 Found 55 hits with Last Name = 'robins' and Initial = 'mj'
Found 55 hits with Last Name = 'robins' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435
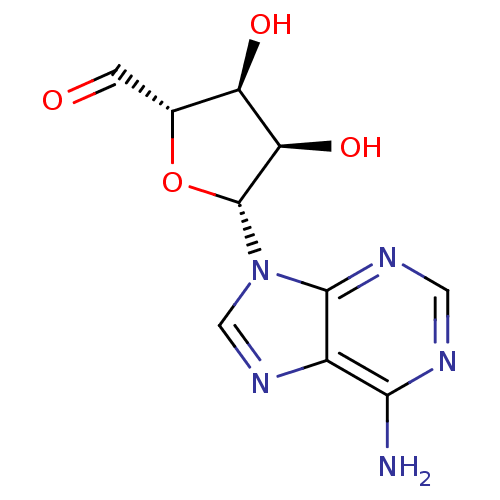
(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051435

(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50046747
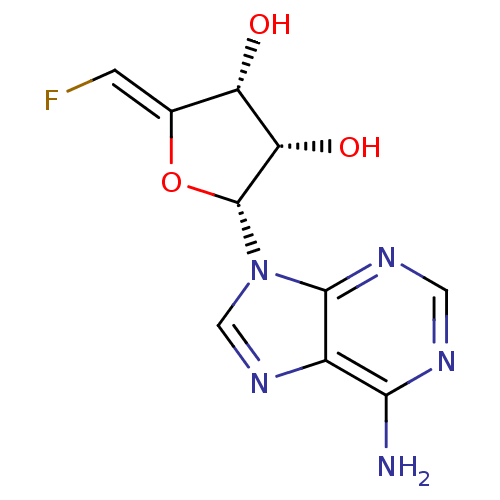
(2-(6-Amino-purin-9-yl)-5-fluoromethylene-tetrahydr...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O\C(=C/F)[C@H](O)[C@@H]1O Show InChI InChI=1S/C10H10FN5O3/c11-1-4-6(17)7(18)10(19-4)16-3-15-5-8(12)13-2-14-9(5)16/h1-3,6-7,10,17-18H,(H2,12,13,14)/b4-1-/t6-,7-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase |
J Med Chem 36: 883-7 (1993)
BindingDB Entry DOI: 10.7270/Q2HM593F |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50051436
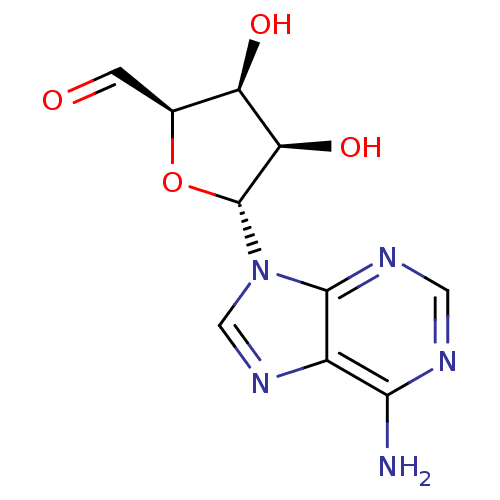
((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6+,7+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase |
J Med Chem 36: 883-7 (1993)
BindingDB Entry DOI: 10.7270/Q2HM593F |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50046748
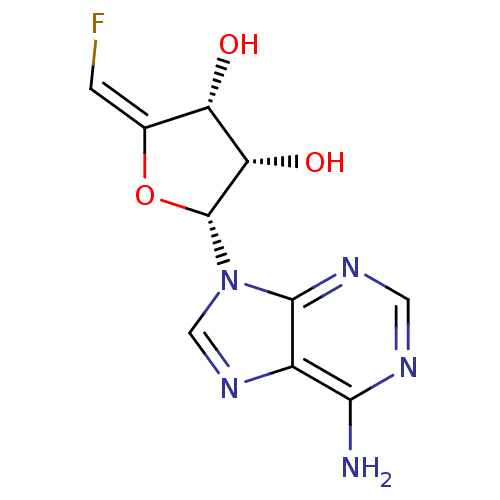
(2-(6-Amino-purin-9-yl)-5-fluoromethylene-tetrahydr...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O\C(=C\F)[C@H](O)[C@@H]1O Show InChI InChI=1S/C10H10FN5O3/c11-1-4-6(17)7(18)10(19-4)16-3-15-5-8(12)13-2-14-9(5)16/h1-3,6-7,10,17-18H,(H2,12,13,14)/b4-1+/t6-,7-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase |
J Med Chem 36: 883-7 (1993)
BindingDB Entry DOI: 10.7270/Q2HM593F |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50051435

(5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase |
J Med Chem 36: 883-7 (1993)
BindingDB Entry DOI: 10.7270/Q2HM593F |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50051436

