/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158303
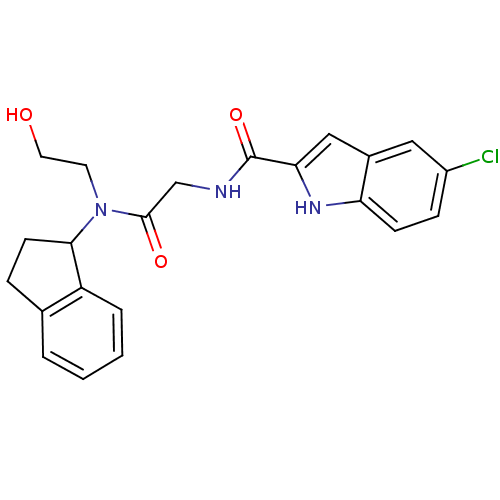
(5-Chloro-1H-indole-2-carboxylic acid {[(2-hydroxy-...)Show SMILES OCCN(C1CCc2ccccc12)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H22ClN3O3/c23-16-6-7-18-15(11-16)12-19(25-18)22(29)24-13-21(28)26(9-10-27)20-8-5-14-3-1-2-4-17(14)20/h1-4,6-7,11-12,20,25,27H,5,8-10,13H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221101

((3R,4S)-1-(6-(4-fluorophenyl)pyrimidin-4-yl)-4-(2,...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H16F4N4/c21-12-3-1-11(2-4-12)19-7-20(27-10-26-19)28-8-14(18(25)9-28)13-5-16(23)17(24)6-15(13)22/h1-7,10,14,18H,8-9,25H2/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221085
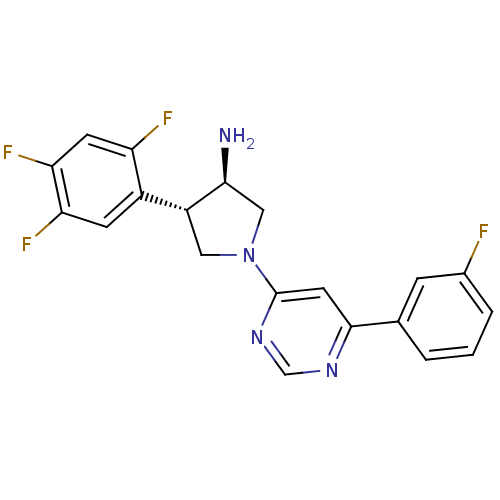
((3R,4S)-1-(6-(3-fluorophenyl)pyrimidin-4-yl)-4-(2,...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1cccc(F)c1 Show InChI InChI=1S/C20H16F4N4/c21-12-3-1-2-11(4-12)19-7-20(27-10-26-19)28-8-14(18(25)9-28)13-5-16(23)17(24)6-15(13)22/h1-7,10,14,18H,8-9,25H2/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221085

((3R,4S)-1-(6-(3-fluorophenyl)pyrimidin-4-yl)-4-(2,...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1cccc(F)c1 Show InChI InChI=1S/C20H16F4N4/c21-12-3-1-2-11(4-12)19-7-20(27-10-26-19)28-8-14(18(25)9-28)13-5-16(23)17(24)6-15(13)22/h1-7,10,14,18H,8-9,25H2/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158295
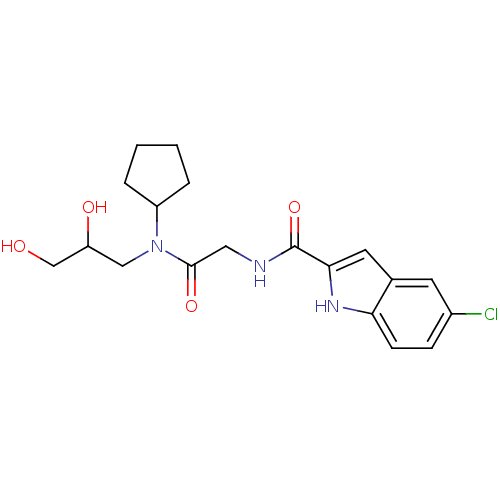
