 Found 291 hits with Last Name = 'morris' and Initial = 'sw'
Found 291 hits with Last Name = 'morris' and Initial = 'sw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181669
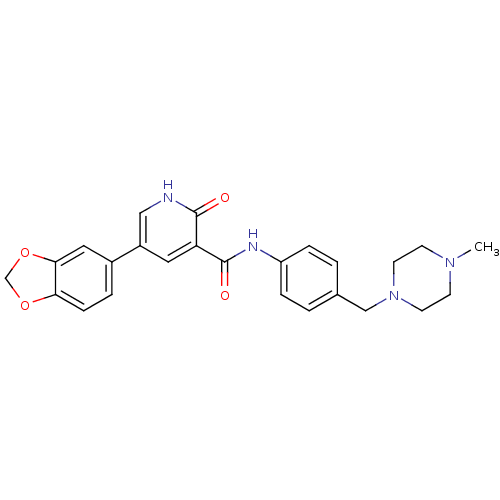
(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C25H26N4O4/c1-28-8-10-29(11-9-28)15-17-2-5-20(6-3-17)27-25(31)21-12-19(14-26-24(21)30)18-4-7-22-23(13-18)33-16-32-22/h2-7,12-14H,8-11,15-16H2,1H3,(H,26,30)(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181675

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-11-13-31(14-12-30)10-2-3-19-4-7-22(8-5-19)29-27(33)23-15-21(17-28-26(23)32)20-6-9-24-25(16-20)35-18-34-24/h4-9,15-17H,2-3,10-14,18H2,1H3,(H,28,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181669

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C25H26N4O4/c1-28-8-10-29(11-9-28)15-17-2-5-20(6-3-17)27-25(31)21-12-19(14-26-24(21)30)18-4-7-22-23(13-18)33-16-32-22/h2-7,12-14H,8-11,15-16H2,1H3,(H,26,30)(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181670

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2cc(c[nH]c2=O)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C24H24N4O4/c1-27-8-10-28(11-9-27)19-5-3-18(4-6-19)26-24(30)20-12-17(14-25-23(20)29)16-2-7-21-22(13-16)32-15-31-21/h2-7,12-14H,8-11,15H2,1H3,(H,25,29)(H,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181675