((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H](C=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H11N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h1-4,6-7,10,17-18H,(H2,11,12,13)/t4-,6+,7+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369258
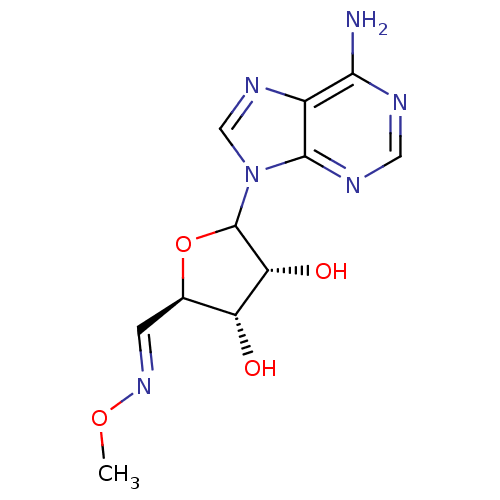
(CHEMBL606276)Show SMILES CO\N=C\[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H14N6O4/c1-20-16-2-5-7(18)8(19)11(21-5)17-4-15-6-9(12)13-3-14-10(6)17/h2-5,7-8,11,18-19H,1H3,(H2,12,13,14)/b16-2+/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50368896
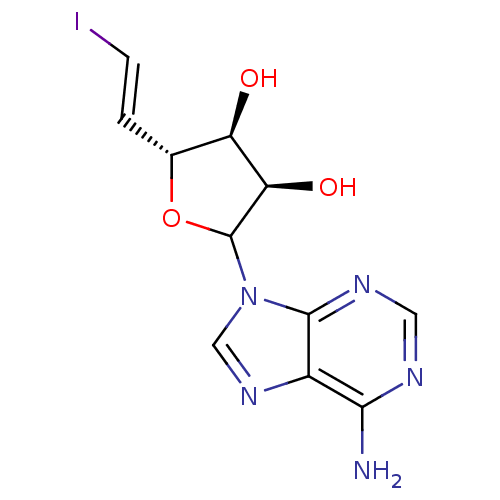
(CHEMBL608056)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=C\I)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12IN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50028378
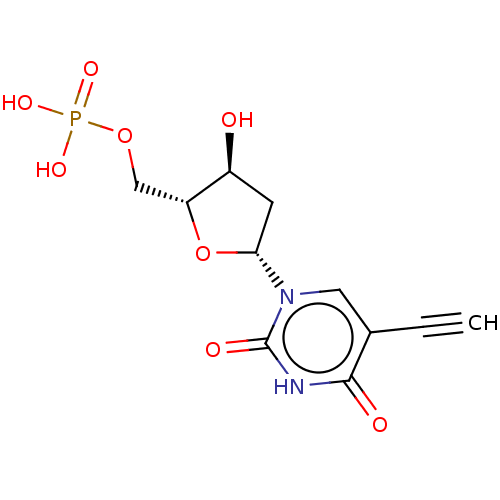
(CHEMBL3143871 | Phosphoric acid mono-[5-(5-ethynyl...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(C#C)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C11H13N2O8P/c1-2-6-4-13(11(16)12-10(6)15)9-3-7(14)8(21-9)5-20-22(17,18)19/h1,4,7-9,14H,3,5H2,(H,12,15,16)(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei |
J Med Chem 24: 1385-8 (1982)
BindingDB Entry DOI: 10.7270/Q2MS3RSP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369257
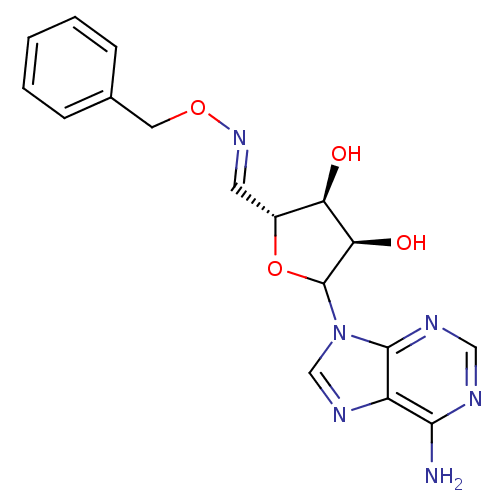
(CHEMBL605902)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=N\OCc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H18N6O4/c18-15-12-16(20-8-19-15)23(9-21-12)17-14(25)13(24)11(27-17)6-22-26-7-10-4-2-1-3-5-10/h1-6,8-9,11,13-14,17,24-25H,7H2,(H2,18,19,20)/b22-6+/t11-,13-,14-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407233