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES OCC(O)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H24ClN3O4/c20-13-5-6-16-12(7-13)8-17(22-16)19(27)21-9-18(26)23(10-15(25)11-24)14-3-1-2-4-14/h5-8,14-15,22,24-25H,1-4,9-11H2,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158245
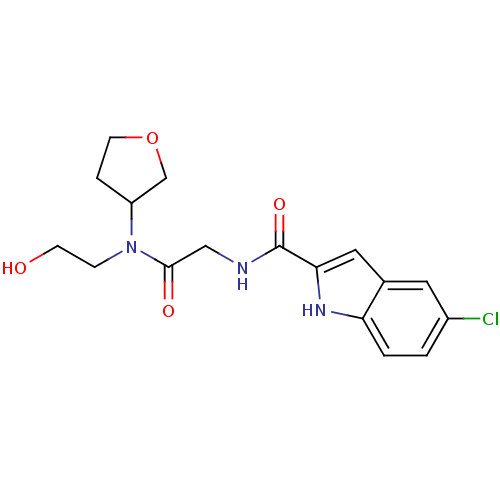
(5-Chloro-1H-indole-2-carboxylic acid {[(2-hydroxy-...)Show SMILES OCCN(C1CCOC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C17H20ClN3O4/c18-12-1-2-14-11(7-12)8-15(20-14)17(24)19-9-16(23)21(4-5-22)13-3-6-25-10-13/h1-2,7-8,13,20,22H,3-6,9-10H2,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158304
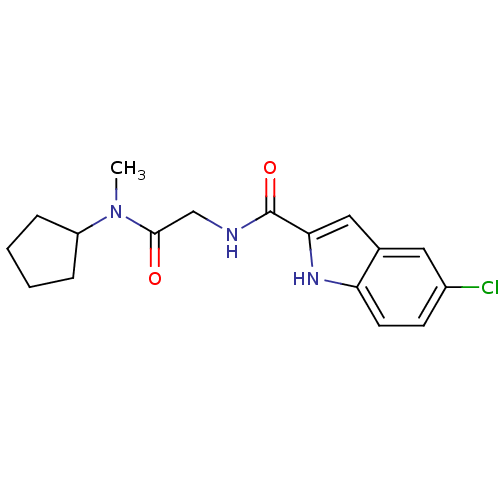
(5-Chloro-1H-indole-2-carboxylic acid [(cyclopentyl...)Show SMILES CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C17H20ClN3O2/c1-21(13-4-2-3-5-13)16(22)10-19-17(23)15-9-11-8-12(18)6-7-14(11)20-15/h6-9,13,20H,2-5,10H2,1H3,(H,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221088
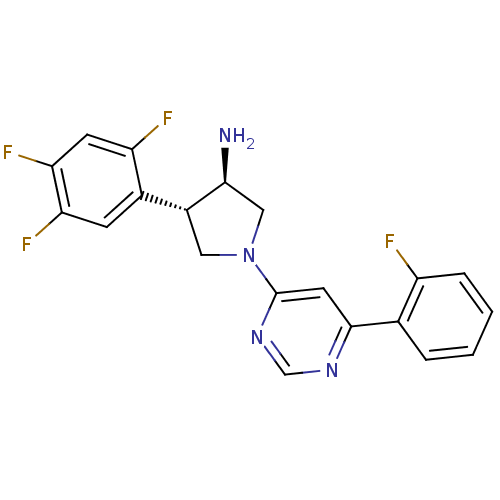
((3R,4S)-1-(6-(2-fluorophenyl)pyrimidin-4-yl)-4-(2,...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1ccccc1F Show InChI InChI=1S/C20H16F4N4/c21-14-4-2-1-3-11(14)19-7-20(27-10-26-19)28-8-13(18(25)9-28)12-5-16(23)17(24)6-15(12)22/h1-7,10,13,18H,8-9,25H2/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187283
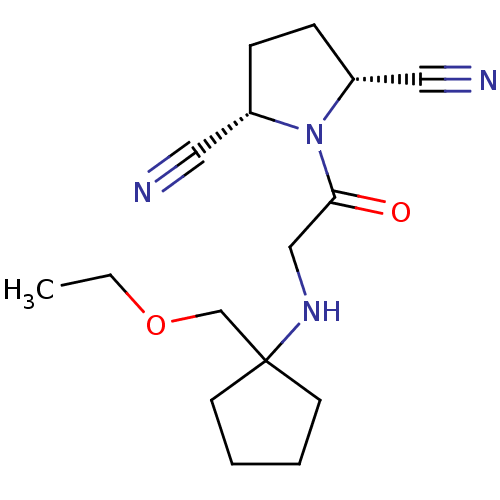
((2R,5S)-1-(2-(1-(ethoxymethyl)cyclopentylamino)ace...)Show SMILES CCOCC1(CCCC1)NCC(=O)N1[C@@H](CC[C@@H]1C#N)C#N Show InChI InChI=1S/C16H24N4O2/c1-2-22-12-16(7-3-4-8-16)19-11-15(21)20-13(9-17)5-6-14(20)10-18/h13-14,19H,2-8,11-12H2,1H3/t13-,14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221074
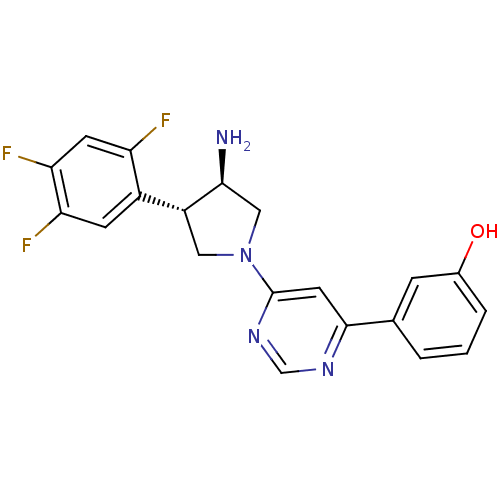
