

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (CALF) | BDBM50408198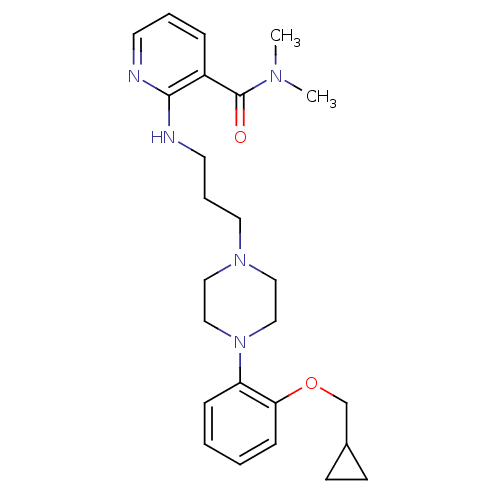 (CHEMBL91278) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408201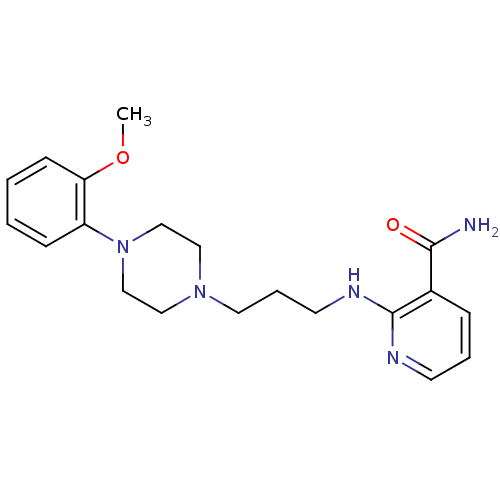 (CHEMBL88512) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50060964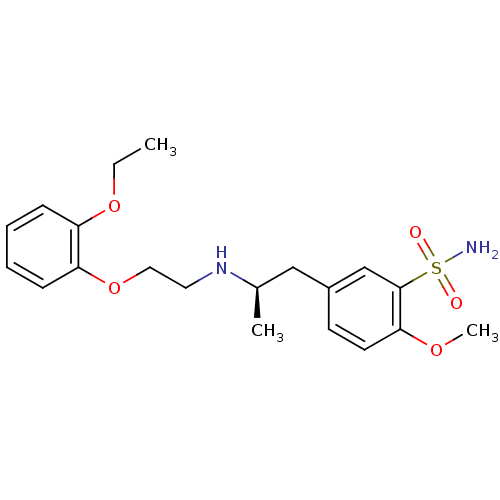 ((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408248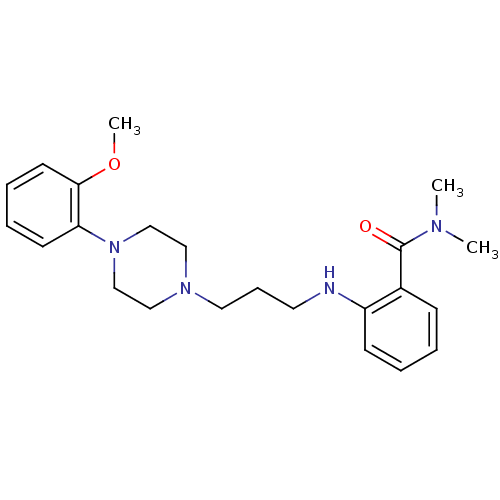 (CHEMBL330060) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408246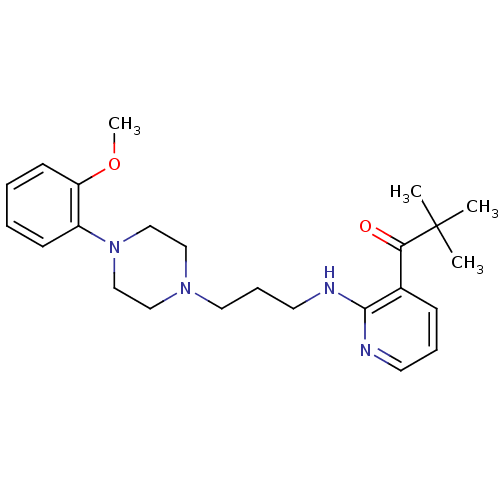 (CHEMBL92261) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50033112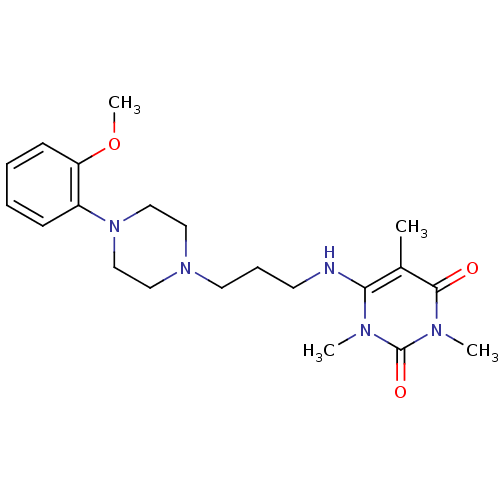 (6-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propyla...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50160165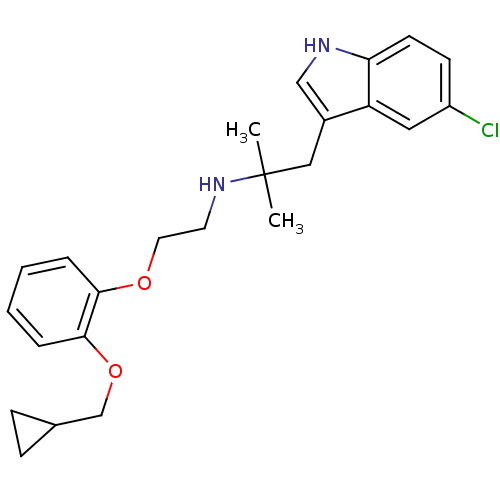 (CHEMBL88272 | RS-17053 | [2-(2-Cyclopropylmethoxy-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408244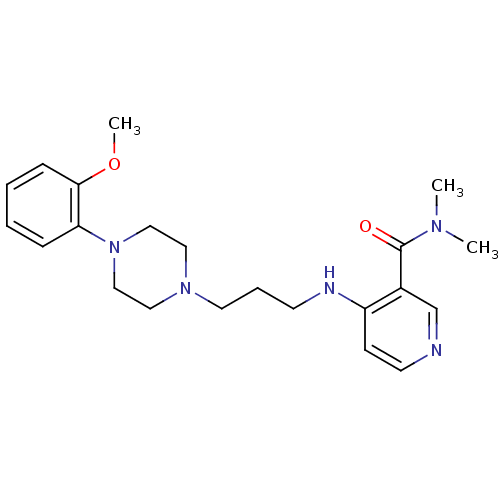 (CHEMBL93736) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408232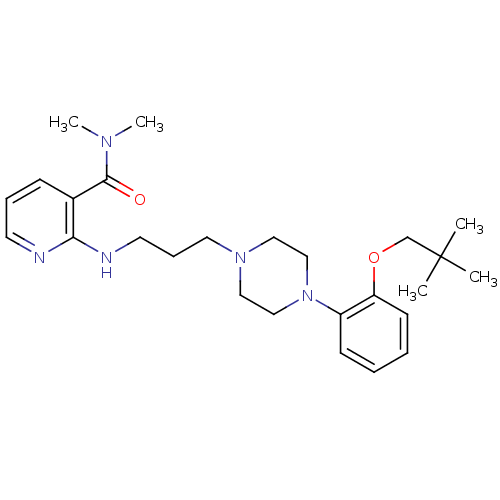 (CHEMBL91093) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408242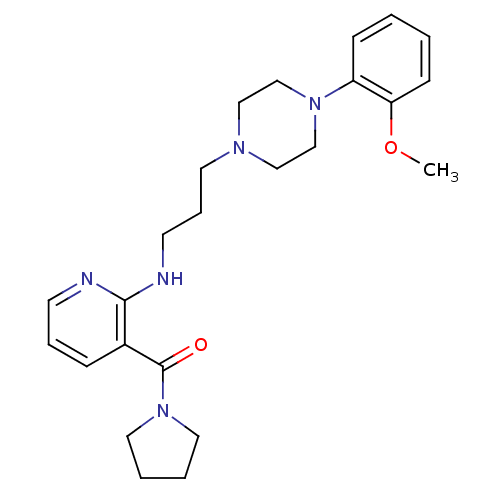 (CHEMBL91876) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM31046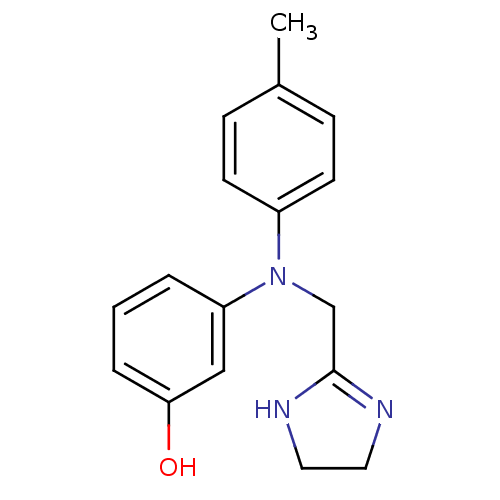 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408229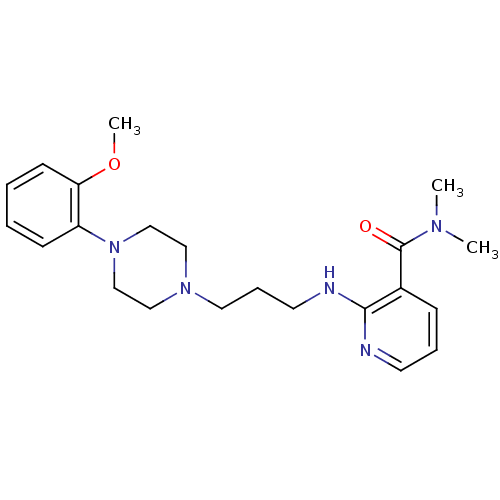 (CHEMBL329160) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound was screened in vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408205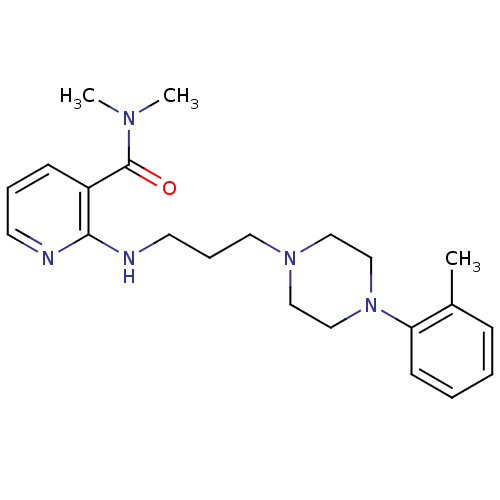 (CHEMBL88435) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408239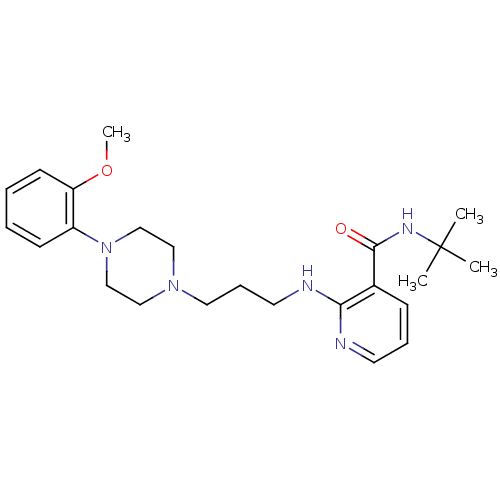 (CHEMBL90874) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408202 (CHEMBL92901) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408238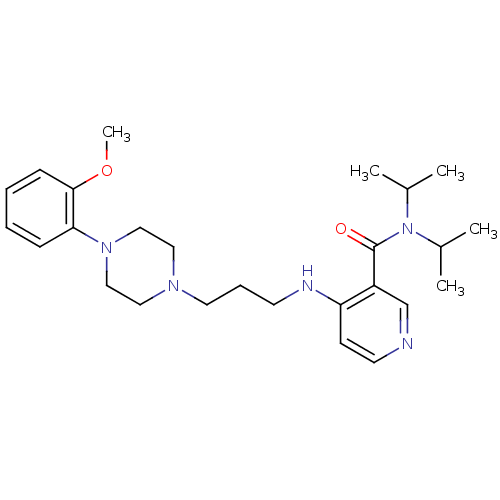 (CHEMBL89916) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408208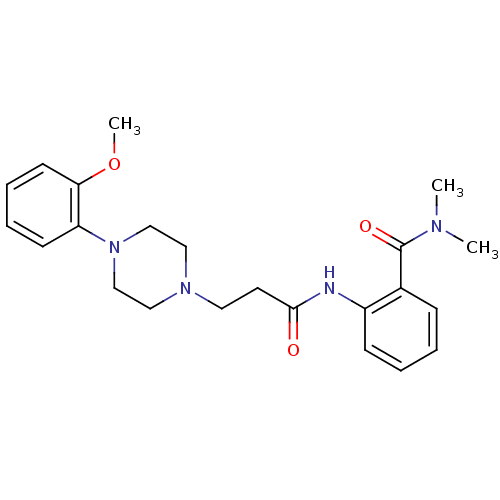 (CHEMBL88820) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408192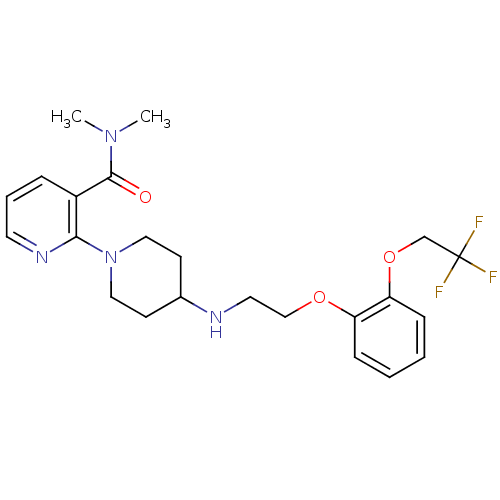 (CHEMBL90869) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408194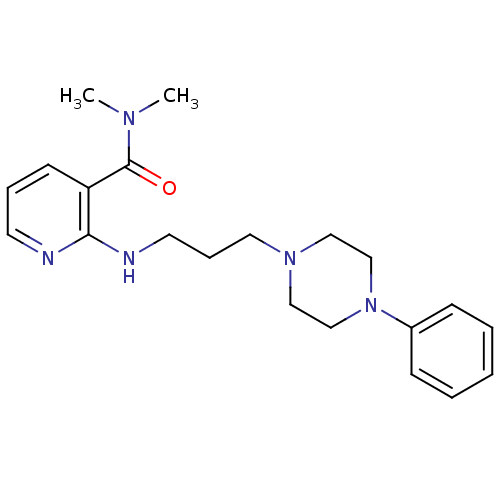 (CHEMBL90287) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50033111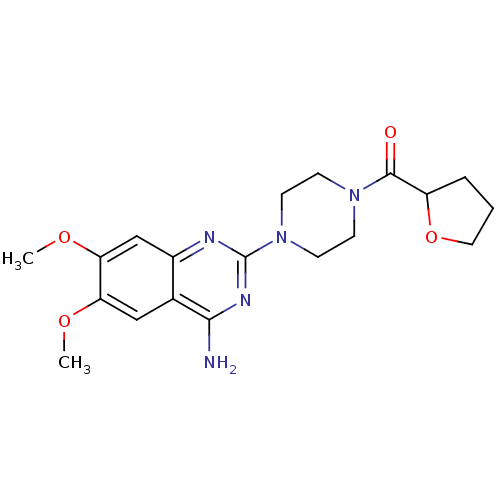 (1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-((tetra...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408225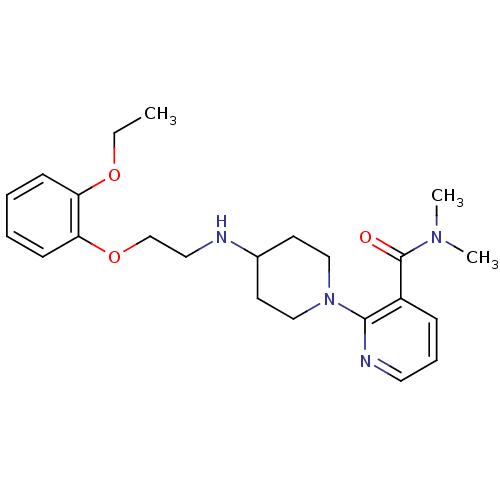 (CHEMBL312935) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50230827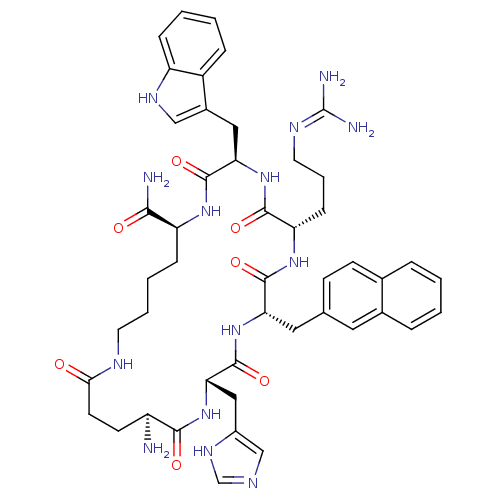 (CHEMBL253364 | MK-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC3 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408193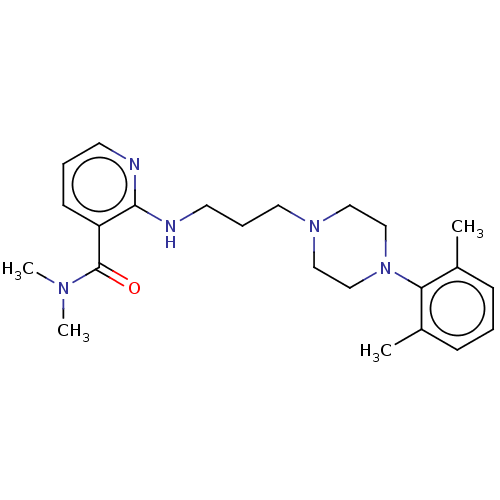 (CHEMBL92109) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484193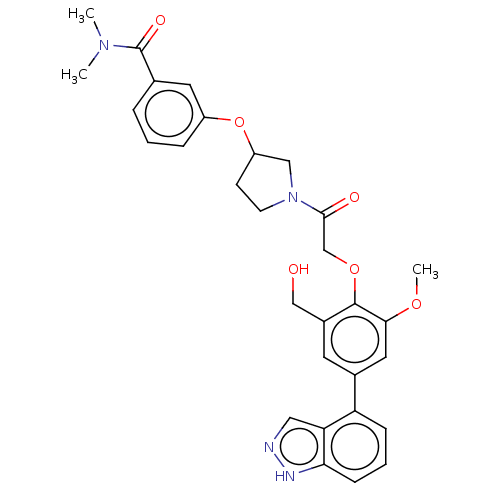 (CDD-1714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine | Assay Description To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... | Proc Natl Acad Sci U S A 118: (2021) Article DOI: 10.1073/pnas.2111172118 BindingDB Entry DOI: 10.7270/Q2W380FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484197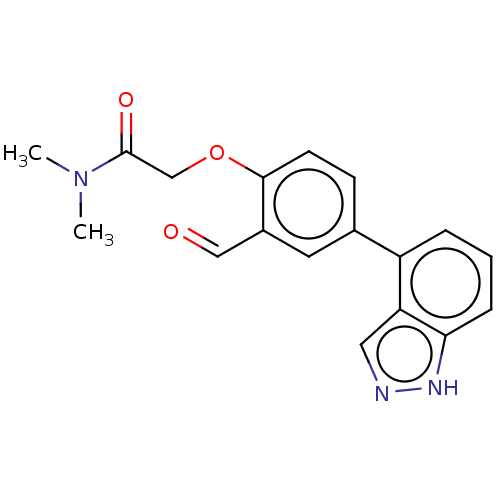 (CDD-1976 | WO2023004291, Compound CDD-1976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine | Assay Description To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... | Proc Natl Acad Sci U S A 118: (2021) Article DOI: 10.1073/pnas.2111172118 BindingDB Entry DOI: 10.7270/Q2W380FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484196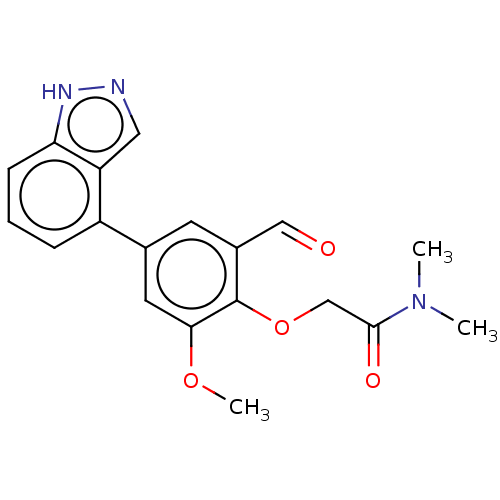 (CDD-1713 | WO2023004291, Compound CDD-1713) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine | Assay Description To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... | Proc Natl Acad Sci U S A 118: (2021) Article DOI: 10.1073/pnas.2111172118 BindingDB Entry DOI: 10.7270/Q2W380FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50408216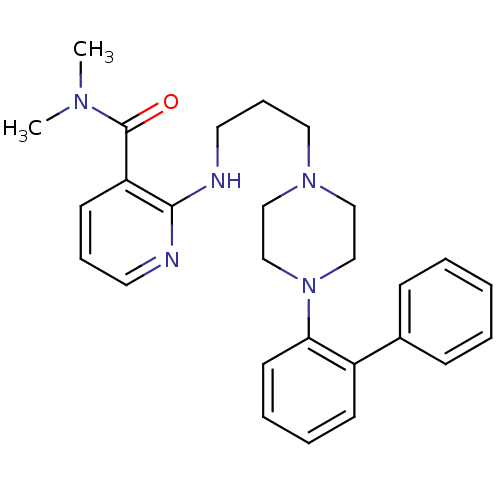 (CHEMBL90746) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50408216 (CHEMBL90746) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Dopamine receptor D2 | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM484198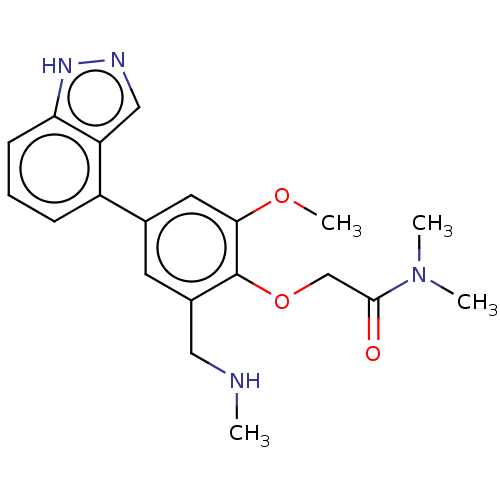 (CDD-1777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine | Assay Description To determine the Ki values of active compounds, 25 nM Mpro was mixed with increasing concentrations of compounds (from 4 nM to 4,000 nM with twofold ... | Proc Natl Acad Sci U S A 118: (2021) Article DOI: 10.1073/pnas.2111172118 BindingDB Entry DOI: 10.7270/Q2W380FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50408197 (CHEMBL91605) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Dopamine receptor D2 | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50408236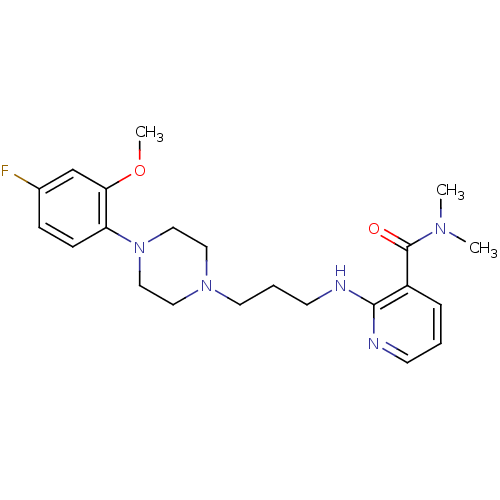 (CHEMBL420620) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50408241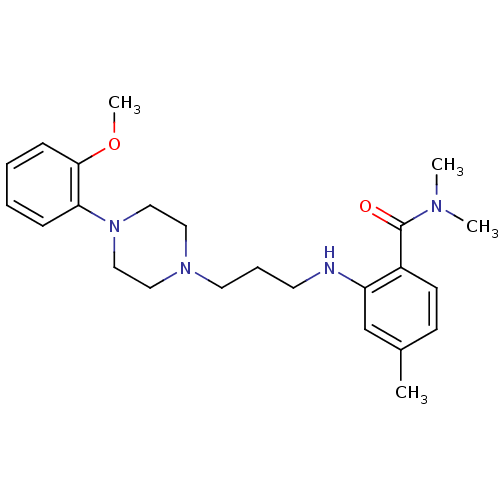 (CHEMBL89030) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50408197 (CHEMBL91605) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against 5-hydroxytryptamine 1A receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50394918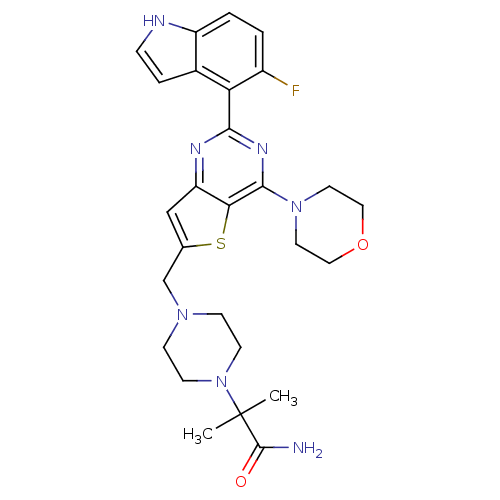 (CHEMBL2165504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins | J Med Chem 55: 5887-900 (2012) Article DOI: 10.1021/jm3003747 BindingDB