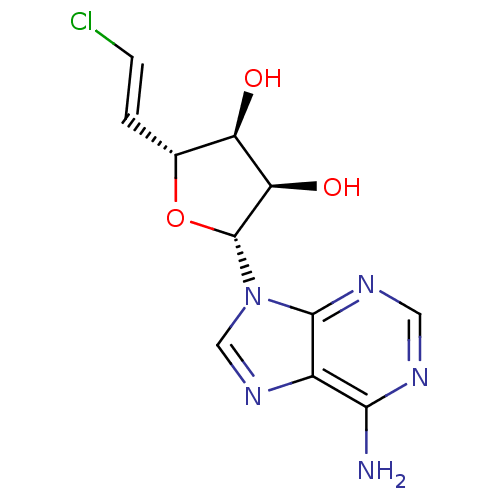
(CHEMBL2092790)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](\C=C\Cl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12ClN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369255

(CHEMBL605900)Show SMILES CCO\N=C\[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H16N6O4/c1-2-21-17-3-6-8(19)9(20)12(22-6)18-5-16-7-10(13)14-4-15-11(7)18/h3-6,8-9,12,19-20H,2H2,1H3,(H2,13,14,15)/b17-3+/t6-,8-,9-,12?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407232

(CHEMBL2092789)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](\C=C\Br)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12BrN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50010239
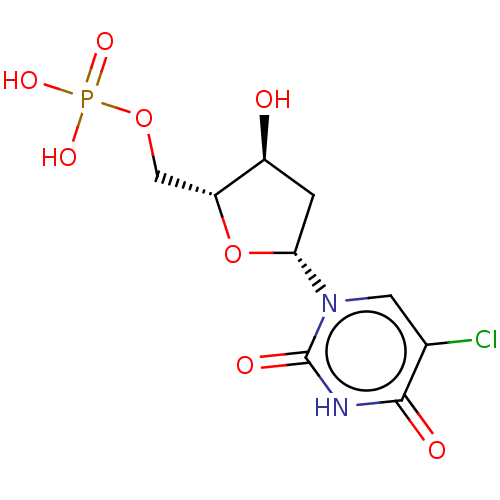
(CHEMBL1236538)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(Cl)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H12ClN2O8P/c10-4-2-12(9(15)11-8(4)14)7-1-5(13)6(20-7)3-19-21(16,17)18/h2,5-7,13H,1,3H2,(H,11,14,15)(H2,16,17,18)/t5-,6+,7+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei |
J Med Chem 24: 1385-8 (1982)
BindingDB Entry DOI: 10.7270/Q2MS3RSP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50408149
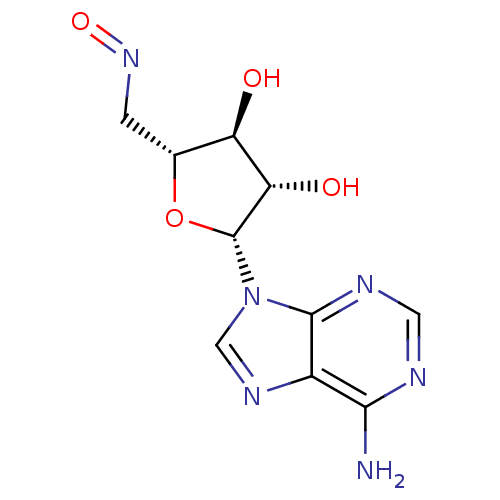
(CHEMBL2093112)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CN=O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C10H12N6O4/c11-8-5-9(13-2-12-8)16(3-14-5)10-7(18)6(17)4(20-10)1-15-19/h2-4,6-7,10,17-18H,1H2,(H2,11,12,13)/t4-,6-,7+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369159
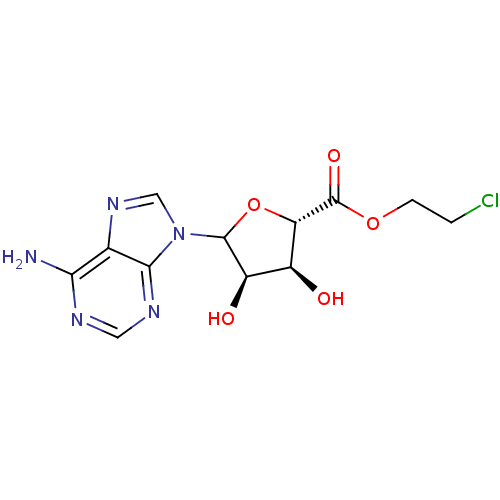
(CHEMBL610148)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)OCCCl |r| Show InChI InChI=1S/C12H14ClN5O5/c13-1-2-22-12(21)8-6(19)7(20)11(23-8)18-4-17-5-9(14)15-3-16-10(5)18/h3-4,6-8,11,19-20H,1-2H2,(H2,14,15,16)/t6-,7+,8-,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369158