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-11-13-31(14-12-30)10-2-3-19-4-7-22(8-5-19)29-27(33)23-15-21(17-28-26(23)32)20-6-9-24-25(16-20)35-18-34-24/h4-9,15-17H,2-3,10-14,18H2,1H3,(H,28,32)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET using biotinylated-poly(GT) peptide as substrate after 60 mins |
Bioorg Med Chem Lett 21: 4592-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.103
BindingDB Entry DOI: 10.7270/Q2N87B4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296352
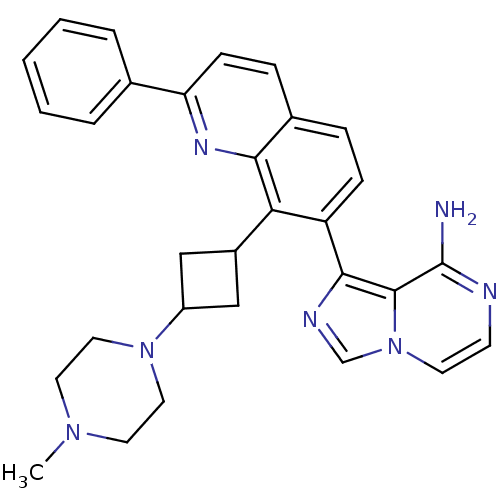
(1-(8-(3-(4-methylpiperazin-1-yl)cyclobutyl)-2-phen...)Show SMILES CN1CCN(CC1)C1CC(C1)c1c(ccc2ccc(nc12)-c1ccccc1)-c1ncn2ccnc(N)c12 |(21.77,-8.21,;20.98,-6.88,;19.44,-6.89,;18.66,-5.58,;19.42,-4.23,;20.96,-4.21,;21.74,-5.54,;18.63,-2.91,;17.14,-2.53,;17.52,-1.04,;19.01,-1.42,;16.74,.28,;15.2,.27,;14.4,1.57,;15.14,2.92,;16.69,2.95,;17.44,4.3,;18.98,4.33,;19.78,2.99,;19.03,1.64,;17.48,1.62,;21.32,3.02,;22.11,1.69,;23.65,1.72,;24.4,3.06,;23.6,4.39,;22.06,4.36,;14.45,-1.08,;15.1,-2.47,;13.98,-3.52,;12.64,-2.77,;11.19,-3.27,;10.03,-2.27,;10.32,-.76,;11.78,-.25,;12.07,1.26,;12.93,-1.27,)| Show InChI InChI=1S/C30H31N7/c1-35-13-15-36(16-14-35)23-17-22(18-23)26-24(28-29-30(31)32-11-12-37(29)19-33-28)9-7-21-8-10-25(34-27(21)26)20-5-3-2-4-6-20/h2-12,19,22-23H,13-18H2,1H3,(H2,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R by cell based assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296353
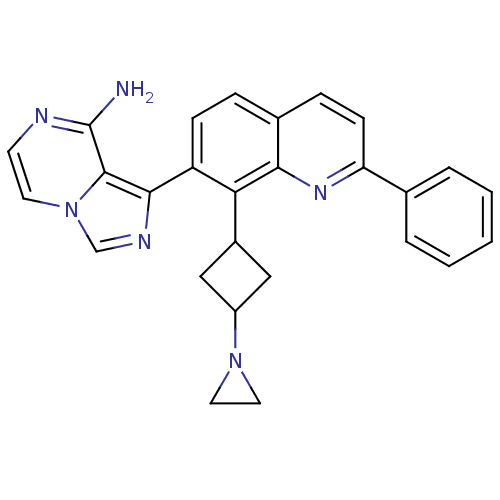
(1-(8-(3-(aziridin-1-yl)cyclobutyl)-2-phenylquinoli...)Show SMILES Nc1nccn2cnc(-c3ccc4ccc(nc4c3C3CC(C3)N3CC3)-c3ccccc3)c12 |(-8.5,-15.87,;-8.8,-17.38,;-10.25,-17.89,;-10.54,-19.41,;-9.38,-20.41,;-7.93,-19.91,;-6.59,-20.65,;-5.47,-19.61,;-6.12,-18.22,;-5.37,-16.87,;-6.17,-15.56,;-5.43,-14.22,;-3.88,-14.18,;-3.13,-12.84,;-1.59,-12.81,;-.79,-14.14,;-1.55,-15.49,;-3.09,-15.51,;-3.84,-16.85,;-3.05,-18.18,;-3.43,-19.67,;-1.94,-20.05,;-1.56,-18.56,;-1.15,-21.37,;-1.13,-22.9,;.19,-22.11,;.75,-14.12,;1.54,-15.44,;3.07,-15.42,;3.82,-14.07,;3.02,-12.74,;1.49,-12.78,;-7.64,-18.4,)| Show InChI InChI=1S/C27H24N6/c28-27-26-25(30-16-33(26)11-10-29-27)21-8-6-18-7-9-22(17-4-2-1-3-5-17)31-24(18)23(21)19-14-20(15-19)32-12-13-32/h1-11,16,19-20H,12-15H2,(H2,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296355
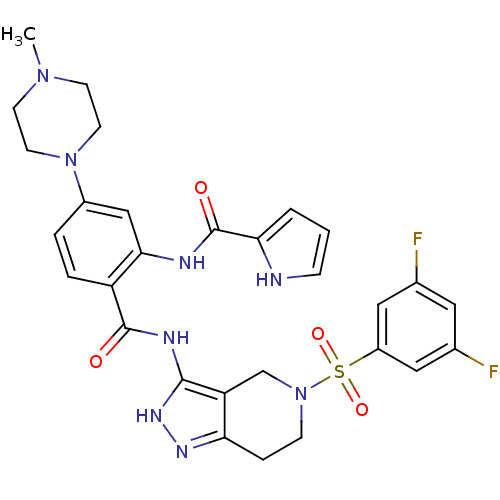
(CHEMBL553443 | N-(2-(5-(3,5-difluorophenylsulfonyl...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2[nH]nc3CCN(Cc23)S(=O)(=O)c2cc(F)cc(F)c2)c(NC(=O)c2ccc[nH]2)c1 Show InChI InChI=1S/C29H30F2N8O4S/c1-37-9-11-38(12-10-37)20-4-5-22(26(16-20)33-29(41)25-3-2-7-32-25)28(40)34-27-23-17-39(8-6-24(23)35-36-27)44(42,43)21-14-18(30)13-19(31)15-21/h2-5,7,13-16,32H,6,8-12,17H2,1H3,(H,33,41)(H2,34,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged IGF1R by trans-phosphorylation assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50296350
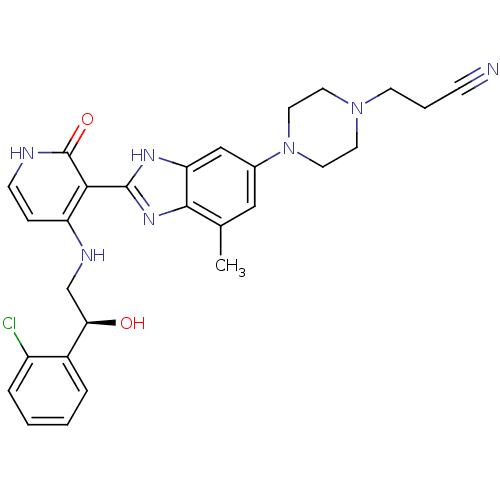
((S)-3-(4-(2-(4-(2-(2-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2ccccc2Cl)cc[nH]c1=O)N1CCN(CCC#N)CC1 |r| Show InChI InChI=1S/C28H30ClN7O2/c1-18-15-19(36-13-11-35(12-14-36)10-4-8-30)16-23-26(18)34-27(33-23)25-22(7-9-31-28(25)38)32-17-24(37)20-5-2-3-6-21(20)29/h2-3,5-7,9,15-16,24,37H,4,10-14,17H2,1H3,(H,33,34)(H2,31,32,38)/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM4293
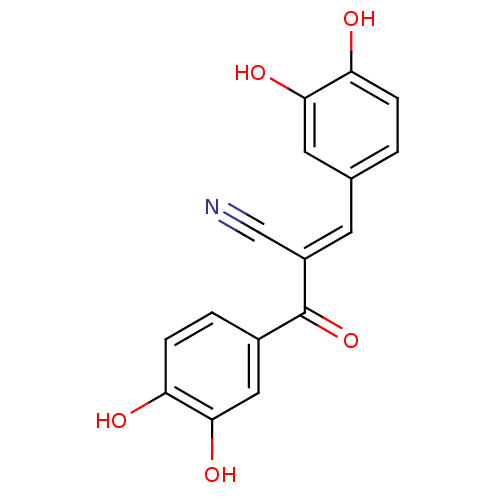
((2E)-3-(3,4-dihydroxyphenyl)-2-[(3,4-dihydroxyphen...)Show InChI InChI=1S/C16H11NO5/c17-8-11(5-9-1-3-12(18)14(20)6-9)16(22)10-2-4-13(19)15(21)7-10/h1-7,18-21H/b11-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296350

