 Found 118 hits with Last Name = 'palomo' and Initial = 'ma'
Found 118 hits with Last Name = 'palomo' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50016816
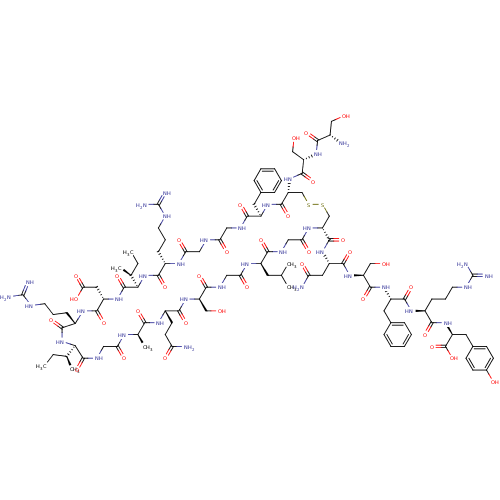
(CHEMBL412913 | S-S-C-F-G-G-R-I-D-R-I-G-A-Q-S-G-L-G...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](CSSC[C@@H](NC(=O)CNC(=O)[C@@H](CC(C)C)NC(=O)CNC(=O)[C@@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO)[C@@H](C)CC Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55+,60-,61-,62-,63-,64-,65+,66+,67-,68-,69-,70-,71+,72-,73-,74+,75?,84+,85+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Apparent binding affinity for non-vasorelaxant receptor |
J Med Chem 32: 67-72 (1989)
BindingDB Entry DOI: 10.7270/Q2FN156B |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50452851
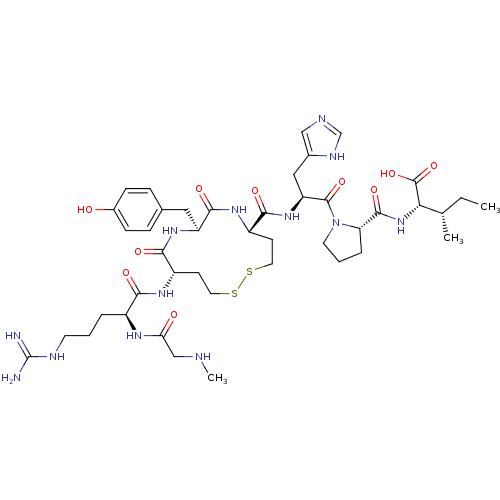
(CHEMBL2373017)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCSSCC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |wU:8.7,4.4,15.16,53.55,2.1,wD:32.34,25.26,36.38,(-13.57,-1.73,;-12.05,-1.44,;-11.05,-2.61,;-11.56,-4.06,;-9.54,-2.32,;-8.53,-3.49,;-7.02,-3.2,;-6.51,-1.75,;-6.01,-4.37,;-6.36,-5.86,;-5.05,-6.66,;-3.88,-5.66,;-4.48,-4.24,;-3.68,-2.92,;-4.42,-1.57,;-2.14,-2.95,;-1.02,-4.01,;-1.37,-5.51,;-.36,-6.68,;-1.16,-7.99,;-2.66,-7.64,;-2.79,-6.1,;-1.34,-1.64,;.2,-1.67,;.94,-3.02,;1,-.35,;.23,.98,;1,2.31,;.23,3.65,;1,4.98,;2.54,4.98,;3.31,3.65,;4.85,3.65,;5.62,4.98,;7.16,4.98,;7.93,3.65,;7.93,6.31,;7.16,7.65,;7.93,8.98,;7.16,10.31,;7.93,11.65,;7.16,12.98,;7.93,14.32,;5.62,12.98,;9.47,6.31,;10.24,7.65,;9.47,8.98,;11.78,7.65,;12.55,8.98,;14.09,8.98,;5.62,2.31,;7.16,2.35,;4.84,.82,;5.16,-.69,;6.57,-1.31,;6.73,-2.85,;8.14,-3.47,;8.3,-5,;7.05,-5.91,;7.21,-7.44,;5.64,-5.28,;5.48,-3.75,;3.83,-1.46,;3.67,-2.99,;2.68,-.43,;-9.03,-.87,;-7.51,-.58,;-10.03,.3,)| Show InChI InChI=1S/C43H65N13O10S2/c1-4-24(2)35(42(65)66)55-40(63)33-8-6-16-56(33)41(64)32(20-26-21-47-23-49-26)54-38(61)30-14-18-68-67-17-13-29(37(60)53-31(39(62)52-30)19-25-9-11-27(57)12-10-25)51-36(59)28(50-34(58)22-46-3)7-5-15-48-43(44)45/h9-12,21,23-24,28-33,35,46,57H,4-8,13-20,22H2,1-3H3,(H,47,49)(H,50,58)(H,51,59)(H,52,62)(H,53,60)(H,54,61)(H,55,63)(H,65,66)(H4,44,45,48)/t24-,28-,29-,30-,31-,32-,33-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228335
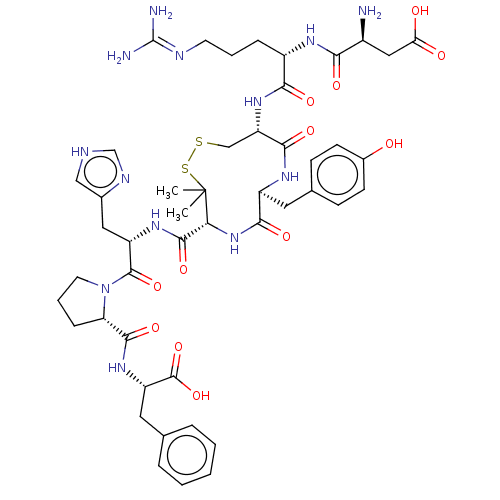
(CHEMBL263034)Show SMILES CC1(C)SSC[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:42.44,59.63,46.47,6.6,wD:63.66,30.30,21.21,10.10,(2.55,2.37,;2.49,1.14,;3.45,.38,;1.48,2.3,;,2.73,;-1.48,2.3,;-2.49,1.14,;-3.89,1.78,;-4.04,3.32,;-3.03,4.03,;-5.44,3.96,;-6.69,3.07,;-8.09,3.72,;-9.35,2.83,;-10.75,3.47,;-12.01,2.58,;-13.13,3.1,;-11.9,1.36,;-5.58,5.49,;-6.98,6.13,;-7.98,5.42,;-7.12,7.67,;-6.12,8.38,;-8.53,8.31,;-8.67,9.84,;-7.67,10.56,;-9.79,10.36,;-2.71,-.39,;-3.93,-.56,;-2.07,-1.79,;-.77,-2.62,;-1.21,-4.1,;-2.71,-4.47,;-3.77,-3.35,;-5.27,-3.7,;-5.71,-5.18,;-6.9,-5.47,;-4.65,-6.3,;-3.15,-5.94,;.77,-2.62,;1.12,-3.8,;2.06,-1.79,;2.7,-.39,;4.23,-.6,;4.7,-1.75,;5.18,.61,;6.71,.39,;7.66,1.6,;7.09,3.03,;7.91,4.32,;6.93,5.5,;5.5,4.92,;5.6,3.39,;7.29,-1.04,;6.53,-2.01,;8.82,-1.25,;9.86,-.14,;11.25,-.81,;11.04,-2.34,;9.52,-2.61,;8.83,-3.98,;9.51,-5.01,;7.3,-4.08,;6.61,-5.46,;5.07,-5.56,;4.38,-6.93,;2.85,-7.03,;2.16,-8.41,;3.02,-9.7,;4.56,-9.6,;5.24,-8.22,;7.46,-6.75,;8.69,-6.67,;6.91,-7.85,)| Show InChI InChI=1S/C47H63N13O12S2/c1-47(2)37(43(69)56-32(20-27-22-51-24-53-27)44(70)60-17-7-11-35(60)42(68)57-33(45(71)72)19-25-8-4-3-5-9-25)59-40(66)31(18-26-12-14-28(61)15-13-26)55-41(67)34(23-73-74-47)58-39(65)30(10-6-16-52-46(49)50)54-38(64)29(48)21-36(62)63/h3-5,8-9,12-15,22,24,29-35,37,61H,6-7,10-11,16-21,23,48H2,1-2H3,(H,51,53)(H,54,64)(H,55,67)(H,56,69)(H,57,68)(H,58,65)(H,59,66)(H,62,63)(H,71,72)(H4,49,50,52)/t29-,30-,31-,32-,33-,34-,35-,37+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50030815
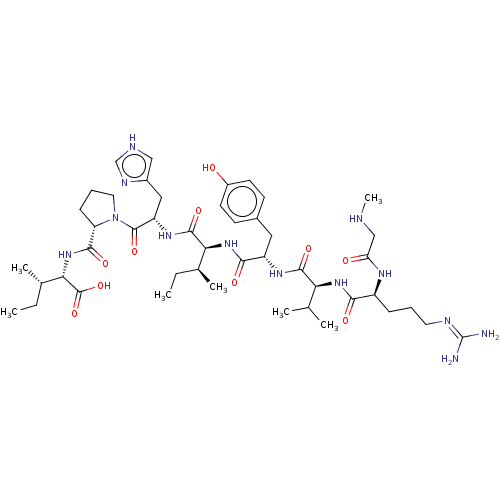