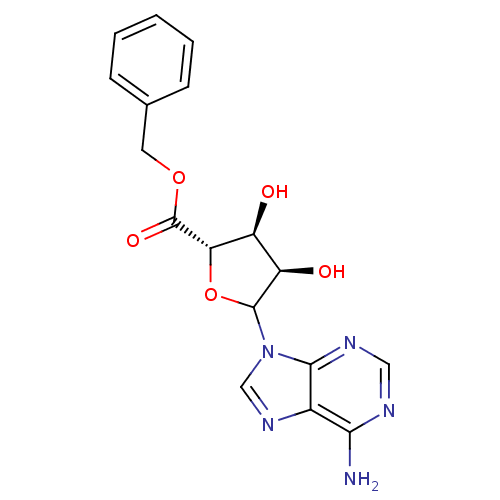
(CHEMBL612224)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C17H17N5O5/c18-14-10-15(20-7-19-14)22(8-21-10)16-12(24)11(23)13(27-16)17(25)26-6-9-4-2-1-3-5-9/h1-5,7-8,11-13,16,23-24H,6H2,(H2,18,19,20)/t11-,12+,13-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50331791
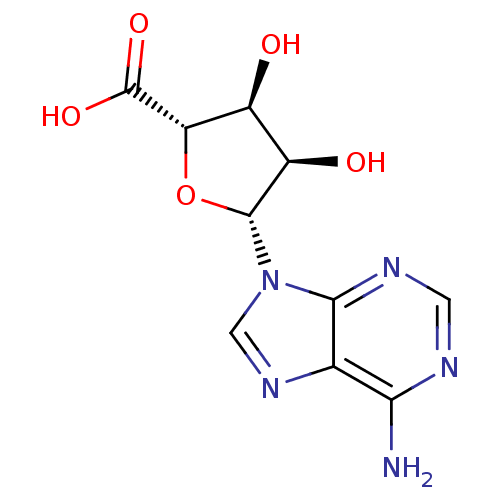
((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydr...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(O)=O |r| Show InChI InChI=1S/C10H11N5O5/c11-7-3-8(13-1-12-7)15(2-14-3)9-5(17)4(16)6(20-9)10(18)19/h1-2,4-6,9,16-17H,(H,18,19)(H2,11,12,13)/t4-,5+,6-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369161
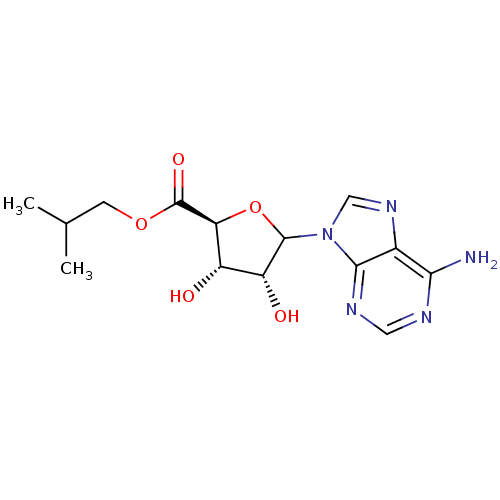
(CHEMBL610383)Show SMILES CC(C)COC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N5O5/c1-6(2)3-23-14(22)10-8(20)9(21)13(24-10)19-5-18-7-11(15)16-4-17-12(7)19/h4-6,8-10,13,20-21H,3H2,1-2H3,(H2,15,16,17)/t8-,9+,10-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369156
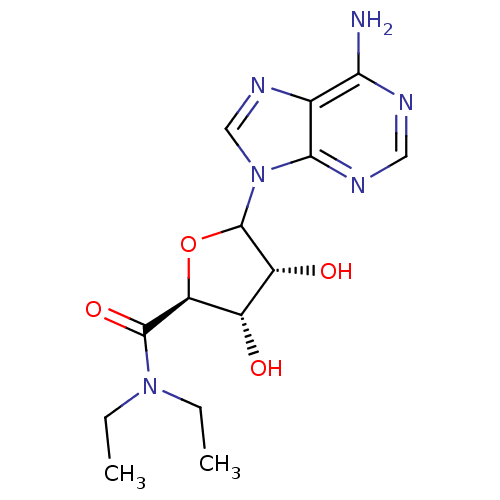
(CHEMBL608072)Show SMILES CCN(CC)C(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H20N6O4/c1-3-19(4-2)13(23)10-8(21)9(22)14(24-10)20-6-18-7-11(15)16-5-17-12(7)20/h5-6,8-10,14,21-22H,3-4H2,1-2H3,(H2,15,16,17)/t8-,9+,10-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50408148
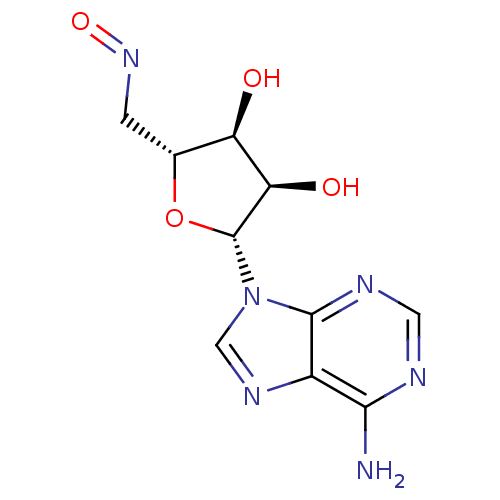
(CHEMBL1288616)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CN=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12N6O4/c11-8-5-9(13-2-12-8)16(3-14-5)10-7(18)6(17)4(20-10)1-15-19/h2-4,6-7,10,17-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method |
J Med Chem 40: 1608-18 (1997)