((S)-3-(4-(2-(4-(2-(2-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2ccccc2Cl)cc[nH]c1=O)N1CCN(CCC#N)CC1 |r| Show InChI InChI=1S/C28H30ClN7O2/c1-18-15-19(36-13-11-35(12-14-36)10-4-8-30)16-23-26(18)34-27(33-23)25-22(7-9-31-28(25)38)32-17-24(37)20-5-2-3-6-21(20)29/h2-3,5-7,9,15-16,24,37H,4,10-14,17H2,1H3,(H,33,34)(H2,31,32,38)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM27879
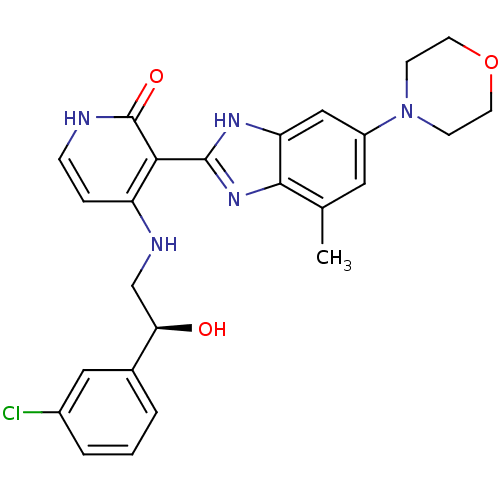
(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296355

(CHEMBL553443 | N-(2-(5-(3,5-difluorophenylsulfonyl...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2[nH]nc3CCN(Cc23)S(=O)(=O)c2cc(F)cc(F)c2)c(NC(=O)c2ccc[nH]2)c1 Show InChI InChI=1S/C29H30F2N8O4S/c1-37-9-11-38(12-10-37)20-4-5-22(26(16-20)33-29(41)25-3-2-7-32-25)28(40)34-27-23-17-39(8-6-24(23)35-36-27)44(42,43)21-14-18(30)13-19(31)15-21/h2-5,7,13-16,32H,6,8-12,17H2,1H3,(H,33,41)(H2,34,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R in human MCF7 cells by western blot analysis |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296348
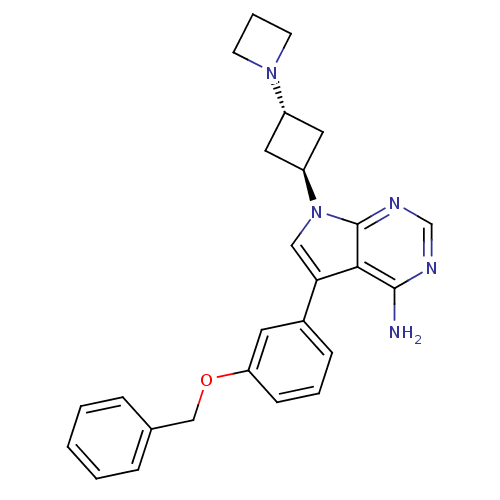
(7-(3-(azetidin-1-yl)cyclobutyl)-5-(3-(benzyloxy)ph...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCC1 |r,wU:26.32,wD:24.27,(12.58,1.25,;12.59,-.29,;11.26,-1.07,;11.26,-2.61,;12.59,-3.38,;13.92,-2.61,;15.4,-3.09,;16.31,-1.83,;15.4,-.58,;15.87,.89,;14.85,2.02,;15.32,3.49,;16.83,3.81,;17.87,2.67,;19.37,2.99,;20.4,1.84,;21.91,2.16,;22.94,1.01,;24.44,1.33,;24.92,2.8,;23.88,3.95,;22.38,3.62,;17.39,1.2,;13.92,-1.06,;15.87,-4.56,;15.17,-5.92,;16.54,-6.63,;17.25,-5.26,;17.01,-8.1,;16.3,-9.45,;17.67,-10.16,;18.37,-8.79,)| Show InChI InChI=1S/C26H27N5O/c27-25-24-23(19-8-4-9-22(12-19)32-16-18-6-2-1-3-7-18)15-31(26(24)29-17-28-25)21-13-20(14-21)30-10-5-11-30/h1-4,6-9,12,15,17,20-21H,5,10-11,13-14,16H2,(H2,27,28,29)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R by cell based assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296356

(CHEMBL541762 | N-(5-(5-(3,5-difluorophenylsulfonyl...)Show SMILES CN1CCN(CC1)c1ccc(cc1NC(=O)c1cc[nH]c1)C(=O)Nc1[nH]nc2c1CN(C2(C)C)S(=O)(=O)c1cc(F)cc(F)c1 Show InChI InChI=1S/C30H32F2N8O4S/c1-30(2)26-23(17-40(30)45(43,44)22-14-20(31)13-21(32)15-22)27(37-36-26)35-28(41)18-4-5-25(39-10-8-38(3)9-11-39)24(12-18)34-29(42)19-6-7-33-16-19/h4-7,12-16,33H,8-11,17H2,1-3H3,(H,34,42)(H2,35,36,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human IGF1R expressed in baculovirus-infected Sf21 cells by scintillation proximity assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50296350

