

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50001265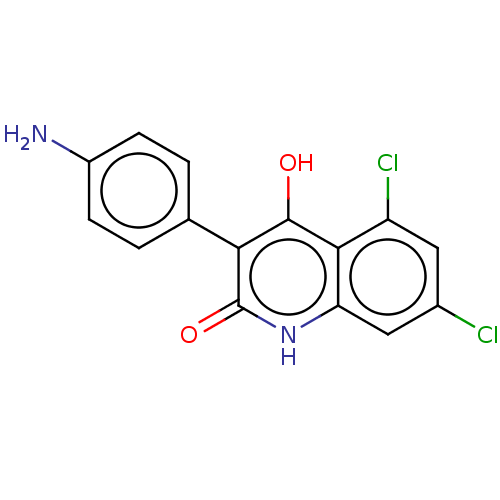 (3-(4-Amino-phenyl)-5,7-dichloro-4-hydroxy-1H-quino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace strychnine-insensitive [3H]-glycine binding to rat cortical membranes. | J Med Chem 35: 3423-5 (1992) BindingDB Entry DOI: 10.7270/Q2RR1X68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50001266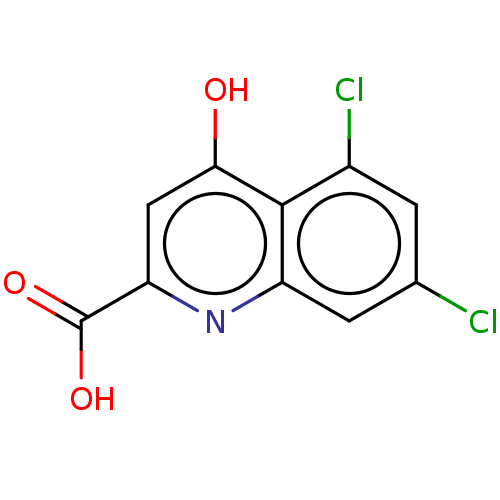 (5,7-DICHLORO-4-HYDROXYQUINOLINE-2-CARBOXYLIC ACID ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace strychnine-insensitive [3H]-glycine binding to rat cortical membranes. | J Med Chem 35: 3423-5 (1992) BindingDB Entry DOI: 10.7270/Q2RR1X68 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50001266 (5,7-DICHLORO-4-HYDROXYQUINOLINE-2-CARBOXYLIC ACID ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50001266 (5,7-DICHLORO-4-HYDROXYQUINOLINE-2-CARBOXYLIC ACID ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50004952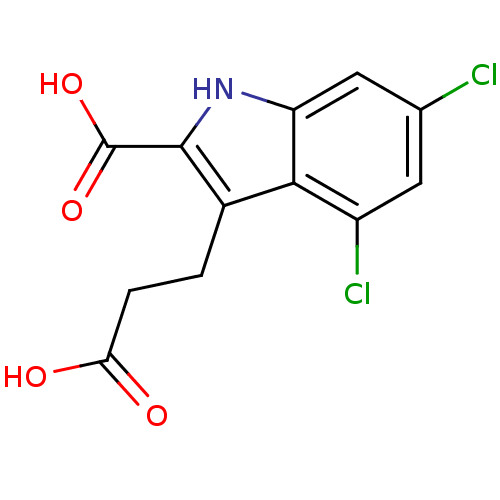 (3-(2-Carboxy-ethyl)-4,6-dichloro-1H-indole-2-carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM22778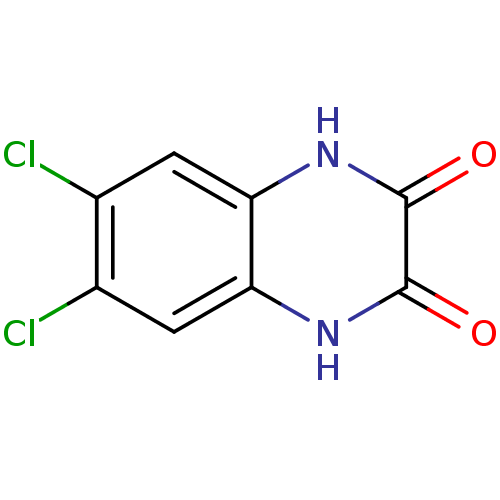 (6,7-Dichloro-1,4-dihydro-quinoxaline-2,3-dione | 6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50001264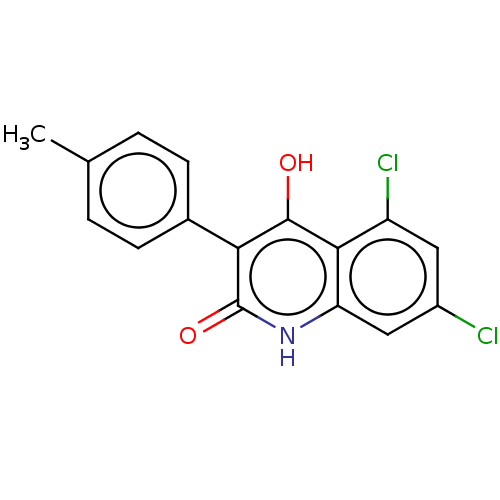 (5,7-Dichloro-4-hydroxy-3-p-tolyl-1H-quinolin-2-one...