Article DOI: 10.1021/jm960828p
BindingDB Entry DOI: 10.7270/Q2PR7WPS |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50368898
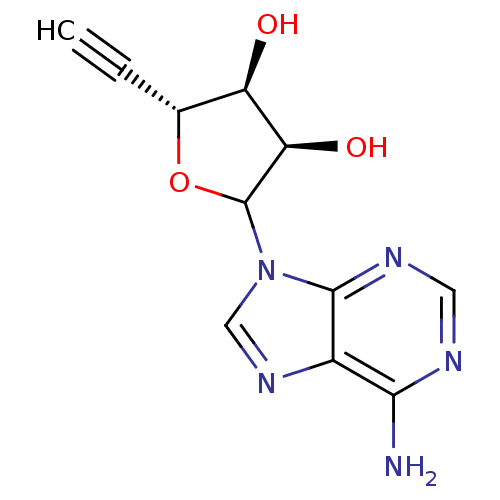
(CHEMBL604208)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](C#C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H11N5O3/c1-2-5-7(17)8(18)11(19-5)16-4-15-6-9(12)13-3-14-10(6)16/h1,3-5,7-8,11,17-18H,(H2,12,13,14)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 681 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369162
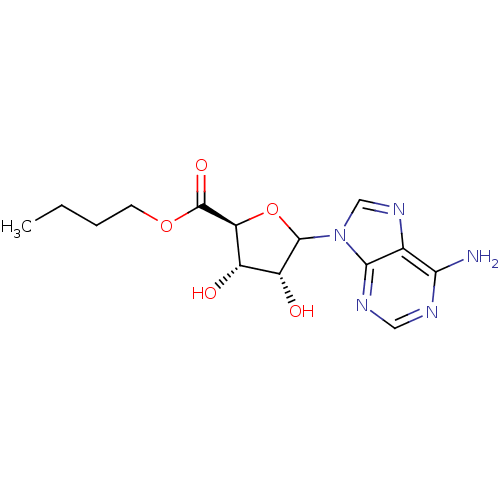
(CHEMBL610384)Show SMILES CCCCOC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N5O5/c1-2-3-4-23-14(22)10-8(20)9(21)13(24-10)19-6-18-7-11(15)16-5-17-12(7)19/h5-6,8-10,13,20-21H,2-4H2,1H3,(H2,15,16,17)/t8-,9+,10-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
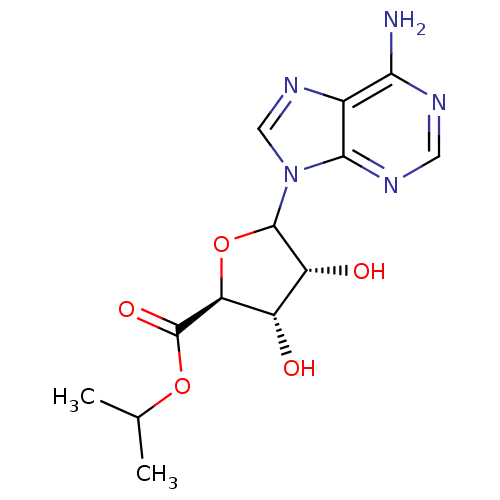
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369157
(CHEMBL608915)Show SMILES CC(C)OC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C13H17N5O5/c1-5(2)22-13(21)9-7(19)8(20)12(23-9)18-4-17-6-10(14)15-3-16-11(6)18/h3-5,7-9,12,19-20H,1-2H3,(H2,14,15,16)/t7-,8+,9-,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369393
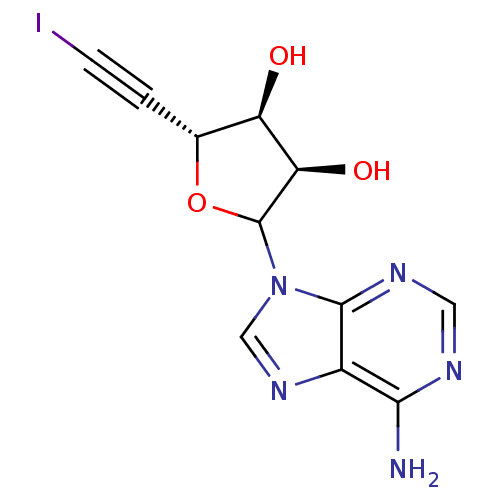
(CHEMBL608312)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](C#CI)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H10IN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h3-5,7-8,11,18-19H,(H2,13,14,15)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivation |
J Med Chem 41: 3857-64 (1998)
Article DOI: 10.1021/jm980163m
BindingDB Entry DOI: 10.7270/Q2DR2W64 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
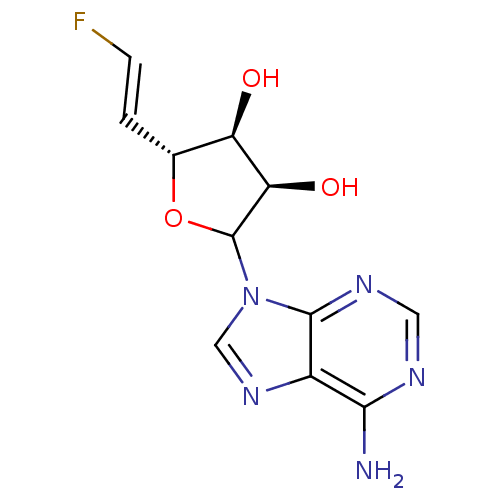
(Homo sapiens (Human)) | BDBM50368895