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor from rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228272
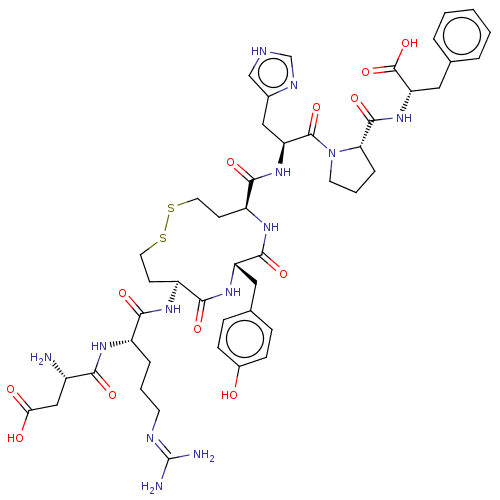
(CHEMBL411997)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CCSSCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:59.63,46.47,9.8,31.31,1.0,wD:20.19,27.44,63.66,(4.96,9.59,;6.07,9.07,;7.34,9.95,;7.21,11.48,;6.1,12.01,;8.22,12.19,;6.2,7.53,;7.31,7.01,;4.93,6.66,;5.06,5.12,;6.45,4.47,;7.72,5.35,;9.11,4.69,;10.38,5.57,;11.77,4.91,;12.78,5.62,;11.87,3.69,;3.79,4.24,;2.68,4.77,;3.92,2.71,;2.65,1.83,;1.49,2.85,;,3.22,;-1.5,2.85,;-2.65,1.83,;-3.19,.39,;-3.01,-1.14,;-2.13,-2.4,;-.77,-3.12,;.77,-3.12,;1.06,-4.32,;2.13,-2.4,;3.16,-3.57,;4.67,-3.26,;5.69,-4.41,;7.19,-4.11,;7.68,-2.65,;8.89,-2.4,;6.66,-1.5,;5.15,-1.8,;3.01,-1.14,;3.19,.39,;4.41,.54,;-3.16,-3.57,;-4.37,-3.32,;-2.67,-5.03,;-3.69,-6.18,;-3.2,-7.64,;-1.69,-7.95,;-1.07,-9.35,;.46,-9.17,;.76,-7.66,;-.58,-6.9,;-5.2,-5.88,;-5.59,-4.71,;-6.21,-7.04,;-5.86,-8.52,;-7.18,-9.31,;-8.34,-8.29,;-7.73,-6.88,;-8.51,-5.55,;-9.74,-5.56,;-7.74,-4.21,;-8.52,-2.88,;-7.75,-1.54,;-8.52,-.21,;-7.76,1.12,;-8.54,2.46,;-10.08,2.45,;-10.84,1.11,;-10.06,-.22,;-10.06,-2.88,;-10.67,-3.95,;-10.68,-1.82,)| Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
pA2 value for Angiotensin II receptor |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228308
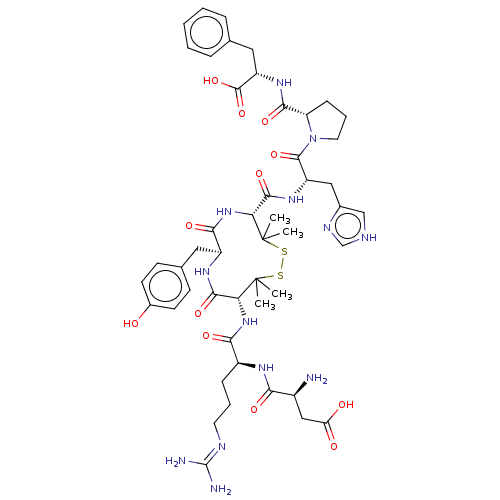
(CHEMBL413740)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]1C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:44.46,8.8,61.65,48.49,wD:65.68,32.32,23.23,12.12,(2.55,2.37,;2.49,1.14,;3.45,.38,;1.48,2.3,;,2.73,;-1.48,2.3,;-2.71,2.19,;-.86,3.37,;-2.49,1.14,;-3.89,1.78,;-4.04,3.32,;-3.03,4.03,;-5.44,3.96,;-6.7,3.07,;-8.09,3.72,;-9.35,2.83,;-10.75,3.47,;-12.01,2.58,;-13.13,3.1,;-11.9,1.36,;-5.58,5.49,;-6.98,6.14,;-7.98,5.42,;-7.12,7.67,;-6.12,8.38,;-8.53,8.31,;-8.67,9.84,;-7.67,10.56,;-9.79,10.36,;-2.71,-.39,;-3.93,-.56,;-2.07,-1.79,;-.77,-2.62,;-1.21,-4.1,;-2.71,-4.47,;-3.77,-3.35,;-5.27,-3.7,;-5.71,-5.18,;-6.9,-5.47,;-4.65,-6.3,;-3.15,-5.94,;.77,-2.62,;1.12,-3.8,;2.06,-1.79,;2.7,-.39,;4.24,-.6,;4.7,-1.75,;5.19,.61,;6.71,.39,;7.66,1.6,;7.09,3.03,;7.91,4.32,;6.93,5.5,;5.5,4.92,;5.6,3.39,;7.29,-1.04,;6.53,-2.01,;8.82,-1.25,;9.86,-.14,;11.25,-.81,;11.04,-2.34,;9.52,-2.61,;8.83,-3.98,;9.52,-5.01,;7.3,-4.08,;6.61,-5.46,;5.07,-5.56,;4.39,-6.94,;2.85,-7.03,;2.16,-8.42,;3.02,-9.7,;4.56,-9.6,;5.24,-8.22,;7.46,-6.75,;8.69,-6.67,;6.91,-7.85,)| Show InChI InChI=1S/C49H67N13O12S2/c1-48(2)37(60-40(67)31(12-8-18-54-47(51)52)56-39(66)30(50)23-36(64)65)43(70)57-32(20-27-14-16-29(63)17-15-27)41(68)61-38(49(3,4)76-75-48)44(71)58-33(22-28-24-53-25-55-28)45(72)62-19-9-13-35(62)42(69)59-34(46(73)74)21-26-10-6-5-7-11-26/h5-7,10-11,14-17,24-25,30-35,37-38,63H,8-9,12-13,18-23,50H2,1-4H3,(H,53,55)(H,56,66)(H,57,70)(H,58,71)(H,59,69)(H,60,67)(H,61,68)(H,64,65)(H,73,74)(H4,51,52,54)/t30-,31-,32-,33-,34-,35-,37+,38+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044356
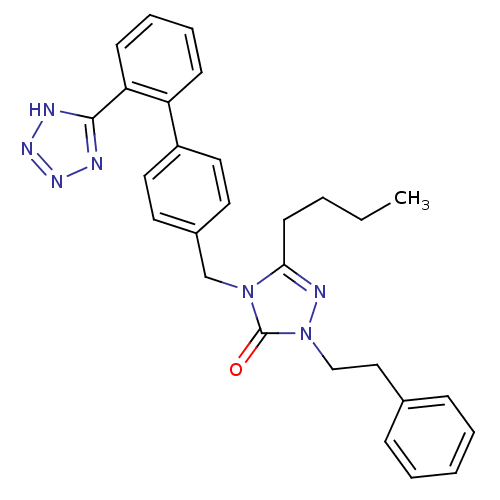
(5-Butyl-2-phenethyl-4-[2'-(1H-tetrazol-5-yl)-biphe...)Show SMILES CCCCc1nn(CCc2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H29N7O/c1-2-3-13-26-31-35(19-18-21-9-5-4-6-10-21)28(36)34(26)20-22-14-16-23(17-15-22)24-11-7-8-12-25(24)27-29-32-33-30-27/h4-12,14-17H,2-3,13,18-20H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044345

(5-Butyl-2-(2-hydroxy-2-phenyl-ethyl)-4-[2'-(1H-tet...)Show SMILES CCCCc1nn(CC(O)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H29N7O2/c1-2-3-13-26-31-35(19-25(36)22-9-5-4-6-10-22)28(37)34(26)18-20-14-16-21(17-15-20)23-11-7-8-12-24(23)27-29-32-33-30-27/h4-12,14-17,25,36H,2-3,13,18-19H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044334
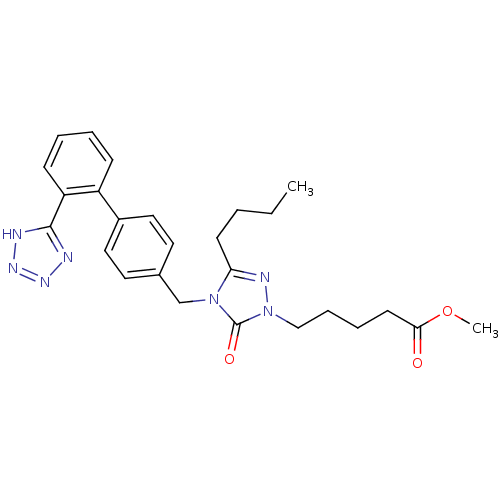