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace strychnine-insensitive [3H]-glycine binding to rat cortical membranes. | J Med Chem 35: 3423-5 (1992) BindingDB Entry DOI: 10.7270/Q2RR1X68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50129080 (3-Carboxymethyl-4,6-dichloro-1H-indole-2-carboxyli...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002707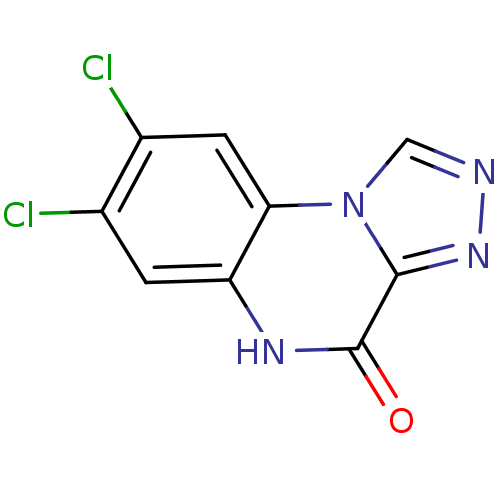 (7,8-Dichloro-5H-[1,2,4]triazolo[4,3-a]quinoxalin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50280807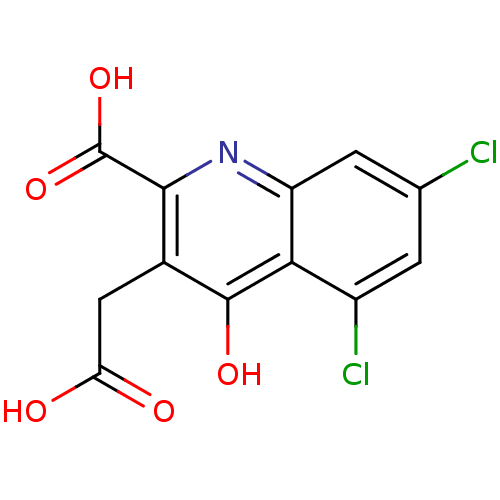 (3-Carboxymethyl-5,7-dichloro-4-oxo-1,4-dihydro-qui...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50001268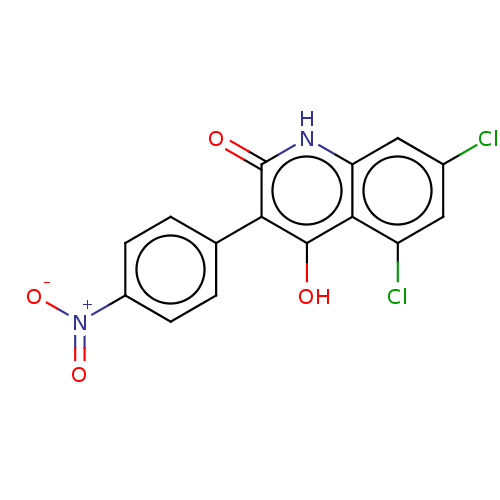 (5,7-Dichloro-4-hydroxy-3-(4-nitro-phenyl)-1H-quino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace strychnine-insensitive [3H]-glycine binding to rat cortical membranes. | J Med Chem 35: 3423-5 (1992) BindingDB Entry DOI: 10.7270/Q2RR1X68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50004960 (4,6-Dichloro-1H-indole-2-carboxylic acid | CHEMBL3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002696 (7,8-Dichloro-5H-imidazo[1,2-a]quinoxalin-4-one | C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002700 (1-Butyl-7,8-dichloro-5H-[1,2,4]triazolo[4,3-a]quin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002710 (7,9-Dichloro-5H-[1,2,4]triazolo[4,3-a]quinoxalin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002701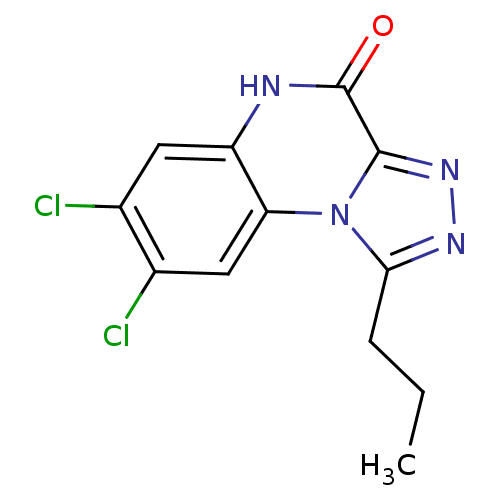 (7,8-Dichloro-1-propyl-5H-[1,2,4]triazolo[4,3-a]qui...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50001260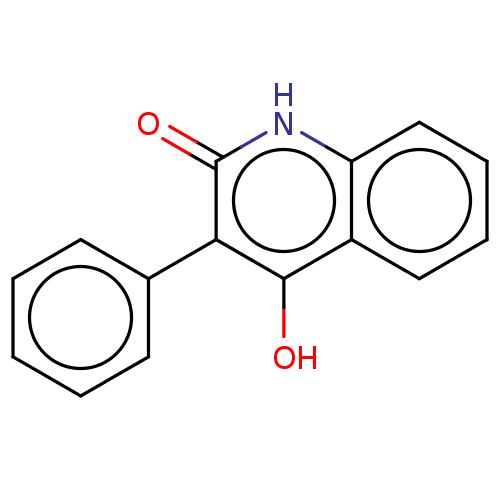 (3-phenyl-4-hydroxyquinolin-2(1H)-one | 4-Hydroxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace strychnine-insensitive [3H]-glycine binding to rat cortical membranes. | J Med Chem 35: 3423-5 (1992) BindingDB Entry DOI: 10.7270/Q2RR1X68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002711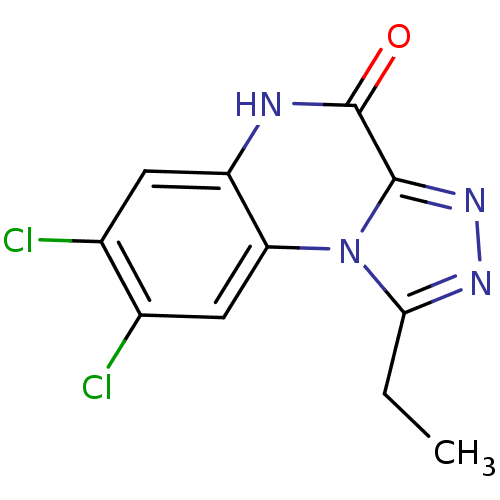 (7,8-Dichloro-1-ethyl-5H-[1,2,4]triazolo[4,3-a]quin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50009092 (3-Carboxymethyl-1H-indole-2-carboxylic acid | CHEM...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 5.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM81975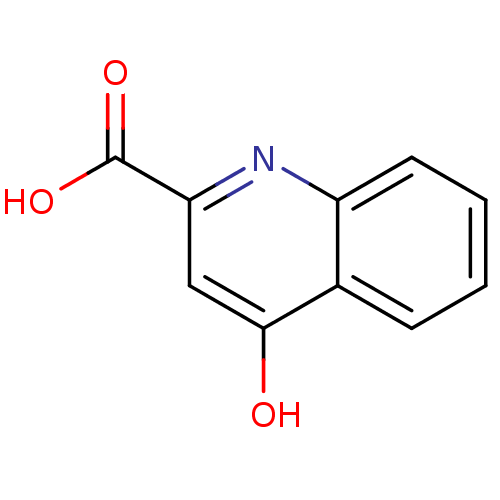 (4-hydroxyquinoline-2-carboxylic acid | CAS_492-27-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM81975 (4-hydroxyquinoline-2-carboxylic acid | CAS_492-27-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace strychnine-insensitive [3H]-glycine binding to rat cortical