Entry DOI: 10.7270/Q2V125XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50408236 (CHEMBL420620) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against 5-hydroxytryptamine 1A receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50408236 (CHEMBL420620) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Dopamine receptor D2 | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50408241 (CHEMBL89030) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Dopamine receptor D2 | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50408229 (CHEMBL329160) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Dopamine receptor D2 | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50408199 (CHEMBL88160) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against 5-hydroxytryptamine 1A receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50408229 (CHEMBL329160) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against 5-hydroxytryptamine 1A receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50408241 (CHEMBL89030) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against 5-hydroxytryptamine 1A receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50408199 (CHEMBL88160) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Dopamine receptor D2 | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50408199 (CHEMBL88160) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50394917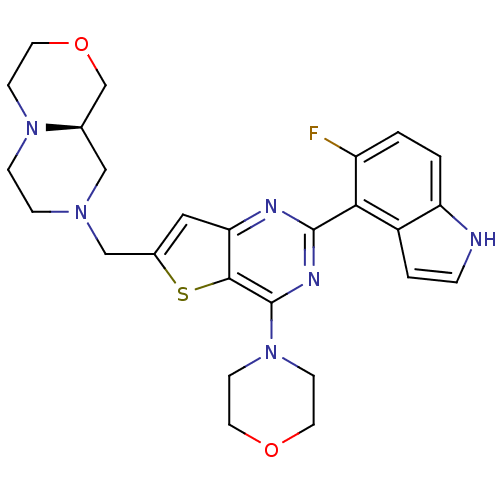 (CHEMBL2165505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins | J Med Chem 55: 5887-900 (2012) Article DOI: 10.1021/jm3003747 BindingDB Entry DOI: 10.7270/Q2V125XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50027084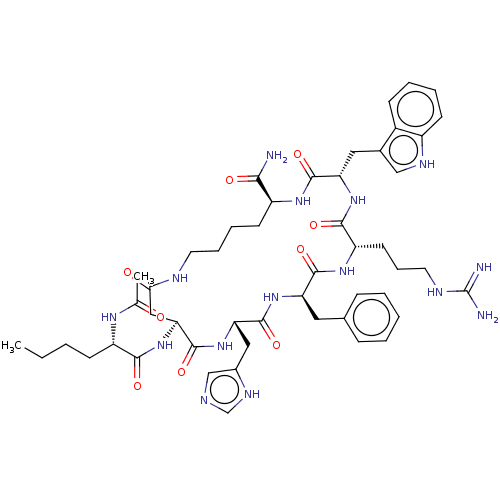 (Melatonan) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC1 receptor expressed in HEK293 cells | J Med Chem 51: 187-95 (2008) Article DOI: 10.1021/jm070461w BindingDB Entry DOI: 10.7270/Q2V125N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50205745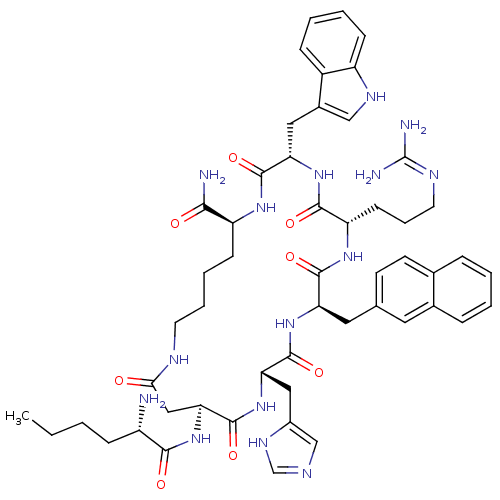 ((3S,6S,9R,12S,15S,23S)-15-[(2S)-2-aminohexanamido]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [125I]NDPalphaMSH from human MC1R expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2492-8 (2007) Article DOI: 10.1016/j.bmcl.2007.02.020 BindingDB Entry DOI: 10.7270/Q2FN15W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50394916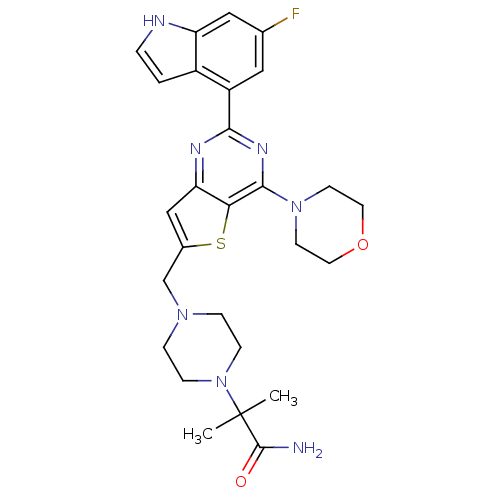 (CHEMBL2165506) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay | J Med Chem 55: 5887-900 (2012) Article DOI: 10.1021/jm3003747 BindingDB Entry DOI: 10.7270/Q2V125XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50394910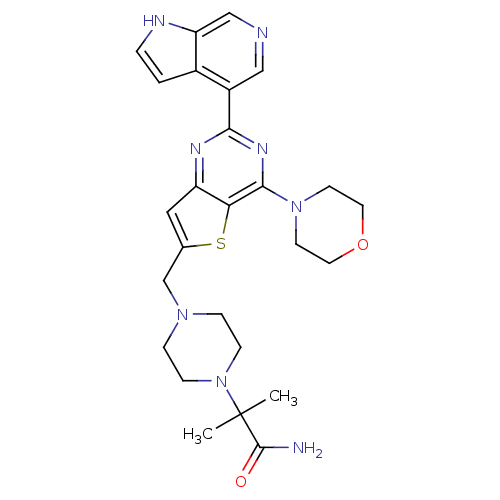 (CHEMBL2165512) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay | J Med Chem 55: 5887-900 (2012) Article DOI: 10.1021/jm3003747 BindingDB Entry DOI: 10.7270/Q2V125XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50205745 ((3S,6S,9R,12S,15S,23S)-15-[(2S)-2-aminohexanamido]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [125I]NDPalphaMSH from human MC4R expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2492-8 (2007) Article DOI: 10.1016/j.bmcl.2007.02.020 BindingDB Entry DOI: 10.7270/Q2FN15W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1832 total ) | Next | Last >> |