(5-{3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCCCC(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H31N7O3/c1-3-4-11-23-29-33(17-8-7-12-24(34)36-2)26(35)32(23)18-19-13-15-20(16-14-19)21-9-5-6-10-22(21)25-27-30-31-28-25/h5-6,9-10,13-16H,3-4,7-8,11-12,17-18H2,1-2H3,(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044355

(2,5-Dibutyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCCCc1nn(CCCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-3-5-11-22-27-31(16-6-4-2)24(32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15H,3-6,11,16-17H2,1-2H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125-I]-labeled angiotensin II from Angiotensin II receptor, type 1 of rat uterine membranes |
Bioorg Med Chem Lett 4: 2591-2596 (1994)
Article DOI: 10.1016/S0960-894X(01)80290-0
BindingDB Entry DOI: 10.7270/Q29Z94VB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044355

(2,5-Dibutyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCCCc1nn(CCCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-3-5-11-22-27-31(16-6-4-2)24(32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15H,3-6,11,16-17H2,1-2H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044357
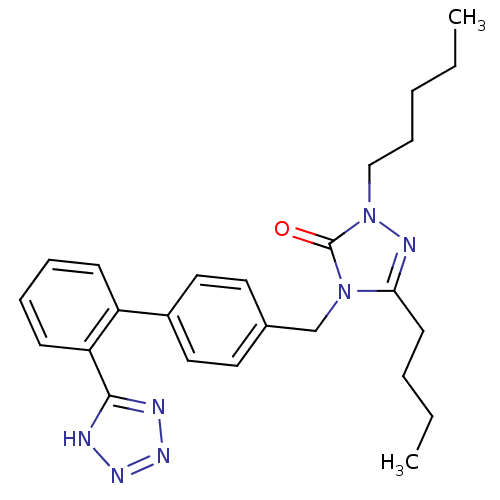
(5-Butyl-2-pentyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCCn1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c1=O Show InChI InChI=1S/C25H31N7O/c1-3-5-9-17-32-25(33)31(23(28-32)12-6-4-2)18-19-13-15-20(16-14-19)21-10-7-8-11-22(21)24-26-29-30-27-24/h7-8,10-11,13-16H,3-6,9,12,17-18H2,1-2H3,(H,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50046078
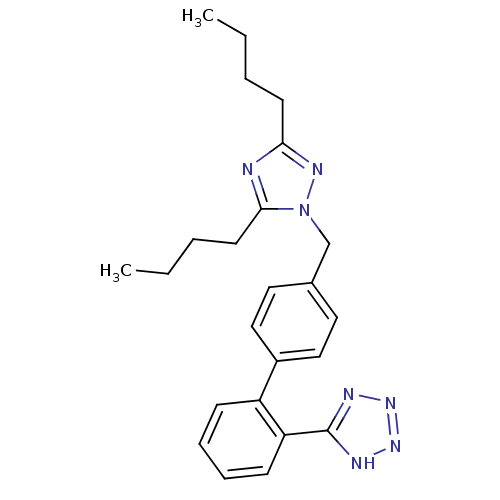
(5-[4'-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-biph...)Show SMILES CCCCc1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C24H29N7/c1-3-5-11-22-25-23(12-6-4-2)31(28-22)17-18-13-15-19(16-14-18)20-9-7-8-10-21(20)24-26-29-30-27-24/h7-10,13-16H,3-6,11-12,17H2,1-2H3,(H,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle & Company
Curated by ChEMBL
| Assay Description
Activity against high affinity Angiotensin II receptor, type 1 was measured from the ability to inhibit [125I]-angiotensin II binding to rat uterine ... |
J Med Chem 36: 101-10 (1993)
BindingDB Entry DOI: 10.7270/Q2KH0MDW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044348
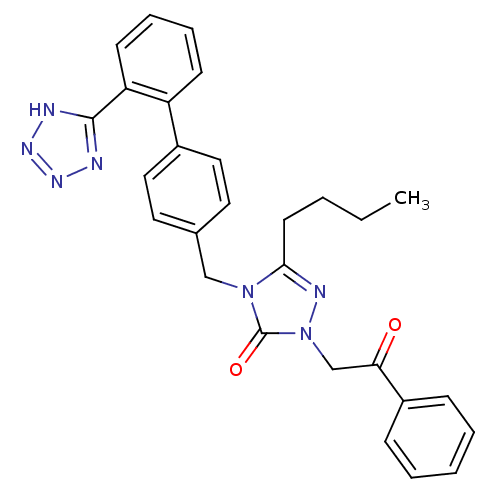
(5-Butyl-2-(2-oxo-2-phenyl-ethyl)-4-[2'-(1H-tetrazo...)Show SMILES CCCCc1nn(CC(=O)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H27N7O2/c1-2-3-13-26-31-35(19-25(36)22-9-5-4-6-10-22)28(37)34(26)18-20-14-16-21(17-15-20)23-11-7-8-12-24(23)27-29-32-33-30-27/h4-12,14-17H,2-3,13,18-19H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044367
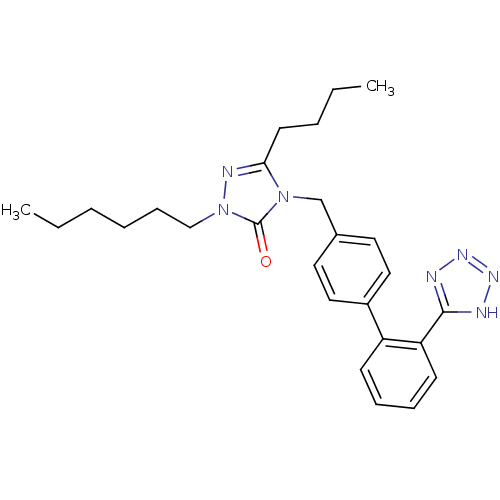
(5-Butyl-2-hexyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-...)Show SMILES CCCCCCn1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c1=O Show InChI InChI=1S/C26H33N7O/c1-3-5-7-10-18-33-26(34)32(24(29-33)13-6-4-2)19-20-14-16-21(17-15-20)22-11-8-9-12-23(22)25-27-30-31-28-25/h8-9,11-12,14-17H,3-7,10,13,18-19H2,1-2H3,(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044361

(CHEMBL67764 | {3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-...)Show SMILES CCCCc1nn(CC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H27N7O3/c1-3-5-10-21-27-31(16-22(32)34-4-2)24(33)30(21)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-28-29-26-23/h6-9,11-14H,3-5,10,15-16H2,1-2H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044338
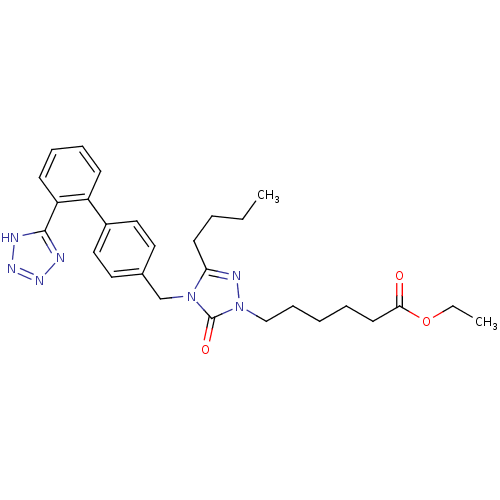
(6-{3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCCCCC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H35N7O3/c1-3-5-13-25-31-35(19-10-6-7-14-26(36)38-4-2)28(37)34(25)20-21-15-17-22(18-16-21)23-11-8-9-12-24(23)27-29-32-33-30-27/h8-9,11-12,15-18H,3-7,10,13-14,19-20H2,1-2H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228309