membranes. | J Med Chem 35: 3423-5 (1992) BindingDB Entry DOI: 10.7270/Q2RR1X68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50280805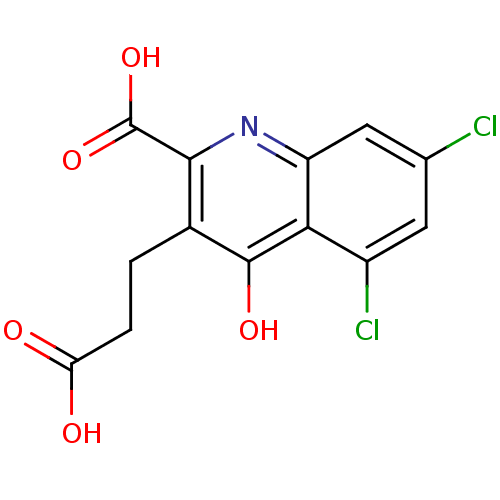 (3-(2-Carboxy-ethyl)-5,7-dichloro-4-oxo-1,4-dihydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 7.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002704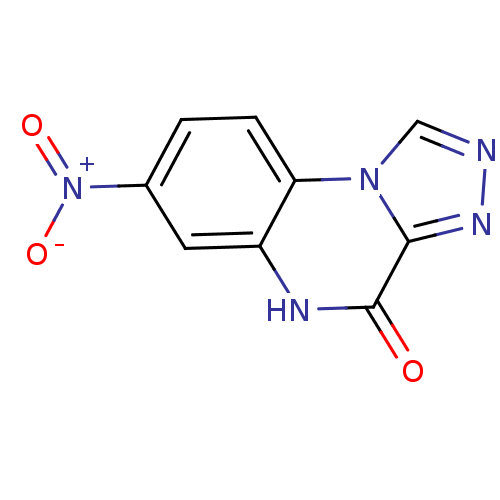 (7-Nitro-5H-[1,2,4]triazolo[4,3-a]quinoxalin-4-one ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002705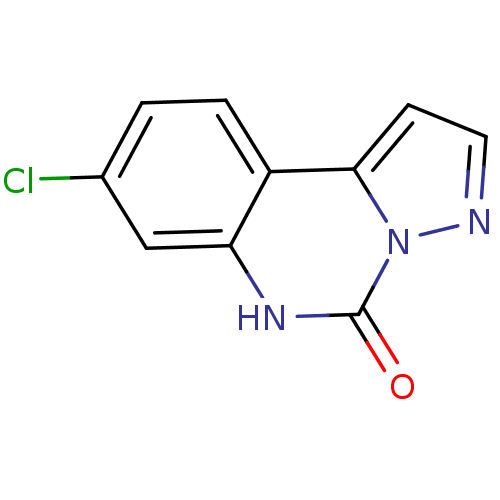 (8-Chloro-6H-pyrazolo[1,5-c]quinazolin-5-one | CHEM...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002706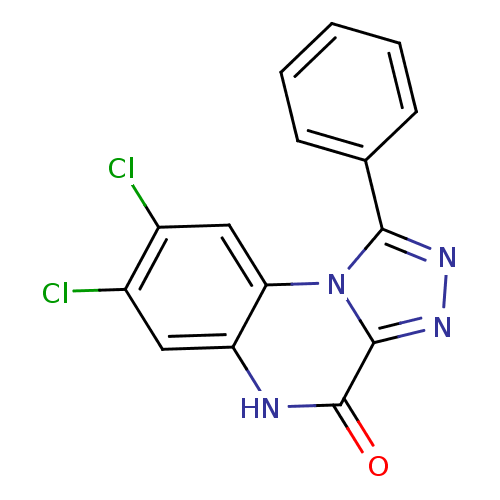 (7,8-Dichloro-1-phenyl-5H-[1,2,4]triazolo[4,3-a]qui...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002703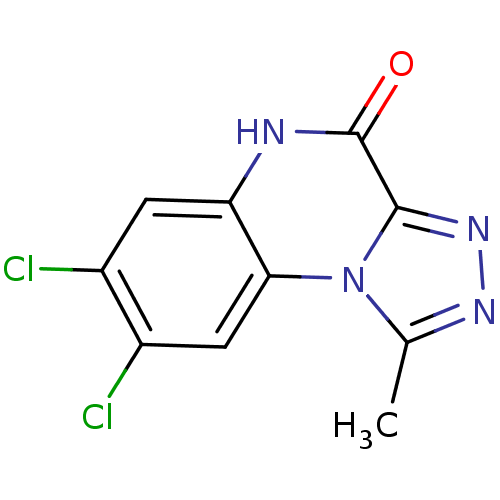 (7,8-Dichloro-1-methyl-5H-[1,2,4]triazolo[4,3-a]qui...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002699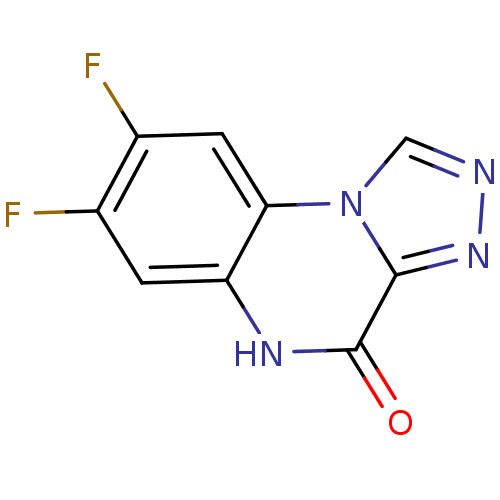 (7,8-Difluoro-5H-[1,2,4]triazolo[4,3-a]quinoxalin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002702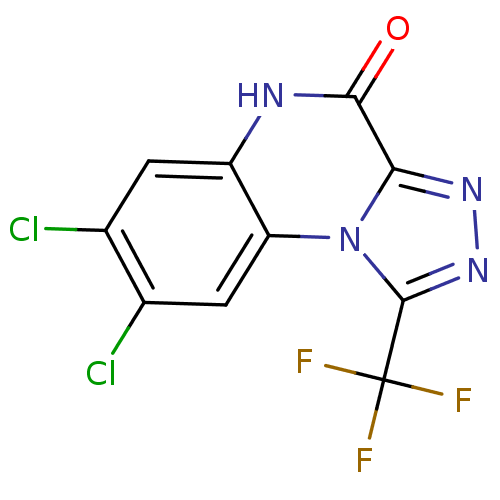 (7,8-Dichloro-1-trifluoromethyl-5H-[1,2,4]triazolo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002712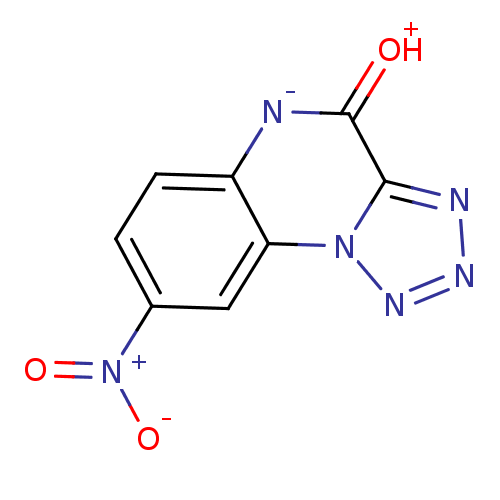 (8-Nitro-5H-1,2,3,5,9b-pentaaza-cyclopenta[a]naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002695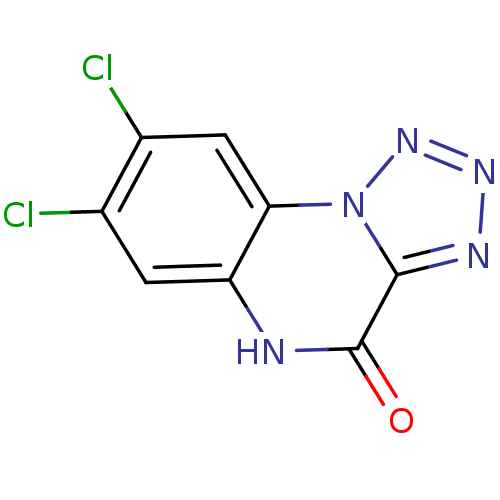 (7,8-Dichloro-5H-1,2,3,5,9b-pentaaza-cyclopenta[a]n...