(CHEMBL610125)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=C\F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12FN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h1-5,7-8,11,18-19H,(H2,13,14,15)/b2-1+/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase |
J Med Chem 37: 3579-87 (1994)
BindingDB Entry DOI: 10.7270/Q2BK1D0X |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
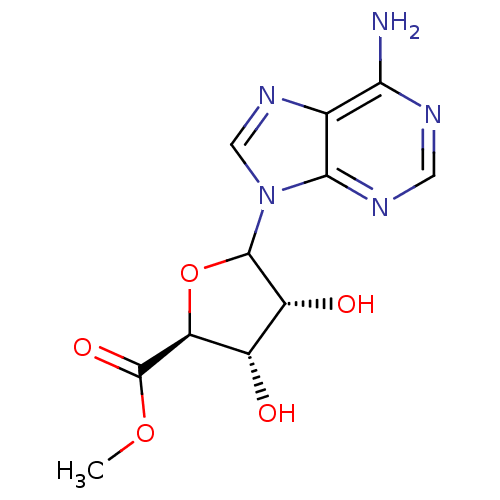
(Homo sapiens (Human)) | BDBM50369160
(CHEMBL608025)Show SMILES COC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13N5O5/c1-20-11(19)7-5(17)6(18)10(21-7)16-3-15-4-8(12)13-2-14-9(4)16/h2-3,5-7,10,17-18H,1H3,(H2,12,13,14)/t5-,6+,7-,10?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
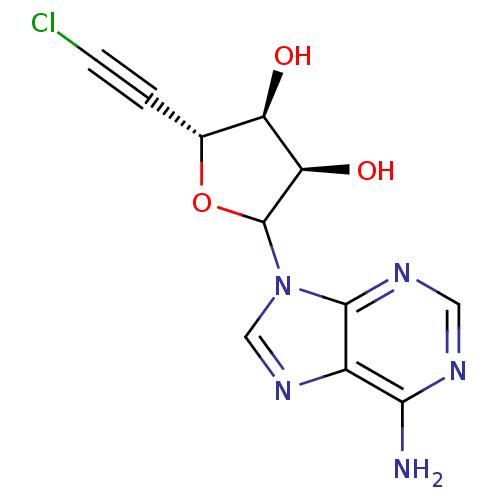
(Homo sapiens (Human)) | BDBM50369392
(CHEMBL608911)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](C#CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H10ClN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h3-5,7-8,11,18-19H,(H2,13,14,15)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivation |
J Med Chem 41: 3857-64 (1998)
Article DOI: 10.1021/jm980163m
BindingDB Entry DOI: 10.7270/Q2DR2W64 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50028376
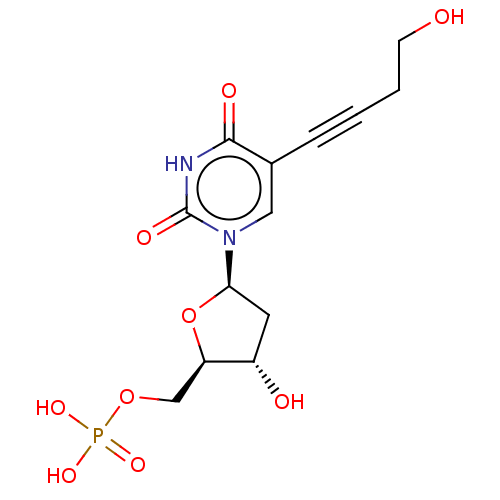
(CHEMBL3143870 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES OCCC#Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C13H17N2O9P/c16-4-2-1-3-8-6-15(13(19)14-12(8)18)11-5-9(17)10(24-11)7-23-25(20,21)22/h6,9-11,16-17H,2,4-5,7H2,(H,14,18,19)(H2,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei |
J Med Chem 24: 1385-8 (1982)
BindingDB Entry DOI: 10.7270/Q2MS3RSP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50407975
