

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50059510 (8-Methoxy-3-phenyl-1,2,3,4-tetrahydro-chromeno[3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101832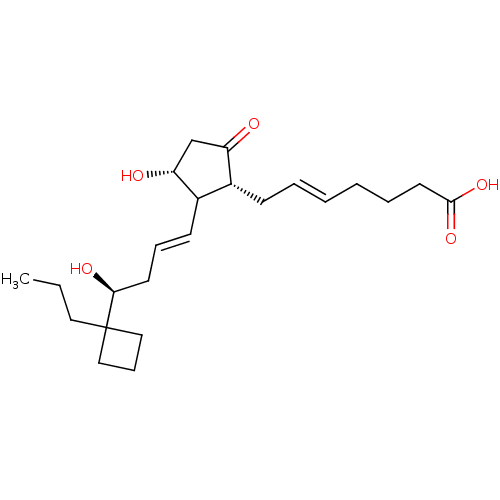 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101823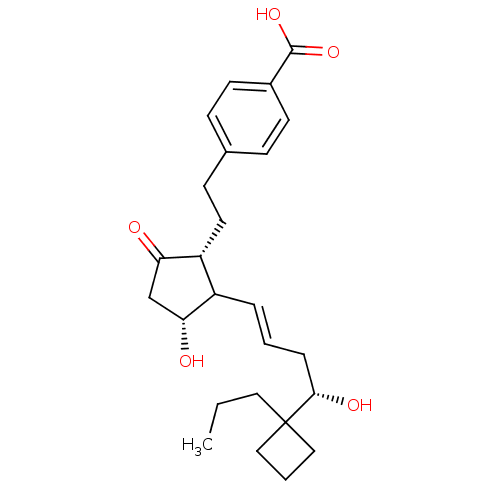 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101827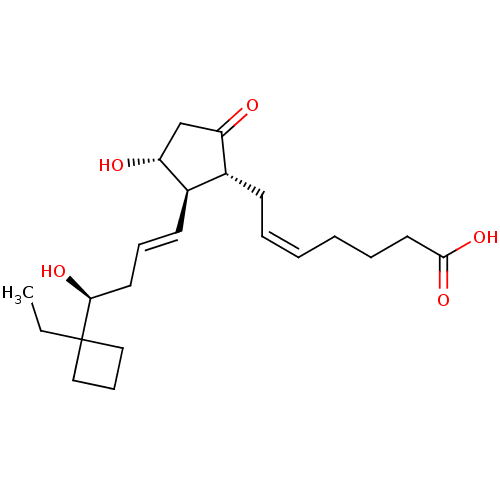 ((Z)-7-{(1R,2R,3R)-2-[(E)-(S)-4-(1-Ethyl-cyclobutyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101832 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101823 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101826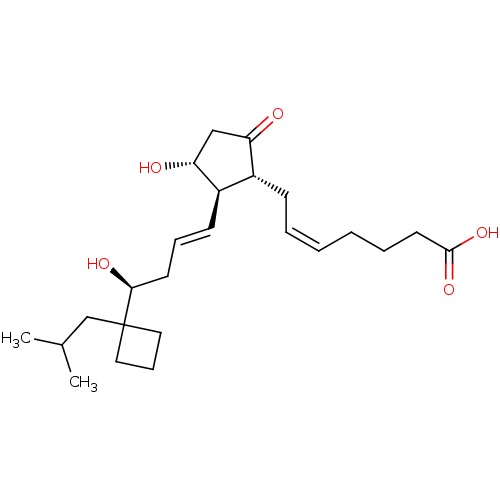 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101833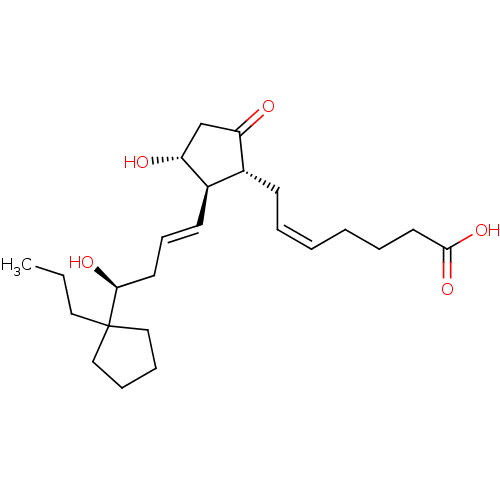 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50281126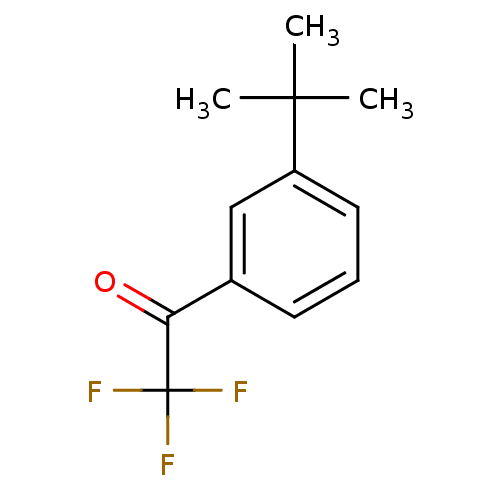 (1-(3-tert-Butyl-phenyl)-2,2,2-trifluoro-ethanone |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.00370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to inhibit Acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 3: 2619-2622 (1993) Article DOI: 10.1016/S0960-894X(01)80727-7 BindingDB Entry DOI: 