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002708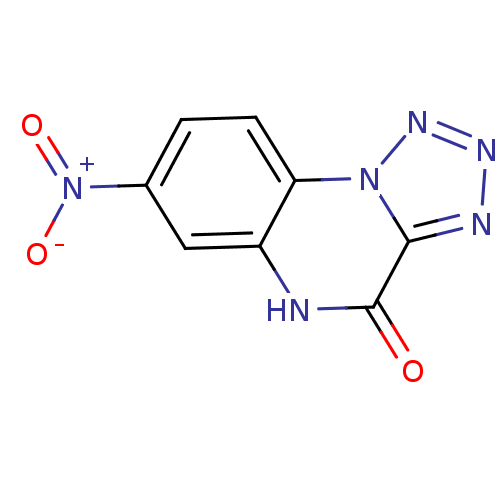 (7-Nitro-5H-1,2,3,5,9b-pentaaza-cyclopenta[a]naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50002709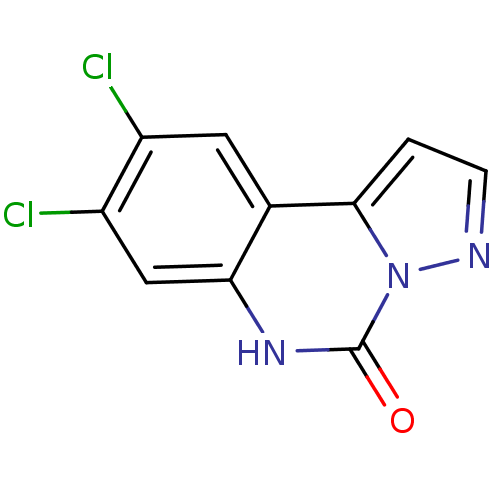 (8,9-Dichloro-6H-pyrazolo[1,5-c]quinazolin-5-one | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]glycine from glycine site on the NMDA receptor. | J Med Chem 35: 3319-24 (1992) BindingDB Entry DOI: 10.7270/Q2WM1F13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50280806 (3-Carboxymethyl-4-oxo-1,4-dihydro-quinoline-2-carb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50009085 (3-Carboxymethyl-quinoline-2-carboxylic acid | CHEM...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]-glycine, by greater than 50%, from NMDA receptor of rat cortical membranes at a dose of 10 microM | Bioorg Med Chem Lett 3: 81-84 (1993) Article DOI: 10.1016/S0960-894X(00)80096-7 BindingDB Entry DOI: 10.7270/Q2MK6CTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017543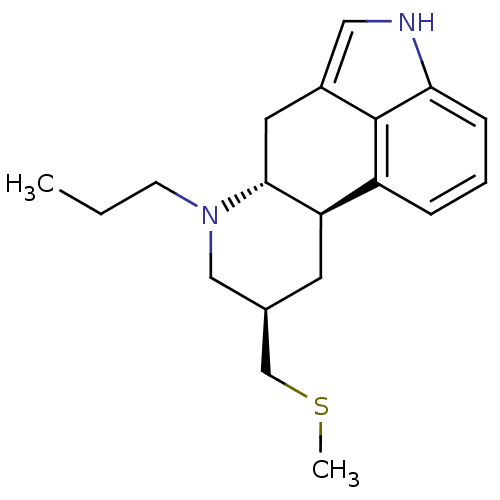 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070046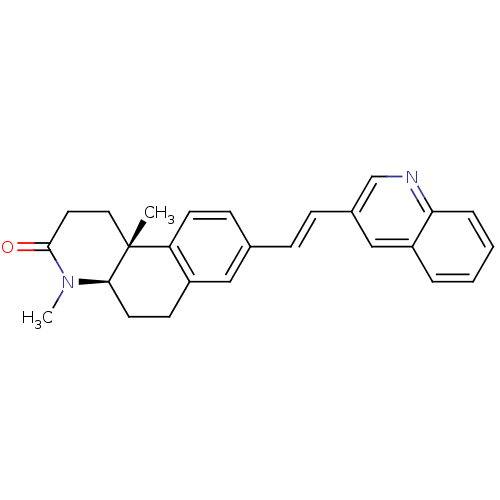 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-2-quinolin-3-yl-v...