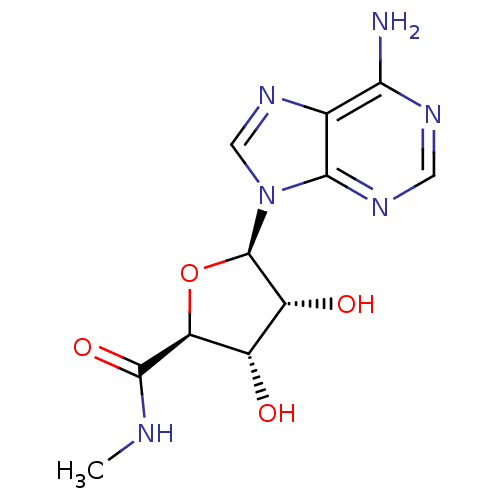
(CHEMBL519809)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H14N6O4/c1-13-10(20)7-5(18)6(19)11(21-7)17-3-16-4-8(12)14-2-15-9(4)17/h2-3,5-7,11,18-19H,1H3,(H,13,20)(H2,12,14,15)/t5-,6+,7-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50028372
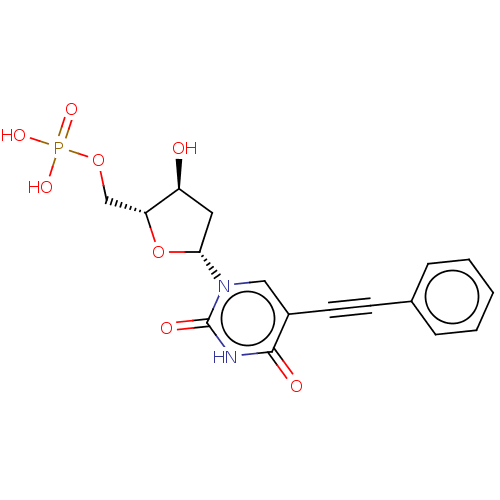
(CHEMBL3143873 | Phosphoric acid mono-[5-(2,4-dioxo...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1cc(C#Cc2ccccc2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C17H17N2O8P/c20-13-8-15(27-14(13)10-26-28(23,24)25)19-9-12(16(21)18-17(19)22)7-6-11-4-2-1-3-5-11/h1-5,9,13-15,20H,8,10H2,(H,18,21,22)(H2,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei |
J Med Chem 24: 1385-8 (1982)
BindingDB Entry DOI: 10.7270/Q2MS3RSP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50367376
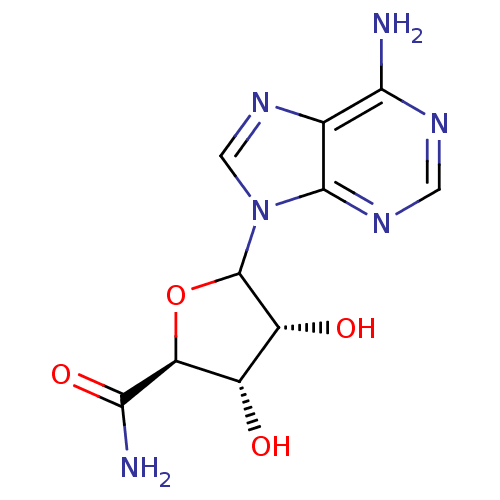
(CHEMBL605866)Show SMILES NC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C10H12N6O4/c11-7-3-9(14-1-13-7)16(2-15-3)10-5(18)4(17)6(20-10)8(12)19/h1-2,4-6,10,17-18H,(H2,12,19)(H2,11,13,14)/t4-,5+,6-,10?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50028375
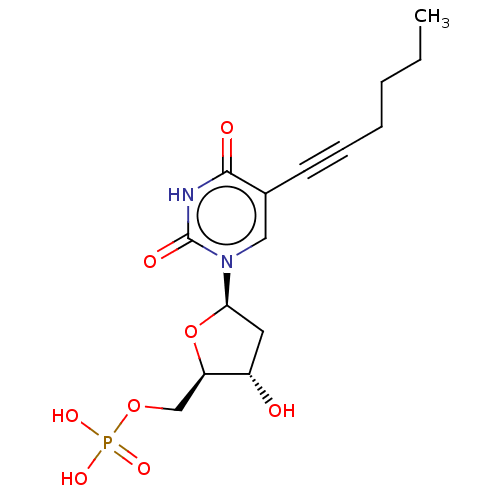
(CHEMBL3143872 | Phosphoric acid mono-[5-(5-hex-1-y...)Show SMILES CCCCC#Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H21N2O8P/c1-2-3-4-5-6-10-8-17(15(20)16-14(10)19)13-7-11(18)12(25-13)9-24-26(21,22)23/h8,11-13,18H,2-4,7,9H2,1H3,(H,16,19,20)(H2,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei |
J Med Chem 24: 1385-8 (1982)
BindingDB Entry DOI: 10.7270/Q2MS3RSP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50028377
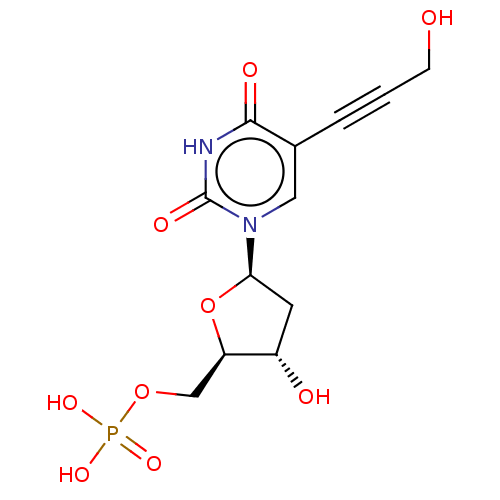