(CHEMBL408560)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CCSSC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:58.62,45.46,9.8,30.30,1.0,wD:26.43,20.19,62.65,(5.51,9.04,;6.57,8.43,;7.91,9.21,;7.91,10.75,;6.84,11.36,;8.97,11.36,;6.57,6.89,;7.64,6.28,;5.24,6.12,;5.24,4.58,;6.58,3.82,;7.91,4.59,;9.25,3.82,;10.58,4.59,;11.92,3.83,;12.99,4.45,;11.93,2.6,;3.91,3.81,;2.84,4.42,;3.91,2.27,;2.58,1.49,;1.48,2.58,;,2.98,;-1.49,2.58,;-2.58,1.49,;-2.98,,;-2.58,-1.48,;-1.49,-2.58,;,-2.97,;,-4.21,;1.48,-2.58,;2.26,-3.92,;1.48,-5.25,;2.25,-6.59,;1.48,-7.92,;-.06,-7.91,;-.68,-8.98,;-.83,-6.57,;-.06,-5.24,;2.58,-1.48,;2.97,,;4.21,,;-3.92,-2.26,;-4.99,-1.65,;-3.91,-3.8,;-5.25,-4.58,;-5.24,-6.12,;-3.91,-6.89,;-3.76,-8.41,;-2.26,-8.72,;-1.49,-7.39,;-2.52,-6.25,;-6.58,-3.81,;-6.59,-2.58,;-7.91,-4.59,;-8.05,-6.1,;-9.55,-6.43,;-10.33,-5.1,;-9.3,-3.96,;-9.62,-2.45,;-10.79,-2.06,;-8.47,-1.42,;-8.78,.09,;-7.63,1.11,;-7.94,2.62,;-6.8,3.65,;-7.11,5.15,;-8.58,5.63,;-9.72,4.6,;-9.41,3.1,;-10.25,.57,;-11.16,-.25,;-10.49,1.78,)| Show InChI InChI=1S/C46H61N13O12S2/c47-29(21-37(61)62)38(63)53-30(8-4-15-51-46(48)49)39(64)54-31-14-17-72-73-23-35(58-41(66)32(55-40(31)65)18-26-10-12-28(60)13-11-26)42(67)56-33(20-27-22-50-24-52-27)44(69)59-16-5-9-36(59)43(68)57-34(45(70)71)19-25-6-2-1-3-7-25/h1-3,6-7,10-13,22,24,29-36,60H,4-5,8-9,14-21,23,47H2,(H,50,52)(H,53,63)(H,54,64)(H,55,65)(H,56,67)(H,57,68)(H,58,66)(H,61,62)(H,70,71)(H4,48,49,51)/t29-,30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044366
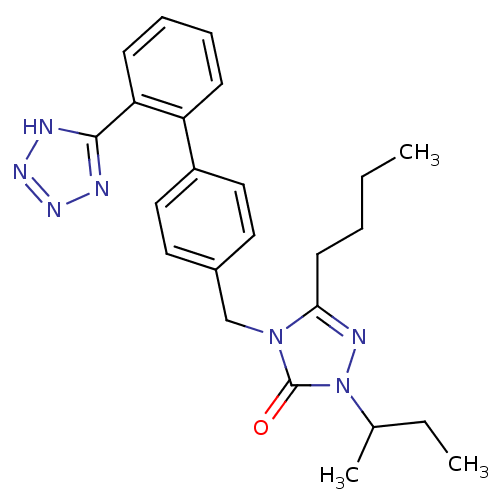
(5-Butyl-2-sec-butyl-4-[2'-(1H-tetrazol-5-yl)-biphe...)Show SMILES CCCCc1nn(C(C)CC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-4-6-11-22-27-31(17(3)5-2)24(32)30(22)16-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15,17H,4-6,11,16H2,1-3H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50283548
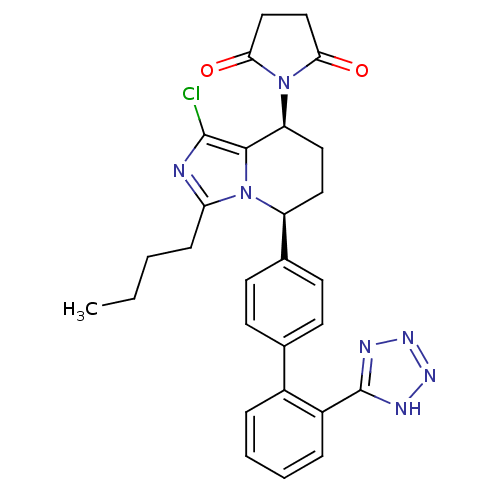
(1-{(5S,8S)-3-Butyl-1-chloro-5-[2'-(2H-tetrazol-5-y...)Show SMILES CCCCc1nc(Cl)c2[C@H](CC[C@@H](c3ccc(cc3)-c3ccccc3-c3nnn[nH]3)n12)N1C(=O)CCC1=O Show InChI InChI=1S/C28H28ClN7O2/c1-2-3-8-23-30-27(29)26-22(36-24(37)15-16-25(36)38)14-13-21(35(23)26)18-11-9-17(10-12-18)19-6-4-5-7-20(19)28-31-33-34-32-28/h4-7,9-12,21-22H,2-3,8,13-16H2,1H3,(H,31,32,33,34)/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125-I]-labeled angiotensin II binding to AT1 receptor in rat uterine membranes |
Bioorg Med Chem Lett 4: 2591-2596 (1994)
Article DOI: 10.1016/S0960-894X(01)80290-0
BindingDB Entry DOI: 10.7270/Q29Z94VB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044369

(5-{3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCCCC(O)=O)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H29N7O3/c1-2-3-10-22-28-32(16-7-6-11-23(33)34)25(35)31(22)17-18-12-14-19(15-13-18)20-8-4-5-9-21(20)24-26-29-30-27-24/h4-5,8-9,12-15H,2-3,6-7,10-11,16-17H2,1H3,(H,33,34)(H,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044365
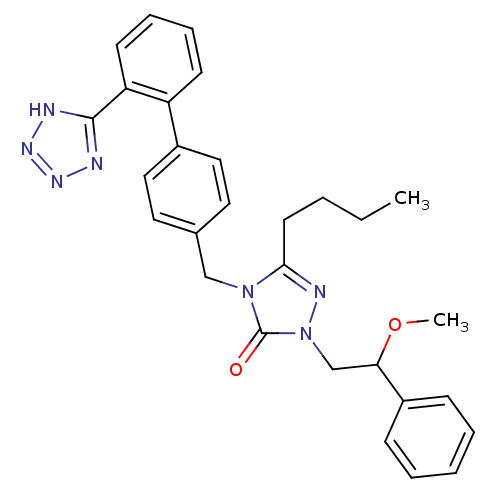
(5-Butyl-2-(2-methoxy-2-phenyl-ethyl)-4-[2'-(2H-tet...)Show SMILES CCCCc1nn(CC(OC)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H31N7O2/c1-3-4-14-27-32-36(20-26(38-2)23-10-6-5-7-11-23)29(37)35(27)19-21-15-17-22(18-16-21)24-12-8-9-13-25(24)28-30-33-34-31-28/h5-13,15-18,26H,3-4,14,19-20H2,1-2H3,(H,30,31,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228312
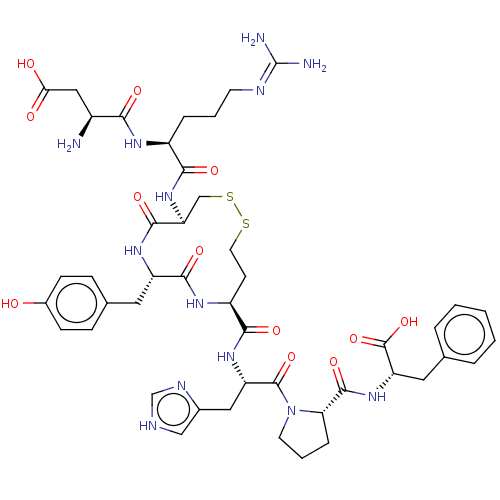
(CHEMBL405557)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:58.62,45.46,9.8,30.30,1.0,wD:20.19,26.43,62.65,(5.51,9.04,;6.57,8.43,;7.91,9.21,;7.91,10.75,;6.84,11.36,;8.97,11.36,;6.57,6.89,;7.64,6.28,;5.24,6.12,;5.24,4.58,;6.58,3.82,;7.91,4.59,;9.25,3.82,;10.58,4.59,;11.92,3.83,;12.99,4.45,;11.93,2.6,;3.91,3.81,;2.84,4.42,;3.91,2.27,;2.58,1.49,;1.48,2.58,;,2.98,;-1.49,2.58,;-2.58,1.49,;-2.98,,;-2.58,-1.48,;-1.49,-2.58,;,-2.97,;,-4.21,;1.48,-2.58,;2.26,-3.92,;1.48,-5.25,;2.25,-6.59,;1.48,-7.92,;-.06,-7.91,;-.68,-8.98,;-.83,-6.57,;-.06,-5.24,;2.58,-1.48,;2.97,,;4.21,,;-3.92,-2.26,;-4.99,-1.65,;-3.91,-3.8,;-5.25,-4.58,;-5.24,-6.12,;-3.91,-6.89,;-3.76,-8.41,;-2.26,-8.72,;-1.49,-7.39,;-2.52,-6.25,;-6.58,-3.81,;-6.59,-2.58,;-7.91,-4.59,;-8.05,-6.1,;-9.55,-6.43,;-10.33,-5.1,;-9.3,-3.96,;-9.62,-2.45,;-10.79,-2.06,;-8.47,-1.42,;-8.78,.09,;-7.63,1.11,;-7.94,2.62,;-6.8,3.65,;-7.11,5.15,;-8.58,5.63,;-9.72,4.6,;-9.41,3.1,;-10.25,.57,;-11.16,-.25,;-10.49,1.78,)| Show