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type 1 enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070049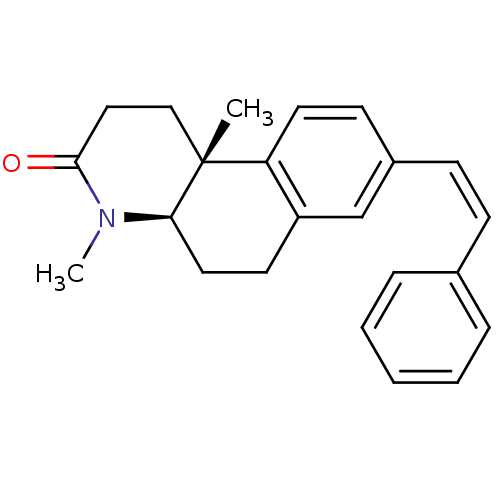 ((4aR,10bR)-4,10b-Dimethyl-8-((Z)-styryl)-1,4,4a,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type I enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50032762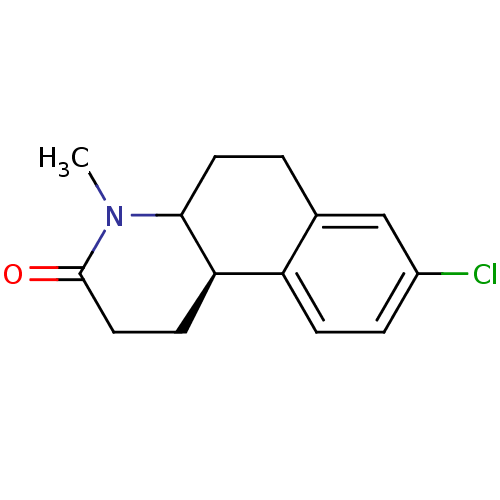 ((4aR,10bR)-8-Chloro-4-methyl-1,4,4a,5,6,10b-hexahy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration required to inhibit Steroid 5-alpha-reductase type 1 in cultured Hs 68 human foreskin fibroblast cells | Bioorg Med Chem Lett 3: 1157-1162 (1993) Article DOI: 10.1016/S0960-894X(00)80306-6 BindingDB Entry DOI: 10.7270/Q2VD70NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50368782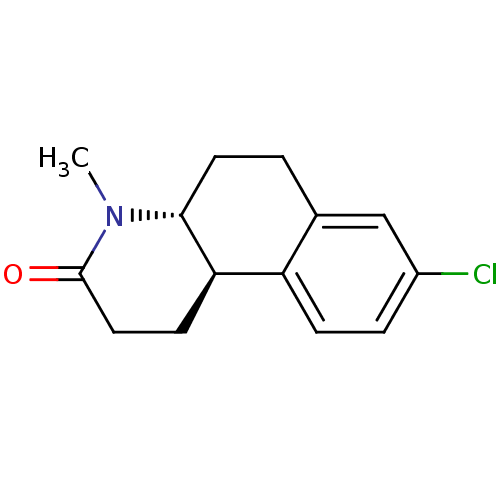 (Bexlosteride | CHEMBL24955 | LY-191704) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50031890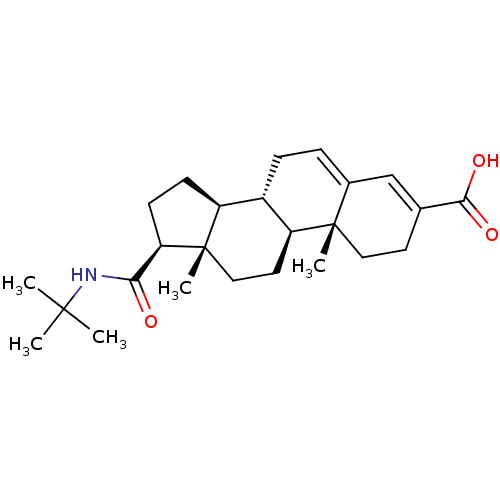 ((10R,13S)-17-tert-Butylcarbamoyl-10,13-dimethyl-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of Type II 5-alpha-reductase in Human Prostate Homogenates (HPH) | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50334788 ((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibition of Type II 5-alpha-reductase in Human Prostate Homogenates (HPH). | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044878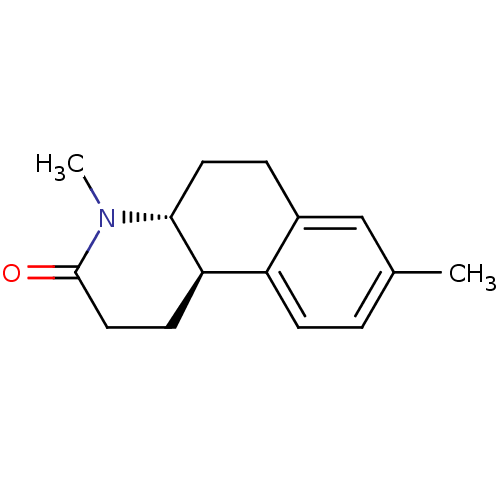 (4,8-Dimethyl-1,4,4a,5,6,10b-hexahydro-2H-benzo[f]q...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044879 ((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070054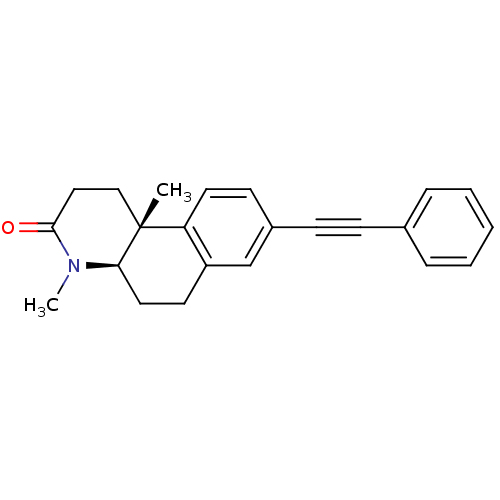 ((4aR,10bR)-4,10b-Dimethyl-8-phenylethynyl-1,4,4a,5...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type I enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070051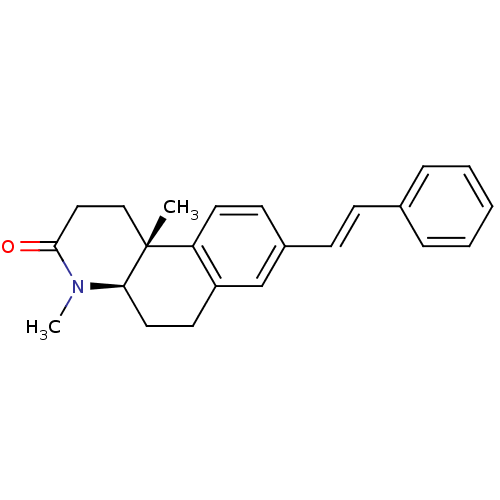 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-styryl)-1,4,4a,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type I enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044881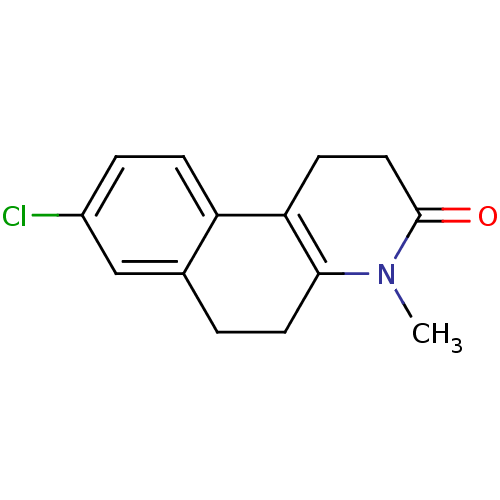 (8-Chloro-4-methyl-1,4,5,6-tetrahydro-2H-benzo[f]qu...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of DHT production in Hs 68 (human genital fibroblast) cells. | J Med Chem 36: 421-3 (1993) BindingDB Entry DOI: 10.7270/Q2T43TQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50070044 ((4aR,10bR)-4,10b-Dimethyl-8-((E)-2-quinolin-2-yl-v...