10.7270/Q2VX0GF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13925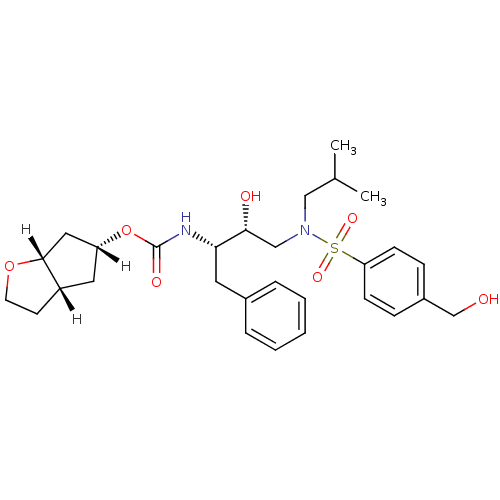 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50229232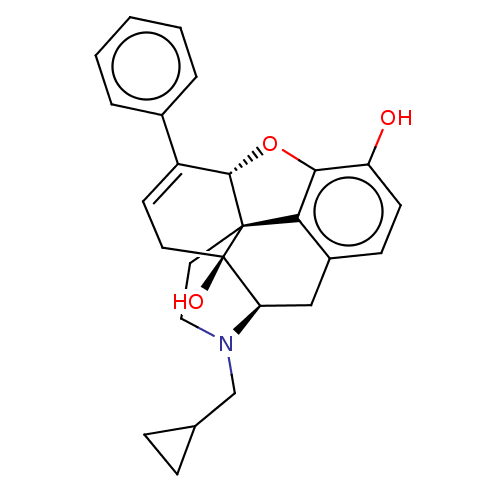 (CHEMBL610527) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to Opioid receptor mu using [3H]DAMGO as radioligand in guinea pig brain membrane | J Med Chem 34: 1715-20 (1991) BindingDB Entry DOI: 10.7270/Q2T43TPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50143314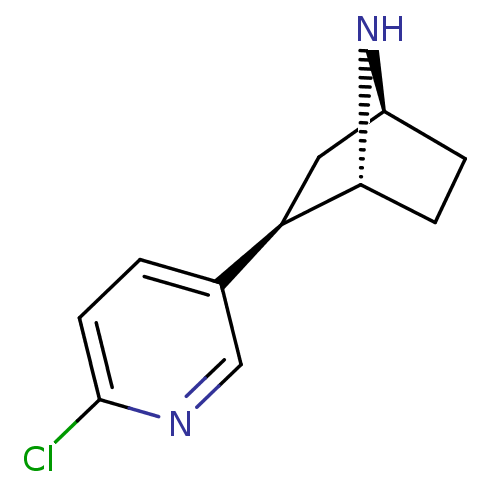 ((+)-Epibatidine | (-)-epibatidine | (1R,2R,4S)-2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-iodo-MLA binding to Nicotinic acetylcholine receptor alpha-7 of rat cerebral cortex | J Med Chem 47: 4588-94 (2004) Article DOI: 10.1021/jm040078g BindingDB Entry DOI: 10.7270/Q2DZ092M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312552 (3'-(3-nitrophenyl)epibatidine | CHEMBL1096352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50281127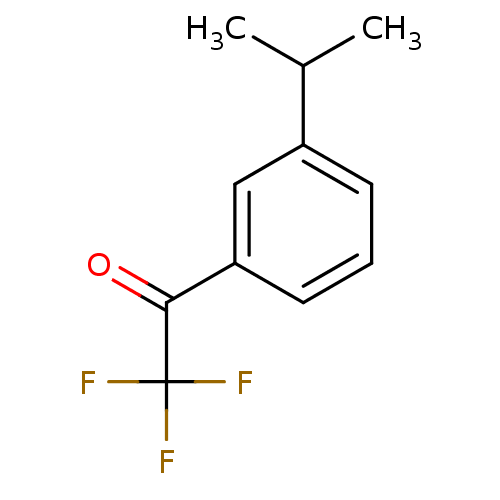 (2,2,2-Trifluoro-1-(3-isopropyl-phenyl)-ethanone | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to inhibit Acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 3: 2619-2622 (1993) Article