(3-(6-((3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)pyr...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1cccc(O)c1 Show InChI InChI=1S/C20H17F3N4O/c21-15-6-17(23)16(22)5-13(15)14-8-27(9-18(14)24)20-7-19(25-10-26-20)11-2-1-3-12(28)4-11/h1-7,10,14,18,28H,8-9,24H2/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221082
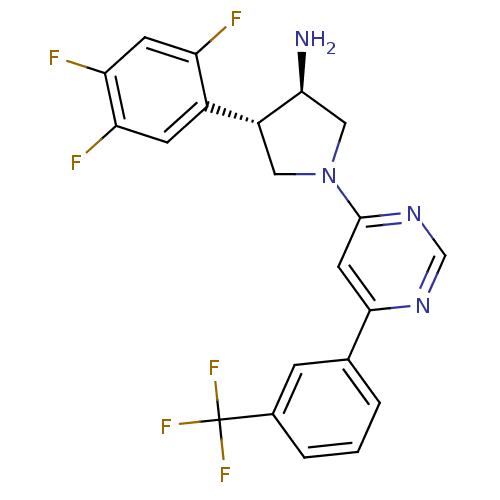
((3R,4S)-1-(6-(3-(trifluoromethyl)phenyl)pyrimidin-...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H16F6N4/c22-15-6-17(24)16(23)5-13(15)14-8-31(9-18(14)28)20-7-19(29-10-30-20)11-2-1-3-12(4-11)21(25,26)27/h1-7,10,14,18H,8-9,28H2/t14-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187258
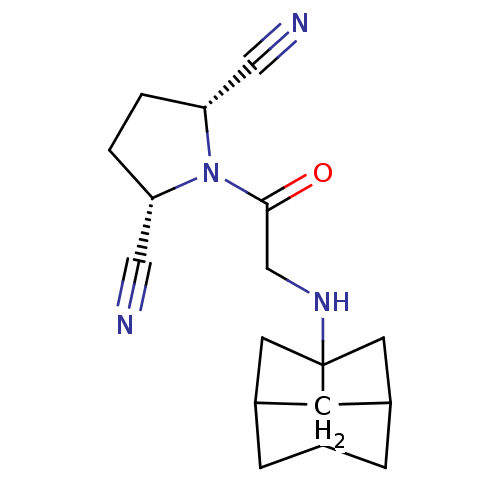
((2S,5R)-1-[2-(hexahydro-2,5-methano-pentalen-3a-yl...)Show SMILES O=C(CNC12CC3CC1CC(C2)C3)N1[C@@H](CC[C@@H]1C#N)C#N |TLB:7:6:8.9:11,THB:9:8:5:10.11.12,9:10:5:7.8,7:8:11:6.5.12| Show InChI InChI=1S/C17H22N4O/c18-8-14-1-2-15(9-19)21(14)16(22)10-20-17-6-11-3-12(7-17)5-13(17)4-11/h11-15,20H,1-7,10H2/t11?,12?,13?,14-,15+,17? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187263
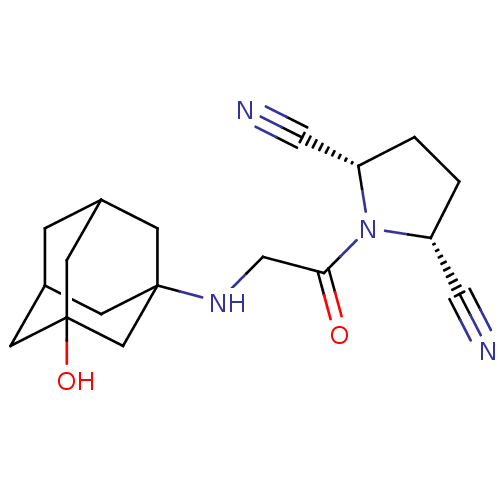
((2S,5R)-1-[2-(3-hydroxy-adamantan-1-ylamino)-acety...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1[C@@H](CC[C@@H]1C#N)C#N |TLB:6:1:9:7.5.4,0:1:9:7.5.4,4:3:10:7.5.6,4:5:10:2.9.3,THB:6:5:10.1.2:9| Show InChI InChI=1S/C18H24N4O2/c19-8-14-1-2-15(9-20)22(14)16(23)10-21-17-4-12-3-13(5-17)7-18(24,6-12)11-17/h12-15,21,24H,1-7,10-11H2/t12?,13?,14-,15+,17?,18? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158310