((S)-3-(4-(2-(4-(2-(2-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2ccccc2Cl)cc[nH]c1=O)N1CCN(CCC#N)CC1 |r| Show InChI InChI=1S/C28H30ClN7O2/c1-18-15-19(36-13-11-35(12-14-36)10-4-8-30)16-23-26(18)34-27(33-23)25-22(7-9-31-28(25)38)32-17-24(37)20-5-2-3-6-21(20)29/h2-3,5-7,9,15-16,24,37H,4,10-14,17H2,1H3,(H,33,34)(H2,31,32,38)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Mus musculus) | BDBM50296348

(7-(3-(azetidin-1-yl)cyclobutyl)-5-(3-(benzyloxy)ph...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCC1 |r,wU:26.32,wD:24.27,(12.58,1.25,;12.59,-.29,;11.26,-1.07,;11.26,-2.61,;12.59,-3.38,;13.92,-2.61,;15.4,-3.09,;16.31,-1.83,;15.4,-.58,;15.87,.89,;14.85,2.02,;15.32,3.49,;16.83,3.81,;17.87,2.67,;19.37,2.99,;20.4,1.84,;21.91,2.16,;22.94,1.01,;24.44,1.33,;24.92,2.8,;23.88,3.95,;22.38,3.62,;17.39,1.2,;13.92,-1.06,;15.87,-4.56,;15.17,-5.92,;16.54,-6.63,;17.25,-5.26,;17.01,-8.1,;16.3,-9.45,;17.67,-10.16,;18.37,-8.79,)| Show InChI InChI=1S/C26H27N5O/c27-25-24-23(19-8-4-9-22(12-19)32-16-18-6-2-1-3-7-18)15-31(26(24)29-17-28-25)21-13-20(14-21)30-10-5-11-30/h1-4,6-9,12,15,17,20-21H,5,10-11,13-14,16H2,(H2,27,28,29)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor-mediated proliferation of mouse NIH/3T3 cells after 48 hrs by MTT assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Mus musculus) | BDBM50296348

(7-(3-(azetidin-1-yl)cyclobutyl)-5-(3-(benzyloxy)ph...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCC1 |r,wU:26.32,wD:24.27,(12.58,1.25,;12.59,-.29,;11.26,-1.07,;11.26,-2.61,;12.59,-3.38,;13.92,-2.61,;15.4,-3.09,;16.31,-1.83,;15.4,-.58,;15.87,.89,;14.85,2.02,;15.32,3.49,;16.83,3.81,;17.87,2.67,;19.37,2.99,;20.4,1.84,;21.91,2.16,;22.94,1.01,;24.44,1.33,;24.92,2.8,;23.88,3.95,;22.38,3.62,;17.39,1.2,;13.92,-1.06,;15.87,-4.56,;15.17,-5.92,;16.54,-6.63,;17.25,-5.26,;17.01,-8.1,;16.3,-9.45,;17.67,-10.16,;18.37,-8.79,)| Show InChI InChI=1S/C26H27N5O/c27-25-24-23(19-8-4-9-22(12-19)32-16-18-6-2-1-3-7-18)15-31(26(24)29-17-28-25)21-13-20(14-21)30-10-5-11-30/h1-4,6-9,12,15,17,20-21H,5,10-11,13-14,16H2,(H2,27,28,29)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R-mediated proliferation of mouse NIH/3T3 cells after 48 hrs by MTT assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296347
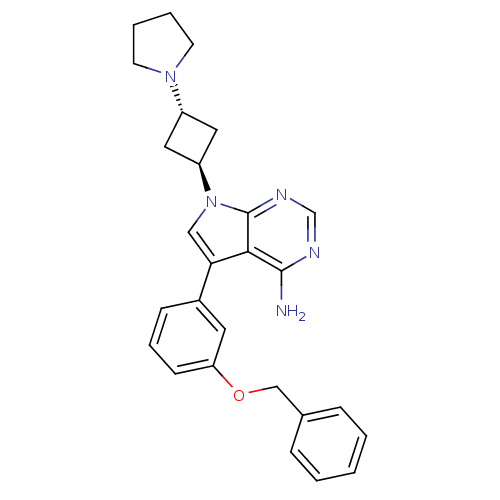
(5-(3-(benzyloxy)phenyl)-7-(3-(pyrrolidin-1-yl)cycl...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCCC1 |r,wU:26.32,wD:24.27,(-6.06,2.61,;-6.05,1.07,;-7.38,.3,;-7.39,-1.25,;-6.05,-2.02,;-4.72,-1.25,;-3.24,-1.73,;-2.33,-.47,;-3.24,.78,;-2.77,2.25,;-3.79,3.38,;-3.32,4.85,;-1.81,5.17,;-.78,4.03,;.73,4.35,;1.76,3.2,;3.27,3.52,;4.29,2.37,;5.8,2.69,;6.28,4.16,;5.24,5.31,;3.74,4.98,;-1.25,2.56,;-4.72,.3,;-2.78,-3.19,;-3.47,-4.56,;-2.1,-5.27,;-1.4,-3.9,;-1.64,-6.73,;-2.54,-7.98,;-1.64,-9.23,;-.18,-8.76,;-.17,-7.22,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(20-9-6-10-23(13-20)33-17-19-7-2-1-3-8-19)16-32(27(25)30-18-29-26)22-14-21(15-22)31-11-4-5-12-31/h1-3,6-10,13,16,18,21-22H,4-5,11-12,14-15,17H2,(H2,28,29,30)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R by cell based assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296357
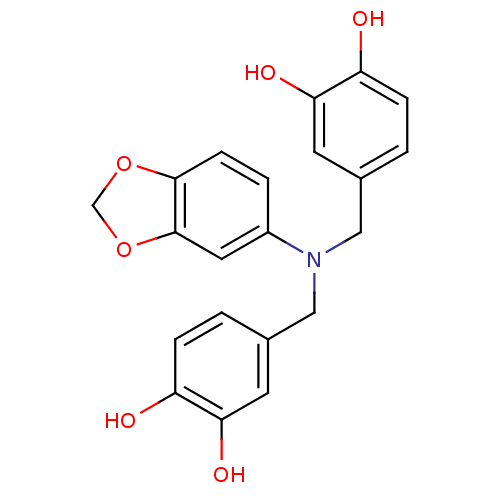
(4,4'-(benzo[d][1,3]dioxol-5-ylazanediyl)bis(methyl...)Show SMILES Oc1ccc(CN(Cc2ccc(O)c(O)c2)c2ccc3OCOc3c2)cc1O Show InChI InChI=1S/C21H19NO6/c23-16-4-1-13(7-18(16)25)10-22(11-14-2-5-17(24)19(26)8-14)15-3-6-20-21(9-15)28-12-27-20/h1-9,23-26H,10-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296356