InChI InChI=1S/C46H61N13O12S2/c47-29(21-37(61)62)38(63)53-30(8-4-15-51-46(48)49)39(64)58-35-23-73-72-17-14-31(54-41(66)32(55-42(35)67)18-26-10-12-28(60)13-11-26)40(65)56-33(20-27-22-50-24-52-27)44(69)59-16-5-9-36(59)43(68)57-34(45(70)71)19-25-6-2-1-3-7-25/h1-3,6-7,10-13,22,24,29-36,60H,4-5,8-9,14-21,23,47H2,(H,50,52)(H,53,63)(H,54,66)(H,55,67)(H,56,65)(H,57,68)(H,58,64)(H,61,62)(H,70,71)(H4,48,49,51)/t29-,30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044346
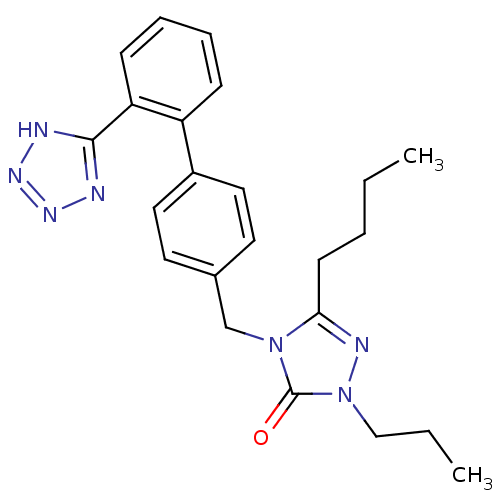
(5-Butyl-2-propyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H27N7O/c1-3-5-10-21-26-30(15-4-2)23(31)29(21)16-17-11-13-18(14-12-17)19-8-6-7-9-20(19)22-24-27-28-25-22/h6-9,11-14H,3-5,10,15-16H2,1-2H3,(H,24,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50283551
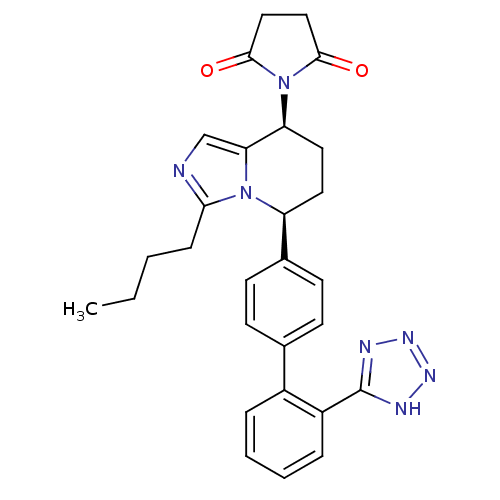
(1-{(5S,8S)-3-Butyl-5-[2'-(2H-tetrazol-5-yl)-biphen...)Show SMILES CCCCc1ncc2[C@H](CC[C@@H](c3ccc(cc3)-c3ccccc3-c3nnn[nH]3)n12)N1C(=O)CCC1=O Show InChI InChI=1S/C28H29N7O2/c1-2-3-8-25-29-17-24-23(35-26(36)15-16-27(35)37)14-13-22(34(24)25)19-11-9-18(10-12-19)20-6-4-5-7-21(20)28-30-32-33-31-28/h4-7,9-12,17,22-23H,2-3,8,13-16H2,1H3,(H,30,31,32,33)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125-I]-labeled angiotensin II binding to AT1 receptor in rat uterine membranes |
Bioorg Med Chem Lett 4: 2591-2596 (1994)
Article DOI: 10.1016/S0960-894X(01)80290-0
BindingDB Entry DOI: 10.7270/Q29Z94VB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044335
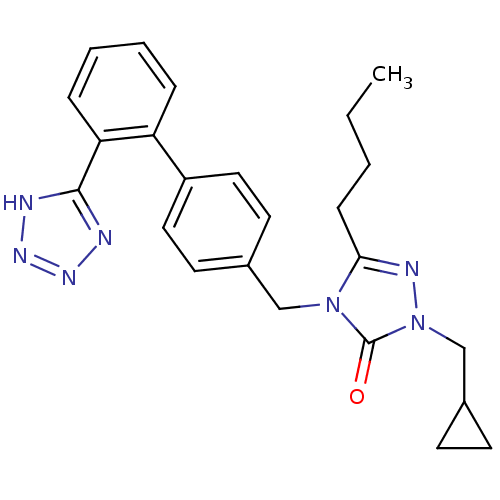
(5-Butyl-2-cyclopropylmethyl-4-[2'-(1H-tetrazol-5-y...)Show SMILES CCCCc1nn(CC2CC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H27N7O/c1-2-3-8-22-27-31(16-18-9-10-18)24(32)30(22)15-17-11-13-19(14-12-17)20-6-4-5-7-21(20)23-25-28-29-26-23/h4-7,11-14,18H,2-3,8-10,15-16H2,1H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228271

(CHEMBL268506)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CS)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:63.65,8.8,20.21,2.2,wD:59.62,4.4,46.46,37.38,26.27,(3.98,-9.71,;3.98,-8.48,;5.32,-7.71,;6.38,-8.33,;5.33,-6.17,;3.99,-5.4,;4,-3.86,;5.07,-3.24,;2.66,-3.08,;2.67,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.34,.77,;-2.4,1.39,;-1.34,-.77,;,-1.54,;1.33,-3.86,;1.33,-5.4,;2.4,-6.01,;-.01,-6.17,;-1.34,-5.4,;-2.41,-6.02,;-.01,-7.71,;-1.34,-8.48,;-2.41,-7.86,;-1.34,-10.02,;-.01,-10.79,;-.01,-12.34,;1.32,-13.11,;1.32,-14.65,;2.65,-15.42,;2.65,-16.65,;3.72,-14.81,;-2.68,-10.79,;-2.68,-12.33,;-1.61,-12.95,;-4.01,-13.1,;-5.08,-12.49,;-4.01,-14.64,;-5.34,-15.42,;-6.41,-14.8,;-5.35,-16.65,;6.66,-5.4,;6.67,-4.17,;8,-6.18,;9.33,-5.41,;10.67,-6.18,;10.67,-7.72,;11.91,-8.61,;11.43,-10.08,;9.89,-10.07,;9.42,-8.61,;9.34,-3.87,;8.28,-3.25,;10.68,-3.1,;12.06,-3.75,;13.1,-2.61,;12.34,-1.27,;10.83,-1.58,;9.68,-.56,;9.93,.65,;8.22,-1.04,;7.07,-.01,;5.61,-.49,;4.45,.53,;2.99,.06,;1.84,1.08,;2.16,2.59,;3.62,3.07,;4.77,2.04,;7.38,1.5,;8.55,1.88,;6.46,2.32,)| Show InChI InChI=1S/C48H67N13O12S/c1-3-26(2)39(45(70)57-34(21-29-23-52-25-54-29)46(71)61-18-8-12-37(61)44(69)58-35(47(72)73)20-27-9-5-4-6-10-27)60-42(67)33(19-28-13-15-30(62)16-14-28)56-43(68)36(24-74)59-41(66)32(11-7-17-53-48(50)51)55-40(65)31(49)22-38(63)64/h4-6,9-10,13-16,23,25-26,31-37,39,62,74H,3,7-8,11-12,17-22,24,49H2,1-2H3,(H,52,54)(H,55,65)(H,56,68)(H,57,70)(H,58,69)(H,59,66)(H,60,67)(H,63,64)(H,72,73)(H4,50,51,53)/t26-,31-,32-,33-,34-,35-,36-,37-,39-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044341
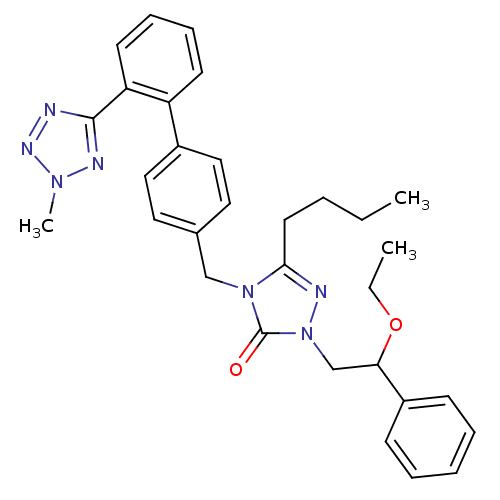