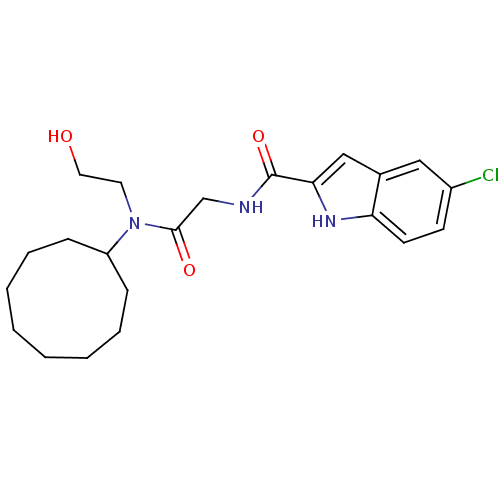
(5-Chloro-1H-indole-2-carboxylic acid {[cyclononyl-...)Show SMILES OCCN(C1CCCCCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H30ClN3O3/c23-17-9-10-19-16(13-17)14-20(25-19)22(29)24-15-21(28)26(11-12-27)18-7-5-3-1-2-4-6-8-18/h9-10,13-14,18,25,27H,1-8,11-12,15H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158315
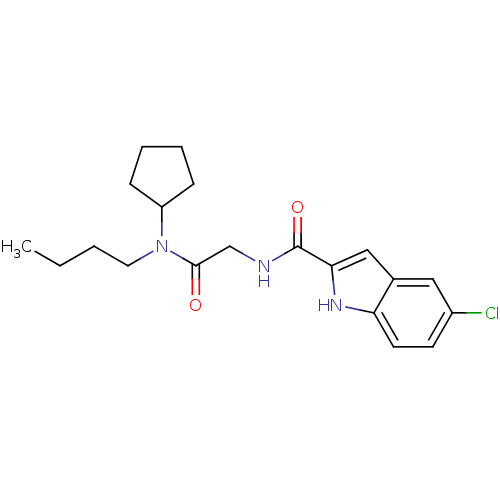
(5-Chloro-1H-indole-2-carboxylic acid [(butyl-cyclo...)Show SMILES CCCCN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O2/c1-2-3-10-24(16-6-4-5-7-16)19(25)13-22-20(26)18-12-14-11-15(21)8-9-17(14)23-18/h8-9,11-12,16,23H,2-7,10,13H2,1H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158261

(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES CC(O)(CO)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O4/c1-20(28,12-25)11-24(15-4-2-3-5-15)18(26)10-22-19(27)17-9-13-8-14(21)6-7-16(13)23-17/h6-9,15,23,25,28H,2-5,10-12H2,1H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187275

((2R,5S)-1-(2-(1-(methoxymethyl)cyclopentylamino)ac...)Show SMILES COCC1(CCCC1)NCC(=O)N1[C@@H](CC[C@@H]1C#N)C#N Show InChI InChI=1S/C15H22N4O2/c1-21-11-15(6-2-3-7-15)18-10-14(20)19-12(8-16)4-5-13(19)9-17/h12-13,18H,2-7,10-11H2,1H3/t12-,13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187261

((2R,5S)-1-(2-(1-(butoxymethyl)cyclopentylamino)ace...)Show SMILES CCCCOCC1(CCCC1)NCC(=O)N1[C@@H](CC[C@@H]1C#N)C#N Show InChI InChI=1S/C18H28N4O2/c1-2-3-10-24-14-18(8-4-5-9-18)21-13-17(23)22-15(11-19)6-7-16(22)12-20/h15-16,21H,2-10,13-14H2,1H3/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187266