(CHEMBL541762 | N-(5-(5-(3,5-difluorophenylsulfonyl...)Show SMILES CN1CCN(CC1)c1ccc(cc1NC(=O)c1cc[nH]c1)C(=O)Nc1[nH]nc2c1CN(C2(C)C)S(=O)(=O)c1cc(F)cc(F)c1 Show InChI InChI=1S/C30H32F2N8O4S/c1-30(2)26-23(17-40(30)45(43,44)22-14-20(31)13-21(32)15-22)27(37-36-26)35-28(41)18-4-5-25(39-10-8-38(3)9-11-39)24(12-18)34-29(42)19-6-7-33-16-19/h4-7,12-16,33H,8-11,17H2,1-3H3,(H,34,42)(H2,35,36,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R in IGF1-stimulated human MCF7 cells by western blot analysis |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296354
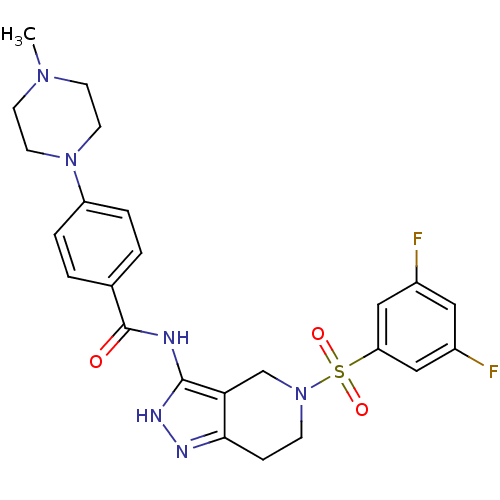
(CHEMBL549369 | N-(5-(3,5-difluorophenylsulfonyl)-4...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CCN(Cc12)S(=O)(=O)c1cc(F)cc(F)c1 Show InChI InChI=1S/C24H26F2N6O3S/c1-30-8-10-31(11-9-30)19-4-2-16(3-5-19)24(33)27-23-21-15-32(7-6-22(21)28-29-23)36(34,35)20-13-17(25)12-18(26)14-20/h2-5,12-14H,6-11,15H2,1H3,(H2,27,28,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R in growth factor-stimulated NHDF |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 341 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181672
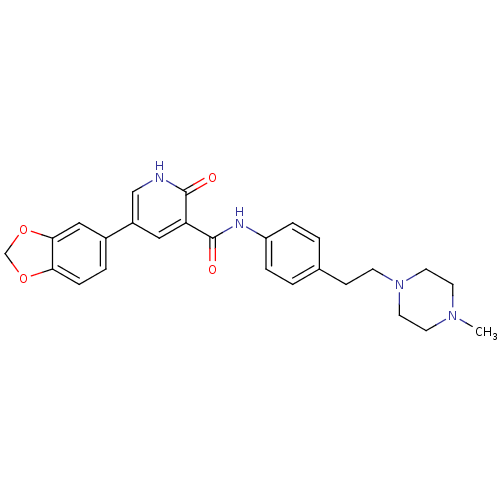
(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C26H28N4O4/c1-29-10-12-30(13-11-29)9-8-18-2-5-21(6-3-18)28-26(32)22-14-20(16-27-25(22)31)19-4-7-23-24(15-19)34-17-33-23/h2-7,14-16H,8-13,17H2,1H3,(H,27,31)(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of 6xHis-tagged NPM-ALK using biotinylated-poly(GT) peptide as substrate after 60 mins |
Bioorg Med Chem Lett 21: 4592-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.103
BindingDB Entry DOI: 10.7270/Q2N87B4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IRK using biotinylated-poly(GT) peptide as substrate after 60 mins |
Bioorg Med Chem Lett 21: 4592-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.103
BindingDB Entry DOI: 10.7270/Q2N87B4V |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181670