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Steroid 5-alpha-reductase type 1 enzyme based on the conversion of [3H]-T to [3H]-DHT in nuclear membrane preparations fr... | Bioorg Med Chem Lett 8: 395-8 (1999) BindingDB Entry DOI: 10.7270/Q2CN732C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044879 ((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration required to inhibit Steroid 5-alpha-reductase type 1 in cultured Hs 68 human foreskin fibroblast cells | Bioorg Med Chem Lett 3: 1157-1162 (1993) Article DOI: 10.1016/S0960-894X(00)80306-6 BindingDB Entry DOI: 10.7270/Q2VD70NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50403176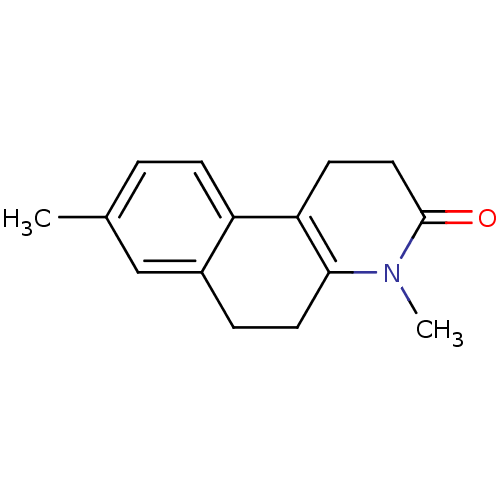 (CHEMBL279420) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration required to inhibit Steroid 5-alpha-reductase type 1 in cultured Hs 68 human foreskin fibroblast cells | Bioorg Med Chem Lett 3: 1157-1162 (1993) Article DOI: 10.1016/S0960-894X(00)80306-6 BindingDB Entry DOI: 10.7270/Q2VD70NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50044878 (4,8-Dimethyl-1,4,4a,5,6,10b-hexahydro-2H-benzo[f]q...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration required to inhibit Steroid 5-alpha-reductase type 1 in cultured Hs 68 human foreskin fibroblast cells | Bioorg Med Chem Lett 3: 1157-1162 (1993) Article DOI: 10.1016/S0960-894X(00)80306-6 BindingDB Entry DOI: 10.7270/Q2VD70NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 130 total ) | Next | Last >> |