((2R,5S)-1-(2-(1-(hydroxymethyl)cyclopentylamino)ac...)Show SMILES OCC1(CCCC1)NCC(=O)N1[C@@H](CC[C@@H]1C#N)C#N Show InChI InChI=1S/C14H20N4O2/c15-7-11-3-4-12(8-16)18(11)13(20)9-17-14(10-19)5-1-2-6-14/h11-12,17,19H,1-6,9-10H2/t11-,12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in cynomolgus monkey plasma |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221098
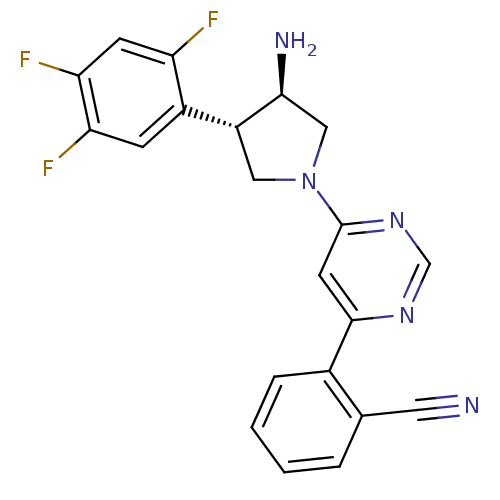
(2-(6-((3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)pyr...)Show SMILES N[C@H]1CN(C[C@@H]1c1cc(F)c(F)cc1F)c1cc(ncn1)-c1ccccc1C#N Show InChI InChI=1S/C21H16F3N5/c22-16-6-18(24)17(23)5-14(16)15-9-29(10-19(15)26)21-7-20(27-11-28-21)13-4-2-1-3-12(13)8-25/h1-7,11,15,19H,9-10,26H2/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
Bioorg Med Chem Lett 17: 5638-42 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.081
BindingDB Entry DOI: 10.7270/Q2319WRX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158264
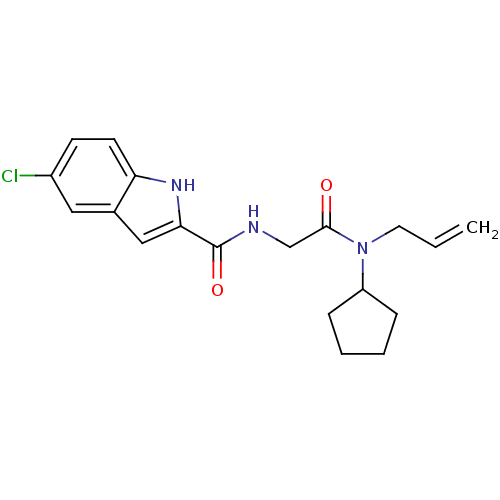
(5-Chloro-1H-indole-2-carboxylic acid [(allyl-cyclo...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)NCC(=O)N(CC=C)C1CCCC1 Show InChI InChI=1S/C19H22ClN3O2/c1-2-9-23(15-5-3-4-6-15)18(24)12-21-19(25)17-11-13-10-14(20)7-8-16(13)22-17/h2,7-8,10-11,15,22H,1,3-6,9,12H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158274

(5-Chloro-1H-indole-2-carboxylic acid {[cyclohexyl-...)Show SMILES OCCN(C1CCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H24ClN3O3/c20-14-6-7-16-13(10-14)11-17(22-16)19(26)21-12-18(25)23(8-9-24)15-4-2-1-3-5-15/h6-7,10-11,15,22,24H,1-5,8-9,12H2,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158276