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2cc(c[nH]c2=O)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C24H24N4O4/c1-27-8-10-28(11-9-27)19-5-3-18(4-6-19)26-24(30)20-12-17(14-25-23(20)29)16-2-7-21-22(13-16)32-15-31-21/h2-7,12-14H,8-11,15H2,1H3,(H,25,29)(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
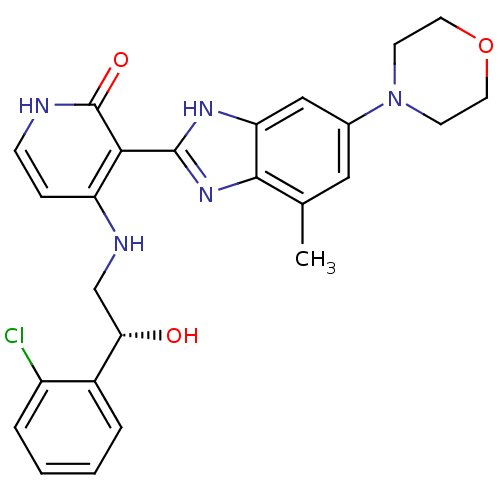
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296351
((R)-4-(2-(2-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@H](O)c2ccccc2Cl)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-12-16(31-8-10-34-11-9-31)13-20-23(15)30-24(29-20)22-19(6-7-27-25(22)33)28-14-21(32)17-4-2-3-5-18(17)26/h2-7,12-13,21,32H,8-11,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50230005

(1-(5-chloro-2-methoxyphenyl)-3-(2-methylquinolin-4...)Show InChI InChI=1S/C18H16ClN3O2/c1-11-9-15(13-5-3-4-6-14(13)20-11)21-18(23)22-16-10-12(19)7-8-17(16)24-2/h3-10H,1-2H3,(H2,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R in growth factor-stimulated NHDF |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50296350

((S)-3-(4-(2-(4-(2-(2-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2ccccc2Cl)cc[nH]c1=O)N1CCN(CCC#N)CC1 |r| Show InChI InChI=1S/C28H30ClN7O2/c1-18-15-19(36-13-11-35(12-14-36)10-4-8-30)16-23-26(18)34-27(33-23)25-22(7-9-31-28(25)38)32-17-24(37)20-5-2-3-6-21(20)29/h2-3,5-7,9,15-16,24,37H,4,10-14,17H2,1H3,(H,33,34)(H2,31,32,38)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50296348

(7-(3-(azetidin-1-yl)cyclobutyl)-5-(3-(benzyloxy)ph...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCC1 |r,wU:26.32,wD:24.27,(12.58,1.25,;12.59,-.29,;11.26,-1.07,;11.26,-2.61,;12.59,-3.38,;13.92,-2.61,;15.4,-3.09,;16.31,-1.83,;15.4,-.58,;15.87,.89,;14.85,2.02,;15.32,3.49,;16.83,3.81,;17.87,2.67,;19.37,2.99,;20.4,1.84,;21.91,2.16,;22.94,1.01,;24.44,1.33,;24.92,2.8,;23.88,3.95,;22.38,3.62,;17.39,1.2,;13.92,-1.06,;15.87,-4.56,;15.17,-5.92,;16.54,-6.63,;17.25,-5.26,;17.01,-8.1,;16.3,-9.45,;17.67,-10.16,;18.37,-8.79,)| Show InChI InChI=1S/C26H27N5O/c27-25-24-23(19-8-4-9-22(12-19)32-16-18-6-2-1-3-7-18)15-31(26(24)29-17-28-25)21-13-20(14-21)30-10-5-11-30/h1-4,6-9,12,15,17,20-21H,5,10-11,13-14,16H2,(H2,27,28,29)/t20-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor by cell based assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50296347

(5-(3-(benzyloxy)phenyl)-7-(3-(pyrrolidin-1-yl)cycl...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCCC1 |r,wU:26.32,wD:24.27,(-6.06,2.61,;-6.05,1.07,;-7.38,.3,;-7.39,-1.25,;-6.05,-2.02,;-4.72,-1.25,;-3.24,-1.73,;-2.33,-.47,;-3.24,.78,;-2.77,2.25,;-3.79,3.38,;-3.32,4.85,;-1.81,5.17,;-.78,4.03,;.73,4.35,;1.76,3.2,;3.27,3.52,;4.29,2.37,;5.8,2.69,;6.28,4.16,;5.24,5.31,;3.74,4.98,;-1.25,2.56,;-4.72,.3,;-2.78,-3.19,;-3.47,-4.56,;-2.1,-5.27,;-1.4,-3.9,;-1.64,-6.73,;-2.54,-7.98,;-1.64,-9.23,;-.18,-8.76,;-.17,-7.22,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(20-9-6-10-23(13-20)33-17-19-7-2-1-3-8-19)16-32(27(25)30-18-29-26)22-14-21(15-22)31-11-4-5-12-31/h1-3,6-10,13,16,18,21-22H,4-5,11-12,14-15,17H2,(H2,28,29,30)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor by cell based assay |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181674
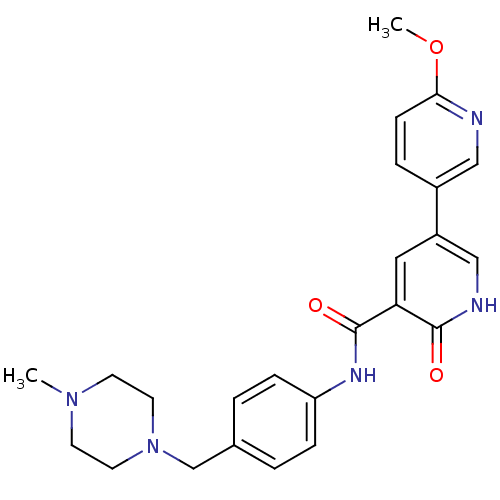
(6'-methoxy-6-oxo-1,6-dihydro-[3,3']bipyridinyl-5-c...)Show SMILES COc1ccc(cn1)-c1c[nH]c(=O)c(c1)C(=O)Nc1ccc(CN2CCN(C)CC2)cc1 Show InChI InChI=1S/C24H27N5O3/c1-28-9-11-29(12-10-28)16-17-3-6-20(7-4-17)27-24(31)21-13-19(15-26-23(21)30)18-5-8-22(32-2)25-14-18/h3-8,13-15H,9-12,16H2,1-2H3,(H,26,30)(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50348945
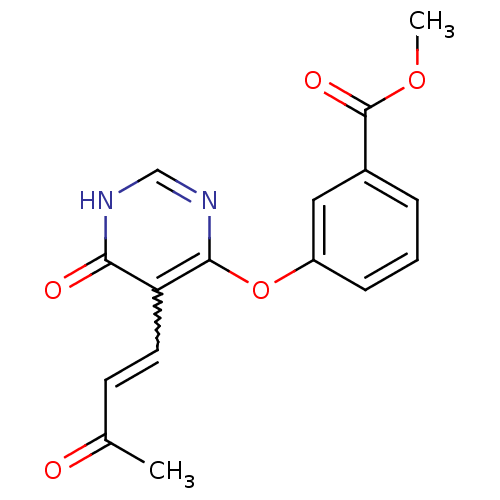
(CHEMBL1807757)Show SMILES COC(=O)c1cccc(Oc2nc[nH]c(=O)c2C=CC(C)=O)c1 |w:17.17| Show InChI InChI=1S/C16H14N2O5/c1-10(19)6-7-13-14(20)17-9-18-15(13)23-12-5-3-4-11(8-12)16(21)22-2/h3-9H,1-2H3,(H,17,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of 6xHis-tagged NPM-ALK using biotinylated-poly(GT) peptide as substrate after 60 mins |
Bioorg Med Chem Lett 21: 4592-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.103
BindingDB Entry DOI: 10.7270/Q2N87B4V |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50296354