(5-Butyl-2-(2-ethoxy-2-phenyl-ethyl)-4-[2'-(2-methy...)Show SMILES CCCCc1nn(CC(OCC)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn(C)n1 Show InChI InChI=1S/C31H35N7O2/c1-4-6-16-29-33-38(22-28(40-5-2)25-12-8-7-9-13-25)31(39)37(29)21-23-17-19-24(20-18-23)26-14-10-11-15-27(26)30-32-35-36(3)34-30/h7-15,17-20,28H,4-6,16,21-22H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044332
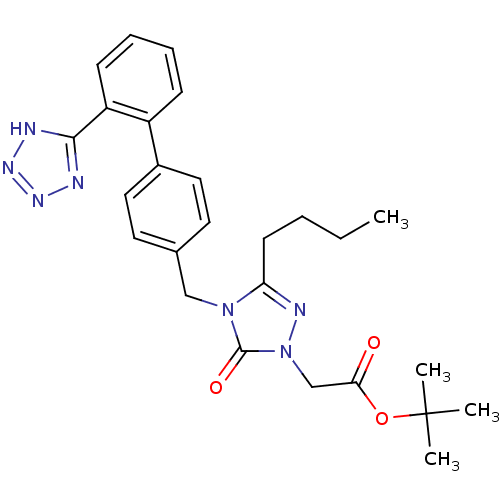
(CHEMBL307310 | {3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5...)Show SMILES CCCCc1nn(CC(=O)OC(C)(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H31N7O3/c1-5-6-11-22-29-33(17-23(34)36-26(2,3)4)25(35)32(22)16-18-12-14-19(15-13-18)20-9-7-8-10-21(20)24-27-30-31-28-24/h7-10,12-15H,5-6,11,16-17H2,1-4H3,(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044344

(2-Benzyl-5-butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(Cc2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H27N7O/c1-2-3-13-25-30-34(19-20-9-5-4-6-10-20)27(35)33(25)18-21-14-16-22(17-15-21)23-11-7-8-12-24(23)26-28-31-32-29-26/h4-12,14-17H,2-3,13,18-19H2,1H3,(H,28,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044340
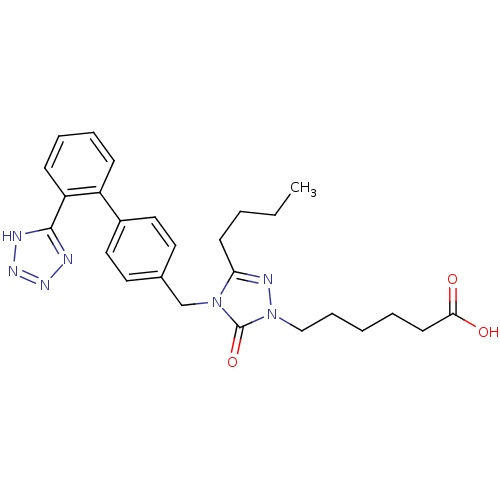
(6-{3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCCCCC(O)=O)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H31N7O3/c1-2-3-11-23-29-33(17-8-4-5-12-24(34)35)26(36)32(23)18-19-13-15-20(16-14-19)21-9-6-7-10-22(21)25-27-30-31-28-25/h6-7,9-10,13-16H,2-5,8,11-12,17-18H2,1H3,(H,34,35)(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044364

(5-Butyl-2-(3-phenyl-propyl)-4-[2'-(1H-tetrazol-5-y...)Show SMILES CCCCc1nn(CCCc2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H31N7O/c1-2-3-15-27-32-36(20-9-12-22-10-5-4-6-11-22)29(37)35(27)21-23-16-18-24(19-17-23)25-13-7-8-14-26(25)28-30-33-34-31-28/h4-8,10-11,13-14,16-19H,2-3,9,12,15,20-21H2,1H3,(H,30,31,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044333
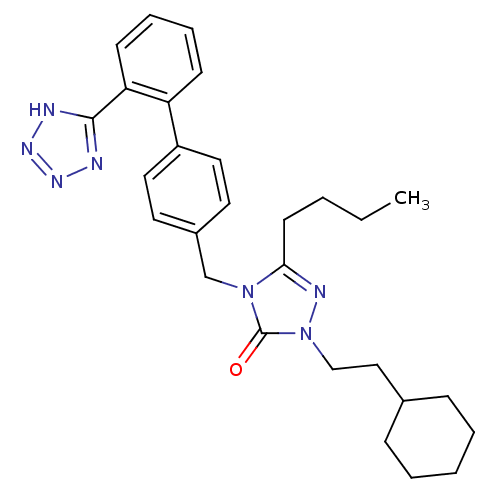
(5-Butyl-2-(2-cyclohexyl-ethyl)-4-[2'-(1H-tetrazol-...)Show SMILES CCCCc1nn(CCC2CCCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H35N7O/c1-2-3-13-26-31-35(19-18-21-9-5-4-6-10-21)28(36)34(26)20-22-14-16-23(17-15-22)24-11-7-8-12-25(24)27-29-32-33-30-27/h7-8,11-12,14-17,21H,2-6,9-10,13,18-20H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044351
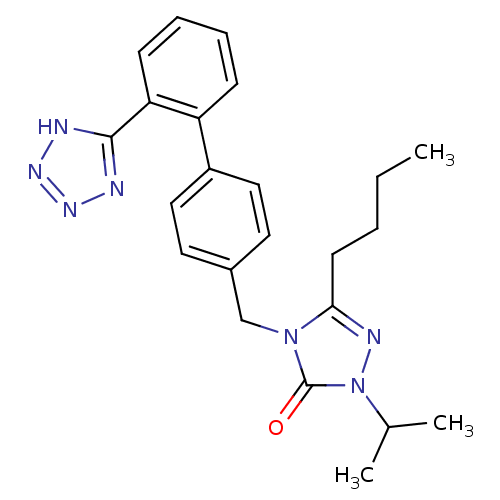
(5-Butyl-2-isopropyl-4-[2'-(1H-tetrazol-5-yl)-biphe...)Show SMILES CCCCc1nn(C(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H27N7O/c1-4-5-10-21-26-30(16(2)3)23(31)29(21)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)22-24-27-28-25-22/h6-9,11-14,16H,4-5,10,15H2,1-3H3,(H,24,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228310
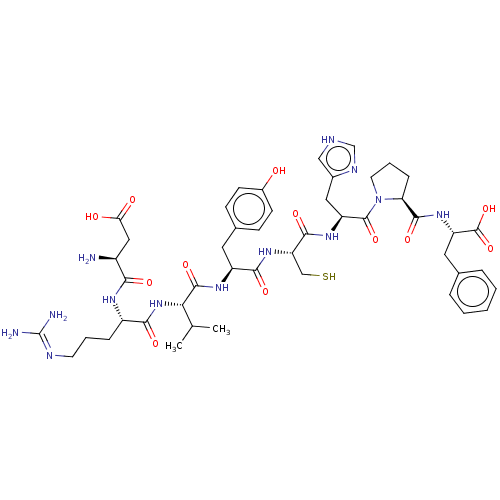
(CHEMBL403983)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:58.61,45.45,39.39,27.26,18.18,7.7,wD:3.3,62.64,(-2.42,-5.99,;-1.35,-5.38,;-.29,-6,;-1.34,-3.84,;-2.68,-3.06,;-4.01,-3.83,;-4.02,-5.06,;-5.35,-3.05,;-5.34,-1.51,;-6.67,-.74,;-6.67,.8,;-8,1.57,;-8,3.11,;-9.06,3.73,;-6.93,3.73,;-6.68,-3.82,;-8.01,-3.05,;-8.01,-1.81,;-9.35,-3.81,;-9.35,-5.04,;-10.68,-3.04,;-12.02,-3.8,;-12.02,-5.03,;-13.08,-3.18,;-.01,-3.07,;-0,-1.84,;1.33,-3.85,;2.66,-3.08,;2.67,-1.54,;4,-.77,;5.33,-1.54,;6.66,-.78,;6.67,.76,;7.74,1.37,;5.34,1.54,;4,.77,;4,-3.85,;5.06,-3.24,;3.99,-5.39,;5.33,-6.17,;6.66,-5.4,;6.66,-4.17,;5.33,-7.71,;4.26,-8.32,;6.66,-8.48,;6.66,-10.02,;7.99,-10.8,;9.33,-10.03,;10.71,-10.67,;11.74,-9.53,;10.97,-8.19,;9.47,-8.51,;5.32,-10.79,;4.25,-10.18,;5.32,-12.34,;6.57,-13.21,;6.1,-14.68,;4.56,-14.69,;4.08,-13.22,;2.62,-12.73,;1.69,-13.55,;2.31,-11.22,;.85,-10.74,;.53,-9.23,;-.92,-8.74,;-1.24,-7.23,;-2.7,-6.75,;-3.85,-7.77,;-3.54,-9.28,;-2.07,-9.76,;-.31,-11.76,;-.06,-12.96,;-1.48,-11.37,)| Show InChI InChI=1S/C47H65N13O12S/c1-25(2)38(59-40(65)31(10-6-16-52-47(49)50)54-39(64)30(48)21-37(62)63)44(69)55-32(18-27-12-14-29(61)15-13-27)41(66)58-35(23-73)42(67)56-33(20-28-22-51-24-53-28)45(70)60-17-7-11-36(60)43(68)57-34(46(71)72)19-26-8-4-3-5-9-26/h3-5,8-9,12-15,22,24-25,30-36,38,61,73H,6-7,10-11,16-21,23,48H2,1-2H3,(H,51,53)(H,54,64)(H,55,69)(H,56,67)(H,57,68)(H,58,66)(H,59,65)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044347