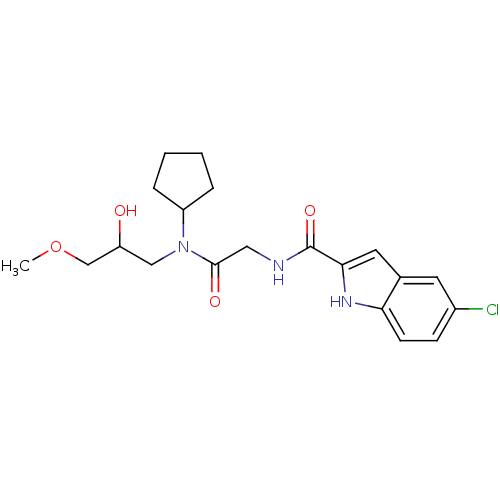
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES COCC(O)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O4/c1-28-12-16(25)11-24(15-4-2-3-5-15)19(26)10-22-20(27)18-9-13-8-14(21)6-7-17(13)23-18/h6-9,15-16,23,25H,2-5,10-12H2,1H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187266

((2R,5S)-1-(2-(1-(hydroxymethyl)cyclopentylamino)ac...)Show SMILES OCC1(CCCC1)NCC(=O)N1[C@@H](CC[C@@H]1C#N)C#N Show InChI InChI=1S/C14H20N4O2/c15-7-11-3-4-12(8-16)18(11)13(20)9-17-14(10-19)5-1-2-6-14/h11-12,17,19H,1-6,9-10H2/t11-,12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187268
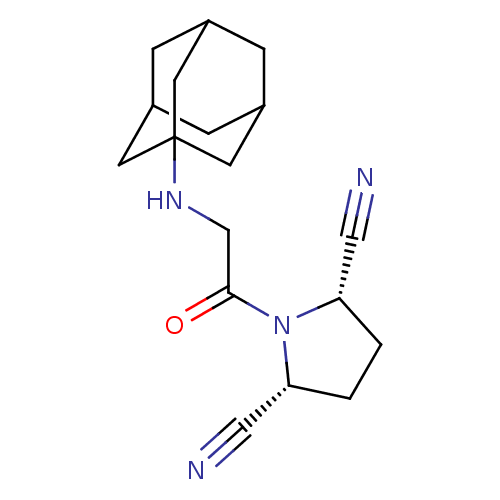
((2S,5R)-1-[2-(adamantan-1-ylamino)-acetyl]-pyrroli...)Show SMILES O=C(CNC12CC3CC(CC(C3)C1)C2)N1[C@@H](CC[C@@H]1C#N)C#N |TLB:3:4:11:7.8.9,13:4:11:7.8.9,THB:13:8:5.4.12:11,9:8:5:12.10.11,9:10:5:7.13.8| Show InChI InChI=1S/C18H24N4O/c19-9-15-1-2-16(10-20)22(15)17(23)11-21-18-6-12-3-13(7-18)5-14(4-12)8-18/h12-16,21H,1-8,11H2/t12?,13?,14?,15-,16+,18? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50187266

((2R,5S)-1-(2-(1-(hydroxymethyl)cyclopentylamino)ac...)Show SMILES OCC1(CCCC1)NCC(=O)N1[C@@H](CC[C@@H]1C#N)C#N Show InChI InChI=1S/C14H20N4O2/c15-7-11-3-4-12(8-16)18(11)13(20)9-17-14(10-19)5-1-2-6-14/h11-12,17,19H,1-6,9-10H2/t11-,12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in beagle dog plasma |
J Med Chem 49: 3068-76 (2006)
Article DOI: 10.1021/jm0600085
BindingDB Entry DOI: 10.7270/Q2BG2NM7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data