DOI: 10.1016/S0960-894X(01)80727-7 BindingDB Entry DOI: 10.7270/Q2VX0GF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561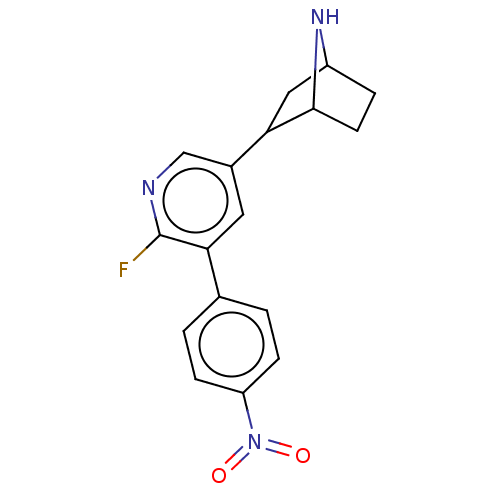 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM86812 (CAS_45263784 | NSC_45263784 | rac-2-(6-fluoro-5-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312557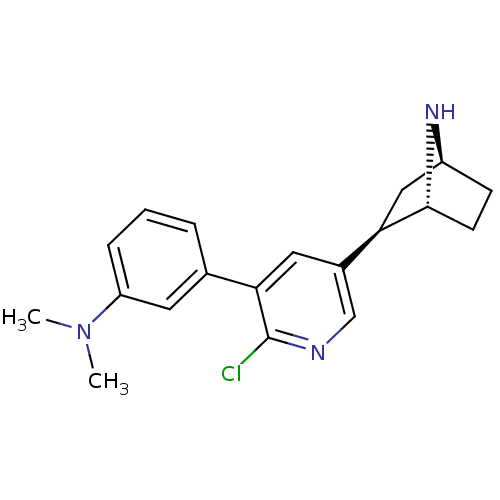 (3'-(3-Dimethylaminophenyl)epibatidine | CHEMBL1084...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50229232 (CHEMBL610527) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to opioid receptor kappa using [3H]-EK as radioligand in guinea pig brain membrane | J Med Chem 34: 1715-20 (1991) BindingDB Entry DOI: 10.7270/Q2T43TPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223209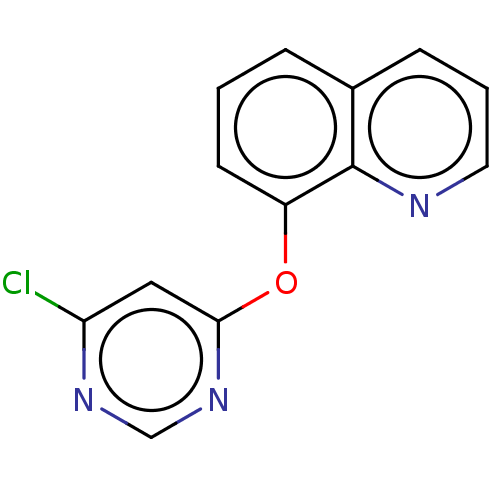 (8-((6-Chloropyrimidin-4-yl)oxy)quinoline (Compound...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476758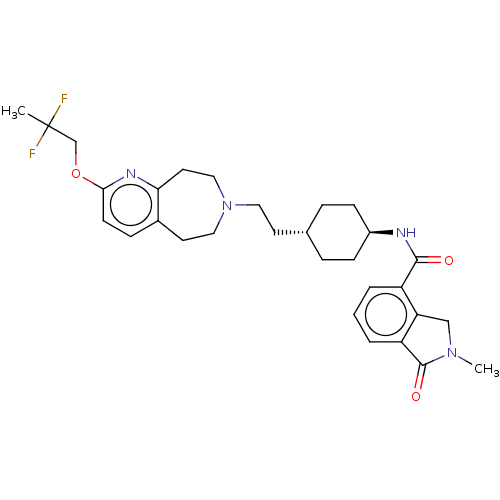 (US10870660, Compound III-024 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312547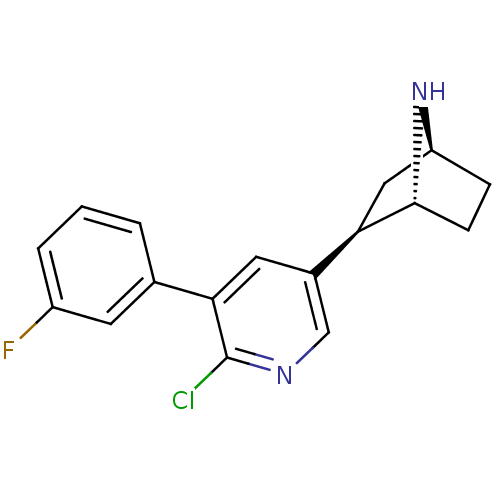 (3'-(3-Fluorophenyl)epibatidine | CHEMBL1097692) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50366138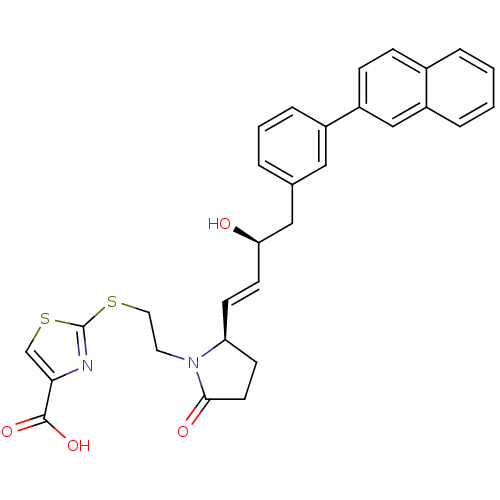 (CHEMBL1957437) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 2235-51 (2012) Article DOI: 10.1016/j.bmc.2012.02.018 BindingDB Entry DOI: 10.7270/Q2542P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50366138 (CHEMBL1957437) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312549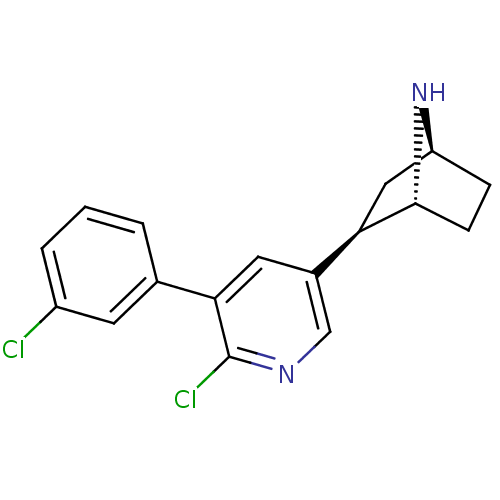 (3'-(3-Chlorophenyl)epibatidine | CHEMBL1098031) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82552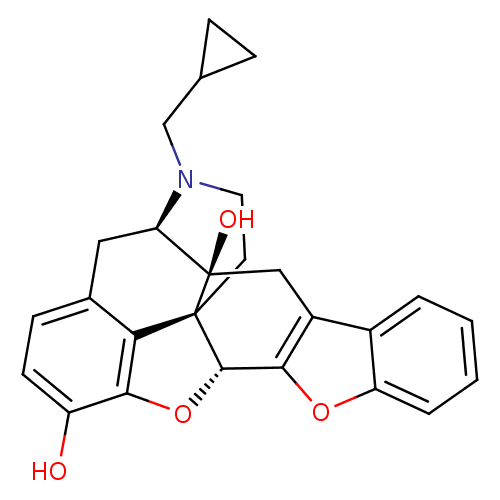 (CAS_111555-58-9 | NTB | naltrindolebenzofuran) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to opioid receptor delta using [3H]DADLE as radioligand in guinea pig brain membrane | J Med Chem 34: 1715-20 (1991) BindingDB Entry DOI: 10.7270/Q2T43TPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 49: 5252-61 (2006) Article DOI: 10.1021/jm060561m BindingDB Entry DOI: 10.7270/Q23R0R41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476918 (US10870660, Compound II-057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312551 (3'-(4-Nitrophenyl)epibatidine | CHEMBL1098033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312550 (3'-(3-Bromophenyl)epibatidine | CHEMBL1098032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593894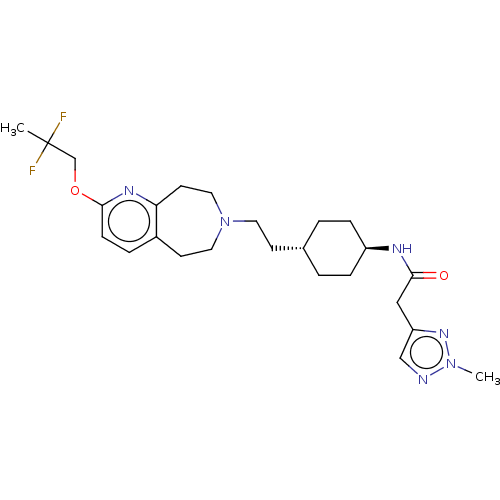 (US11578084, Compound I'-42) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312554 (3'-(3-Aminophenyl)epibatidine | CHEMBL1076992) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312546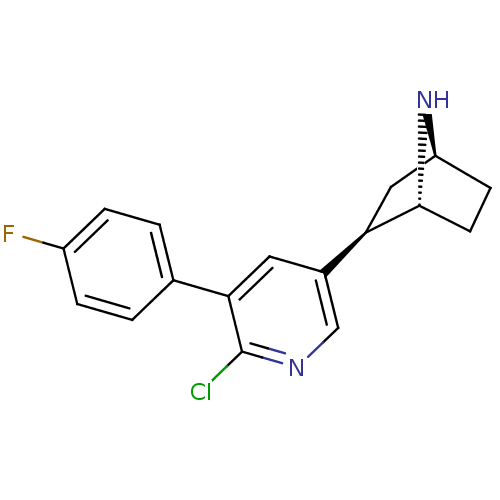 ((1R,2R,4S)-2-(6-chloro-5-(4-fluorophenyl)pyridin-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143314 ((+)-Epibatidine | (-)-epibatidine | (1R,2R,4S)-2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50451343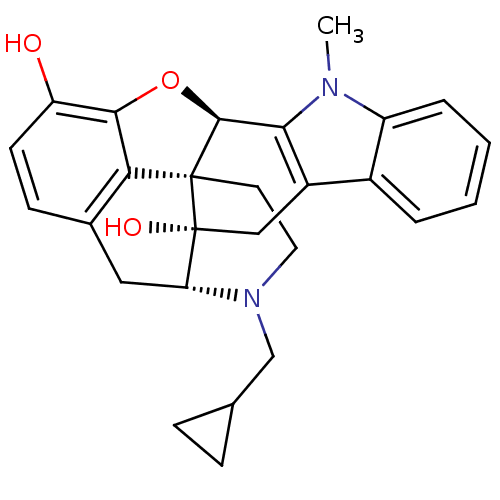 (CHEMBL477154) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to opioid receptor delta using [3H]DADLE as radioligand in guinea pig brain membrane | J Med Chem 34: 1715-20 (1991) BindingDB Entry DOI: 10.7270/Q2T43TPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312556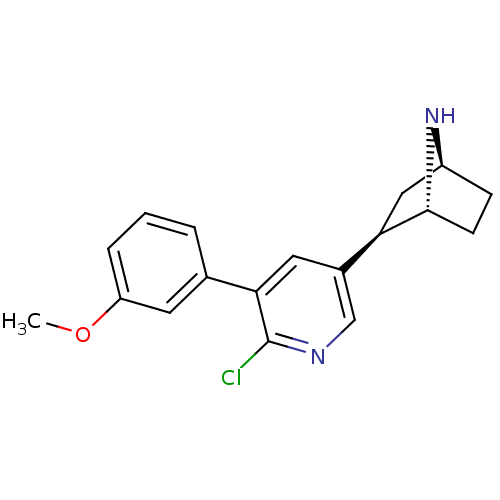 ((1R,2R,4S)-2-(6-chloro-5-(3-methoxyphenyl)pyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325534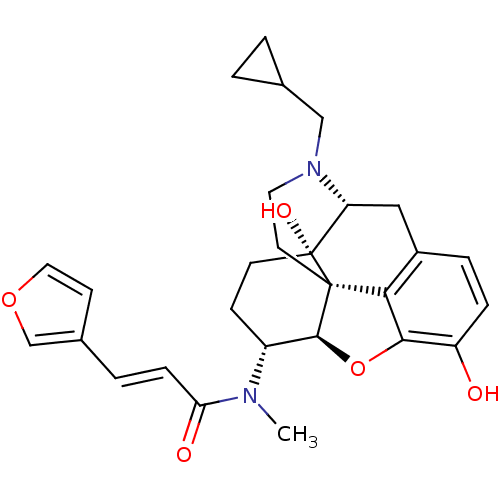 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100717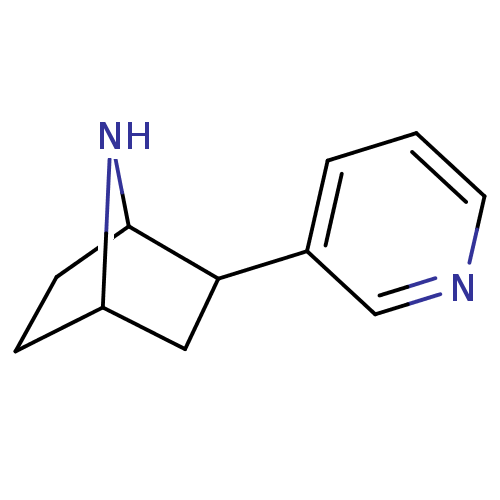 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50100717 (2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by PDSP Ki Database | Bioorg Med Chem 16: 746-54 (2008) Article DOI: 10.1016/j.bmc.2007.10.027 BindingDB Entry DOI: 10.7270/Q2H130KC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50162061 (2-(5-Ethynyl-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hep...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha4-beta2 in rat cerebral cortex | J Med Chem 48: 1221-8 (2005) Article DOI: 10.1021/jm040160b BindingDB Entry DOI: 10.7270/Q26W9BV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021919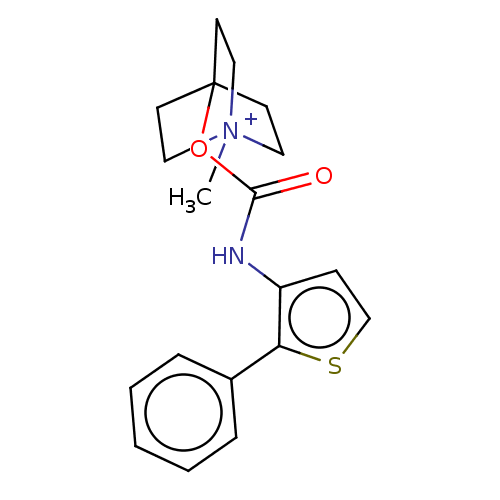 (CHEMBL3298595) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50021919 (CHEMBL3298595) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312545 ((1R,2R,4S)-2-(6-chloro-5-phenylpyridin-3-yl)-7-aza...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Alpha-4-2 of Neuronal acetylcholine receptor subunit alpha-4 (Alpha-4-2) (Rattus norvegicus (Rat)) | BDBM184561 (US9150581, RTI-7527-(+/-)-102 | US9150581, RTI-752...