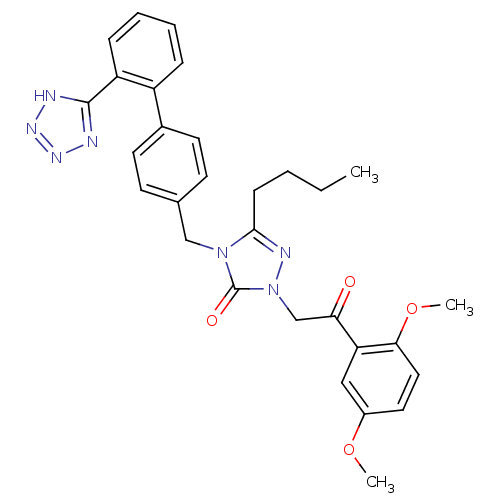
(5-Butyl-2-[2-(2,5-dimethoxy-phenyl)-2-oxo-ethyl]-4...)Show SMILES CCCCc1nn(CC(=O)c2cc(OC)ccc2OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H31N7O4/c1-4-5-10-28-33-37(19-26(38)25-17-22(40-2)15-16-27(25)41-3)30(39)36(28)18-20-11-13-21(14-12-20)23-8-6-7-9-24(23)29-31-34-35-32-29/h6-9,11-17H,4-5,10,18-19H2,1-3H3,(H,31,32,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044370
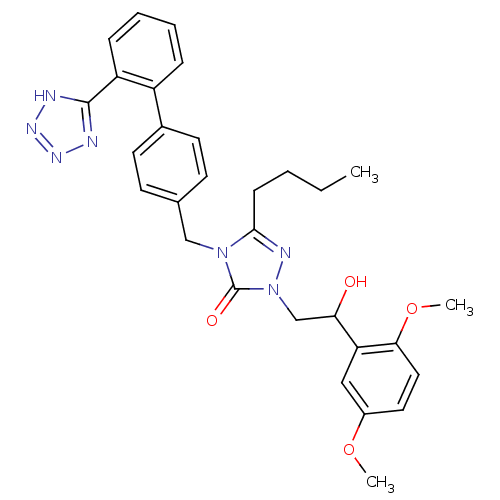
(5-Butyl-2-[2-(2,5-dimethoxy-phenyl)-2-hydroxy-ethy...)Show SMILES CCCCc1nn(CC(O)c2cc(OC)ccc2OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H33N7O4/c1-4-5-10-28-33-37(19-26(38)25-17-22(40-2)15-16-27(25)41-3)30(39)36(28)18-20-11-13-21(14-12-20)23-8-6-7-9-24(23)29-31-34-35-32-29/h6-9,11-17,26,38H,4-5,10,18-19H2,1-3H3,(H,31,32,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044343
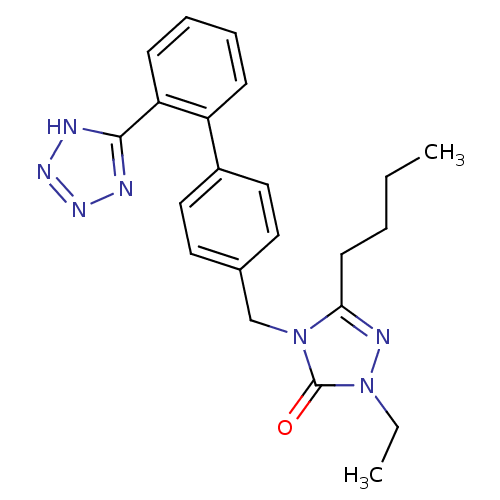
(5-Butyl-2-ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-...)Show SMILES CCCCc1nn(CC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H25N7O/c1-3-5-10-20-25-29(4-2)22(30)28(20)15-16-11-13-17(14-12-16)18-8-6-7-9-19(18)21-23-26-27-24-21/h6-9,11-14H,3-5,10,15H2,1-2H3,(H,23,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044342
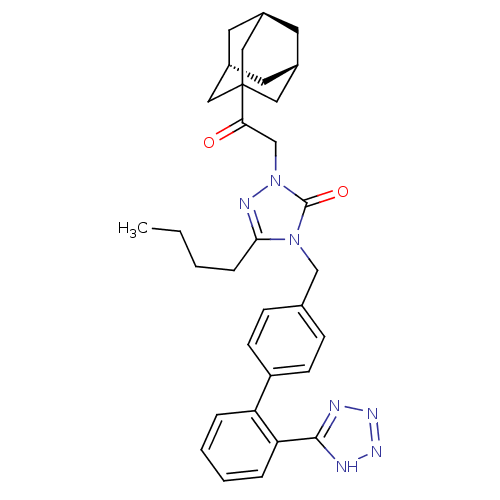
(2-(2-Adamantan-1-yl-2-oxo-ethyl)-5-butyl-4-[2'-(1H...)Show SMILES CCCCc1nn(CC(=O)C23C[C@H]4C[C@H](C[C@H](C4)C2)C3)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |TLB:17:16:19:11.12.13,17:12:19:18.16.15| Show InChI InChI=1S/C32H37N7O2/c1-2-3-8-29-35-39(20-28(40)32-16-22-13-23(17-32)15-24(14-22)18-32)31(41)38(29)19-21-9-11-25(12-10-21)26-6-4-5-7-27(26)30-33-36-37-34-30/h4-7,9-12,22-24H,2-3,8,13-20H2,1H3,(H,33,34,36,37)/t22-,23+,24-,32? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044359
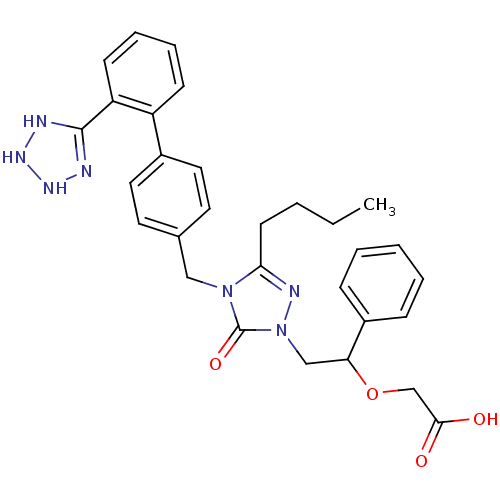
((2-{3-Butyl-4-[2'-(2,3-dihydro-1H-tetrazol-5-yl)-b...)Show SMILES CCCCc1nn(CC(OCC(O)=O)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1C1=NNNN1 |t:40| Show InChI InChI=1S/C30H33N7O4/c1-2-3-13-27-33-37(19-26(41-20-28(38)39)23-9-5-4-6-10-23)30(40)36(27)18-21-14-16-22(17-15-21)24-11-7-8-12-25(24)29-31-34-35-32-29/h4-12,14-17,26,34-35H,2-3,13,18-20H2,1H3,(H,31,32)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50046079
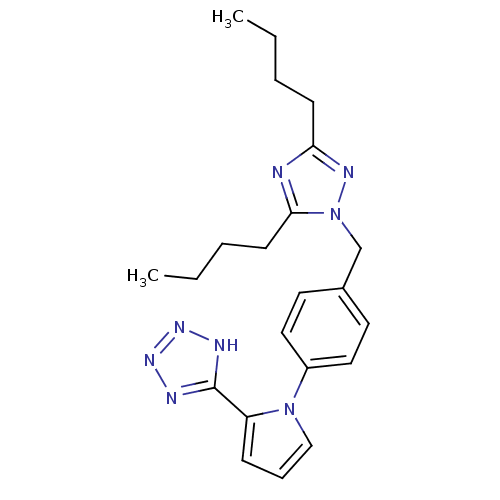
(CHEMBL12810 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol...)Show SMILES CCCCc1nc(CCCC)n(Cc2ccc(cc2)-n2cccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C22H28N8/c1-3-5-9-20-23-21(10-6-4-2)30(26-20)16-17-11-13-18(14-12-17)29-15-7-8-19(29)22-24-27-28-25-22/h7-8,11-15H,3-6,9-10,16H2,1-2H3,(H,24,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle & Company
Curated by ChEMBL
| Assay Description
Activity against high affinity Angiotensin II receptor, type 1 was measured from the ability to inhibit [125I]-angiotensin II binding to rat uterine ... |
J Med Chem 36: 101-10 (1993)
BindingDB Entry DOI: 10.7270/Q2KH0MDW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044336
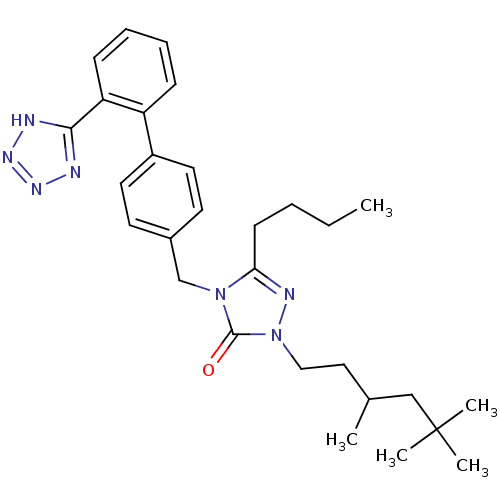
(5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCc1nn(CCC(C)CC(C)(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H39N7O/c1-6-7-12-26-32-36(18-17-21(2)19-29(3,4)5)28(37)35(26)20-22-13-15-23(16-14-22)24-10-8-9-11-25(24)27-30-33-34-31-27/h8-11,13-16,21H,6-7,12,17-20H2,1-5H3,(H,30,31,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044339
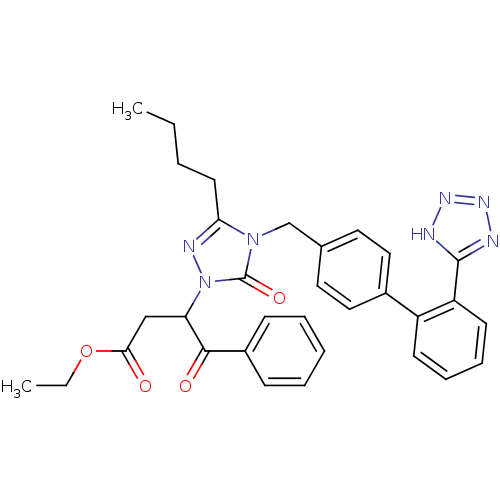
(3-{3-Butyl-5-oxo-4-[2'-(2H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(C(CC(=O)OCC)C(=O)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H33N7O4/c1-3-5-15-28-35-39(27(20-29(40)43-4-2)30(41)24-11-7-6-8-12-24)32(42)38(28)21-22-16-18-23(19-17-22)25-13-9-10-14-26(25)31-33-36-37-34-31/h6-14,16-19,27H,3-5,15,20-21H2,1-2H3,(H,33,34,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50009714