(CHEMBL549369 | N-(5-(3,5-difluorophenylsulfonyl)-4...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CCN(Cc12)S(=O)(=O)c1cc(F)cc(F)c1 Show InChI InChI=1S/C24H26F2N6O3S/c1-30-8-10-31(11-9-30)19-4-2-16(3-5-19)24(33)27-23-21-15-32(7-6-22(21)28-29-23)36(34,35)20-13-17(25)12-18(26)14-20/h2-5,12-14H,6-11,15H2,1H3,(H2,27,28,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition for IGF1R-induced S6 ribosomal protein phosphorylation in growth factor-stimulated NHDF |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50294542
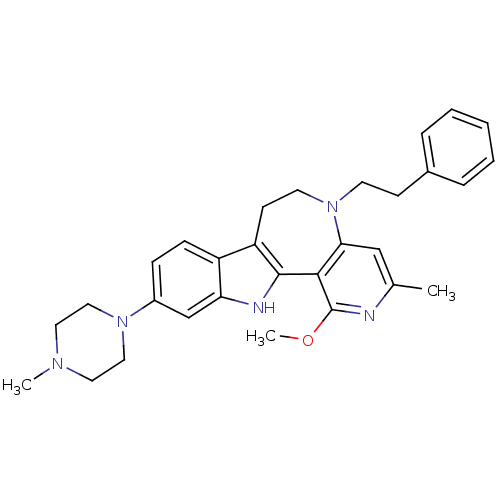
(1-Methoxy-3-methyl-10-(4-methyl-piperazin-1-yl)-5-...)Show SMILES COc1nc(C)cc2N(CCc3ccccc3)CCc3c([nH]c4cc(ccc34)N3CCN(C)CC3)-c12 Show InChI InChI=1S/C30H35N5O/c1-21-19-27-28(30(31-21)36-3)29-25(12-14-35(27)13-11-22-7-5-4-6-8-22)24-10-9-23(20-26(24)32-29)34-17-15-33(2)16-18-34/h4-10,19-20,32H,11-18H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IRK |
Bioorg Med Chem 17: 3308-16 (2009)
Article DOI: 10.1016/j.bmc.2009.03.046
BindingDB Entry DOI: 10.7270/Q2GX4BKJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50201127

(3-(5-(1H-imidazol-1-yl)-7-methyl-1H-benzo[d]imidaz...)Show SMILES Cc1cc(cc2nc([nH]c12)-c1ccc[nH]c1=O)-n1ccnc1 Show InChI InChI=1S/C16H13N5O/c1-10-7-11(21-6-5-17-9-21)8-13-14(10)20-15(19-13)12-3-2-4-18-16(12)22/h2-9H,1H3,(H,18,22)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor/Nucleophosmin
(Homo sapiens (Human)) | BDBM50181670

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2cc(c[nH]c2=O)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C24H24N4O4/c1-27-8-10-28(11-9-27)19-5-3-18(4-6-19)26-24(30)20-12-17(14-25-23(20)29)16-2-7-21-22(13-16)32-15-31-21/h2-7,12-14H,8-11,15H2,1H3,(H,25,29)(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against human NPM-ALK positive anaplastic large cell lymphoma karpas299 cell line |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181672

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C26H28N4O4/c1-29-10-12-30(13-11-29)9-8-18-2-5-21(6-3-18)28-26(32)22-14-20(16-27-25(22)31)19-4-7-23-24(15-19)34-17-33-23/h2-7,14-16H,8-13,17H2,1H3,(H,27,31)(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181671