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RESEARCH TRIANGLE INSTITUTE; THE REGENTS OF THE UNIVERSITY OF MICHIGAN; VIRGINIA COMMONWEALTH UNIVERSITY US Patent | Assay Description The Ki values for the inhibition of [3H]epibatidine binding at the α4β2 nAChR in male rat cerebral cortex for compounds are listed in Table... | US Patent US9150581 (2015) BindingDB Entry DOI: 10.7270/Q2319TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476915 (US10870660, Compound II-047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351401 (CHEMBL1819091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50312555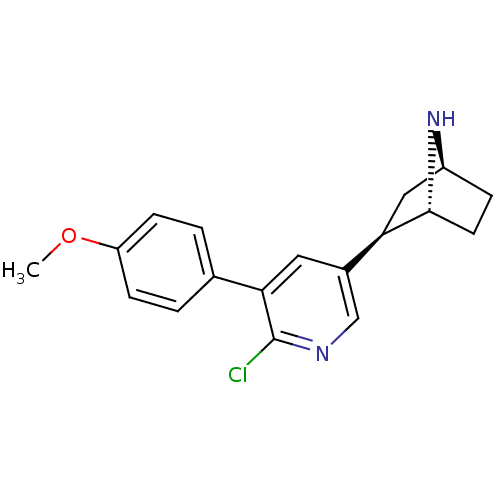 (3'-(4-Methoxylphenyl)epibatidine | CHEMBL1085916) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat cerebral cortex after 4 hrs by scintillation counting | J Nat Prod 73: 306-12 (2010) Article DOI: 10.1021/np9006124 BindingDB Entry DOI: 10.7270/Q2TQ61PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50100715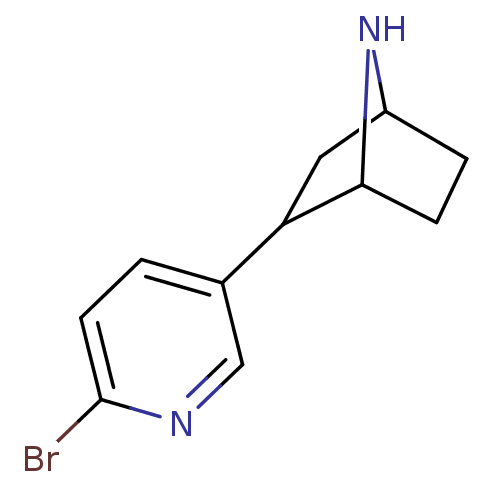 (2-(6-Bromo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]hepta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding at the nicotinic acetylcholine receptor alpha4-beta2 in male rat cerebral cortex | J Med Chem 44: 2229-37 (2001) BindingDB Entry DOI: 10.7270/Q2BV7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53263 total ) | Next | Last >> |