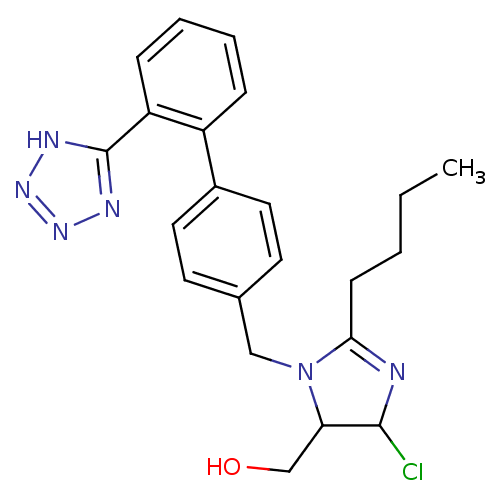
(CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...)Show SMILES CCCCC1=NC(Cl)C(CO)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C22H25ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,19,21,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044350

(5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCc1n[nH]c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C20H21N7O/c1-2-3-8-18-21-24-20(28)27(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)19-22-25-26-23-19/h4-7,9-12H,2-3,8,13H2,1H3,(H,24,28)(H,22,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044352

(5-Butyl-2-(1,1-dimethyl-2-oxo-2-phenyl-ethyl)-4-[2...)Show SMILES CCCCc1nn(c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(C)(C)C(=O)c1ccccc1 Show InChI InChI=1S/C30H31N7O2/c1-4-5-15-26-33-37(30(2,3)27(38)23-11-7-6-8-12-23)29(39)36(26)20-21-16-18-22(19-17-21)24-13-9-10-14-25(24)28-31-34-35-32-28/h6-14,16-19H,4-5,15,20H2,1-3H3,(H,31,32,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50228196
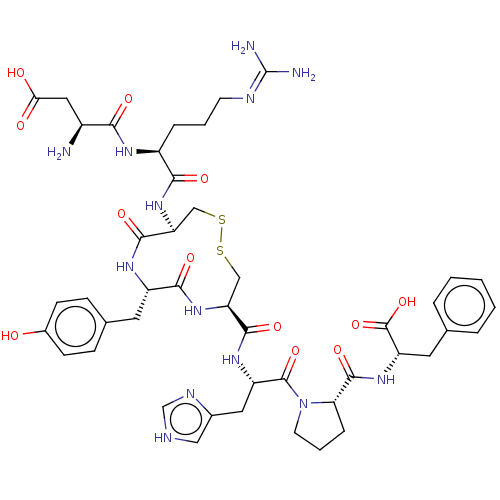
(CHEMBL405464)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:57.61,44.45,9.8,29.29,1.0,wD:25.42,20.19,61.64,(6.12,8.38,;7.12,7.67,;8.53,8.31,;8.67,9.85,;7.67,10.56,;9.79,10.36,;6.98,6.14,;7.98,5.42,;5.58,5.49,;5.44,3.96,;6.7,3.07,;8.1,3.72,;9.35,2.83,;10.75,3.47,;12.01,2.58,;13.13,3.1,;11.9,1.36,;4.03,3.32,;3.03,4.03,;3.89,1.78,;2.49,1.14,;1.48,2.3,;,2.73,;-1.48,2.3,;-2.49,1.14,;-2.71,-.39,;-2.07,-1.79,;-.77,-2.62,;-1.12,-3.8,;.77,-2.62,;1.2,-4.11,;.14,-5.22,;.56,-6.7,;-.5,-7.81,;-2,-7.45,;-2.85,-8.33,;-2.43,-5.96,;-1.36,-4.85,;2.06,-1.79,;2.7,-.39,;3.92,-.56,;-4.24,-.61,;-5,.36,;-4.81,-2.04,;-6.33,-2.26,;-6.9,-3.7,;-5.95,-4.91,;-6.38,-6.37,;-5.1,-7.23,;-3.89,-6.27,;-4.43,-4.83,;-7.29,-1.06,;-6.83,.09,;-8.81,-1.28,;-9.5,-2.64,;-11.02,-2.39,;-11.25,-.86,;-9.87,-.18,;-9.6,1.33,;-10.54,2.13,;-8.15,1.86,;-7.88,3.38,;-6.44,3.91,;-6.17,5.42,;-4.72,5.95,;-4.45,7.46,;-5.64,8.45,;-7.08,7.92,;-7.35,6.41,;-9.06,4.37,;-10.22,3.96,;-8.84,5.58,)| Show InChI InChI=1S/C45H59N13O12S2/c46-28(19-36(60)61)37(62)52-29(8-4-14-50-45(47)48)38(63)56-33-21-71-72-22-34(57-39(64)30(53-40(33)65)16-25-10-12-27(59)13-11-25)41(66)54-31(18-26-20-49-23-51-26)43(68)58-15-5-9-35(58)42(67)55-32(44(69)70)17-24-6-2-1-3-7-24/h1-3,6-7,10-13,20,23,28-35,59H,4-5,8-9,14-19,21-22,46H2,(H,49,51)(H,52,62)(H,53,65)(H,54,66)(H,55,67)(H,56,63)(H,57,64)(H,60,61)(H,69,70)(H4,47,48,50)/t28-,29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
Concentration required to 50% inhibition in specific binding of [125- I]A-II to Angiotensin II receptor in rat uterine membrane |
J Med Chem 33: 1935-40 (1990)
BindingDB Entry DOI: 10.7270/Q2R78D70 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
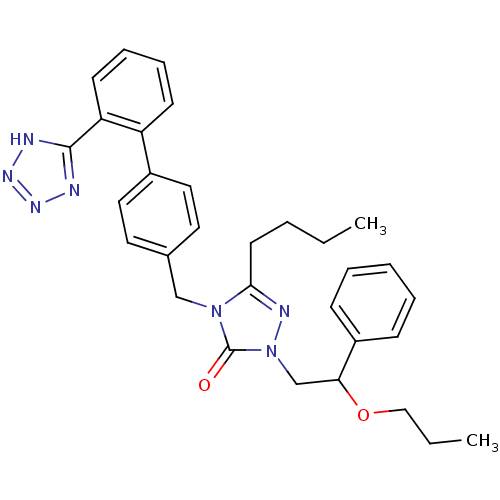
(RAT) | BDBM50044363
(5-Butyl-2-(2-phenyl-2-propoxy-ethyl)-4-[2'-(2H-tet...)Show SMILES CCCCc1nn(CC(OCCC)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H35N7O2/c1-3-5-15-29-34-38(22-28(40-20-4-2)25-11-7-6-8-12-25)31(39)37(29)21-23-16-18-24(19-17-23)26-13-9-10-14-27(26)30-32-35-36-33-30/h6-14,16-19,28H,3-5,15,20-22H2,1-2H3,(H,32,33,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
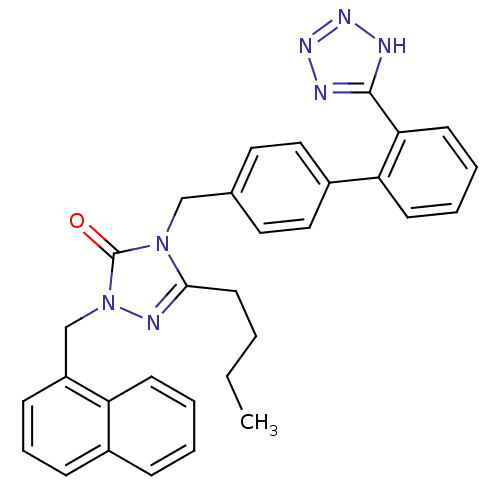
Type-1 angiotensin II receptor B
(RAT) | BDBM50044337
(5-Butyl-2-naphthalen-1-ylmethyl-4-[2'-(1H-tetrazol...)Show SMILES CCCCc1nn(Cc2cccc3ccccc23)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H29N7O/c1-2-3-15-29-34-38(21-25-11-8-10-23-9-4-5-12-26(23)25)31(39)37(29)20-22-16-18-24(19-17-22)27-13-6-7-14-28(27)30-32-35-36-33-30/h4-14,16-19H,2-3,15,20-21H2,1H3,(H,32,33,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