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN(C(=O)c1cc(c[nH]c1=O)-c1ccc2OCOc2c1)c1ccc(CN2CCN(C)CC2)cc1 Show InChI InChI=1S/C26H28N4O4/c1-28-9-11-30(12-10-28)16-18-3-6-21(7-4-18)29(2)26(32)22-13-20(15-27-25(22)31)19-5-8-23-24(14-19)34-17-33-23/h3-8,13-15H,9-12,16-17H2,1-2H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181679
(5-(2-methyl-benzothiazol-5-yl)-2-oxo-1,2-dihydro-p...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4sc(C)nc4c3)cc2)CC1 Show InChI InChI=1S/C26H27N5O2S/c1-17-28-23-14-19(5-8-24(23)34-17)20-13-22(25(32)27-15-20)26(33)29-21-6-3-18(4-7-21)16-31-11-9-30(2)10-12-31/h3-8,13-15H,9-12,16H2,1-2H3,(H,27,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein/Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50181670

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2cc(c[nH]c2=O)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C24H24N4O4/c1-27-8-10-28(11-9-27)19-5-3-18(4-6-19)26-24(30)20-12-17(14-25-23(20)29)16-2-7-21-22(13-16)32-15-31-21/h2-7,12-14H,8-11,15H2,1H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Antiproliferative activity against human BCR-ABL positive chronic myeloid leukemia K562 cell line |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50181672

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C26H28N4O4/c1-29-10-12-30(13-11-29)9-8-18-2-5-21(6-3-18)28-26(32)22-14-20(16-27-25(22)31)19-4-7-23-24(15-19)34-17-33-23/h2-7,14-16H,8-13,17H2,1H3,(H,27,31)(H,28,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against Flt3 |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50348945

(CHEMBL1807757)Show SMILES COC(=O)c1cccc(Oc2nc[nH]c(=O)c2C=CC(C)=O)c1 |w:17.17| Show InChI InChI=1S/C16H14N2O5/c1-10(19)6-7-13-14(20)17-9-18-15(13)23-12-5-3-4-11(8-12)16(21)22-2/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET using biotinylated-poly(GT) peptide as substrate after 60 mins |
Bioorg Med Chem Lett 21: 4592-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.103
BindingDB Entry DOI: 10.7270/Q2N87B4V |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50294542

(1-Methoxy-3-methyl-10-(4-methyl-piperazin-1-yl)-5-...)Show SMILES COc1nc(C)cc2N(CCc3ccccc3)CCc3c([nH]c4cc(ccc34)N3CCN(C)CC3)-c12 Show InChI InChI=1S/C30H35N5O/c1-21-19-27-28(30(31-21)36-3)29-25(12-14-35(27)13-11-22-7-5-4-6-8-22)24-10-9-23(20-26(24)32-29)34-17-15-33(2)16-18-34/h4-10,19-20,32H,11-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IGFR1 |
Bioorg Med Chem 17: 3308-16 (2009)
Article DOI: 10.1016/j.bmc.2009.03.046
BindingDB Entry DOI: 10.7270/Q2GX4BKJ |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50296347

(5-(3-(benzyloxy)phenyl)-7-(3-(pyrrolidin-1-yl)cycl...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCCC1 |r,wU:26.32,wD:24.27,(-6.06,2.61,;-6.05,1.07,;-7.38,.3,;-7.39,-1.25,;-6.05,-2.02,;-4.72,-1.25,;-3.24,-1.73,;-2.33,-.47,;-3.24,.78,;-2.77,2.25,;-3.79,3.38,;-3.32,4.85,;-1.81,5.17,;-.78,4.03,;.73,4.35,;1.76,3.2,;3.27,3.52,;4.29,2.37,;5.8,2.69,;6.28,4.16,;5.24,5.31,;3.74,4.98,;-1.25,2.56,;-4.72,.3,;-2.78,-3.19,;-3.47,-4.56,;-2.1,-5.27,;-1.4,-3.9,;-1.64,-6.73,;-2.54,-7.98,;-1.64,-9.23,;-.18,-8.76,;-.17,-7.22,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(20-9-6-10-23(13-20)33-17-19-7-2-1-3-8-19)16-32(27(25)30-18-29-26)22-14-21(15-22)31-11-4-5-12-31/h1-3,6-10,13,16,18,21-22H,4-5,11-12,14-15,17H2,(H2,28,29,30)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of c-KIT |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50296348

(7-(3-(azetidin-1-yl)cyclobutyl)-5-(3-(benzyloxy)ph...)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@@H](C1)N1CCC1 |r,wU:26.32,wD:24.27,(12.58,1.25,;12.59,-.29,;11.26,-1.07,;11.26,-2.61,;12.59,-3.38,;13.92,-2.61,;15.4,-3.09,;16.31,-1.83,;15.4,-.58,;15.87,.89,;14.85,2.02,;15.32,3.49,;16.83,3.81,;17.87,2.67,;19.37,2.99,;20.4,1.84,;21.91,2.16,;22.94,1.01,;24.44,1.33,;24.92,2.8,;23.88,3.95,;22.38,3.62,;17.39,1.2,;13.92,-1.06,;15.87,-4.56,;15.17,-5.92,;16.54,-6.63,;17.25,-5.26,;17.01,-8.1,;16.3,-9.45,;17.67,-10.16,;18.37,-8.79,)| Show InChI InChI=1S/C26H27N5O/c27-25-24-23(19-8-4-9-22(12-19)32-16-18-6-2-1-3-7-18)15-31(26(24)29-17-28-25)21-13-20(14-21)30-10-5-11-30/h1-4,6-9,12,15,17,20-21H,5,10-11,13-14,16H2,(H2,27,28,29)/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of c-KIT |
J Med Chem 52: 4981-5004 (2010)
Article DOI: 10.1021/jm9002395
BindingDB Entry DOI: 10.7270/Q2P84CTD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data