(CHEMBL3143869 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES OCC#Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C12H15N2O9P/c15-3-1-2-7-5-14(12(18)13-11(7)17)10-4-8(16)9(23-10)6-22-24(19,20)21/h5,8-10,15-16H,3-4,6H2,(H,13,17,18)(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei |
J Med Chem 24: 1385-8 (1982)
BindingDB Entry DOI: 10.7270/Q2MS3RSP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369380
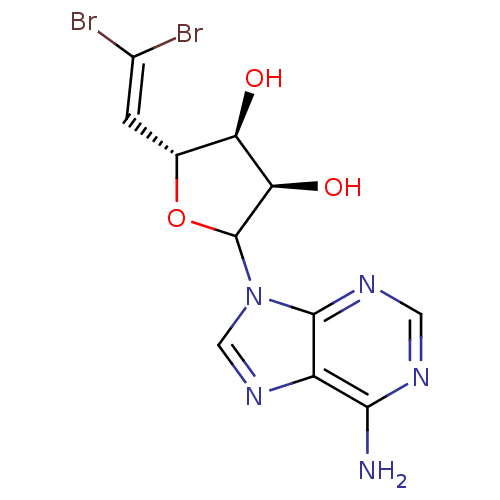
(CHEMBL606502)Show SMILES [#7]-c1ncnc2n(cnc12)-[#6]-1-[#8]-[#6@H](\[#6]=[#6](\Br)Br)-[#6@@H](-[#8])-[#6@H]-1-[#8] |r| Show InChI InChI=1S/C11H11Br2N5O3/c12-5(13)1-4-7(19)8(20)11(21-4)18-3-17-6-9(14)15-2-16-10(6)18/h1-4,7-8,11,19-20H,(H2,14,15,16)/t4-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound |
J Med Chem 41: 3078-83 (1998)
Article DOI: 10.1021/jm9801410
BindingDB Entry DOI: 10.7270/Q2MC90PC |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369381

(CHEMBL612194)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(\Br)=C/Br |r| Show InChI InChI=1S/C11H11Br2N5O3/c12-1-4(13)8-6(19)7(20)11(21-8)18-3-17-5-9(14)15-2-16-10(5)18/h1-3,6-8,11,19-20H,(H2,14,15,16)/b4-1+/t6-,7+,8+,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound |
J Med Chem 41: 3078-83 (1998)
Article DOI: 10.1021/jm9801410
BindingDB Entry DOI: 10.7270/Q2MC90PC |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369394

(CHEMBL608943)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](C#CBr)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H10BrN5O3/c12-2-1-5-7(18)8(19)11(20-5)17-4-16-6-9(13)14-3-15-10(6)17/h3-5,7-8,11,18-19H,(H2,13,14,15)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivation |
J Med Chem 41: 3857-64 (1998)
Article DOI: 10.1021/jm980163m
BindingDB Entry DOI: 10.7270/Q2DR2W64 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
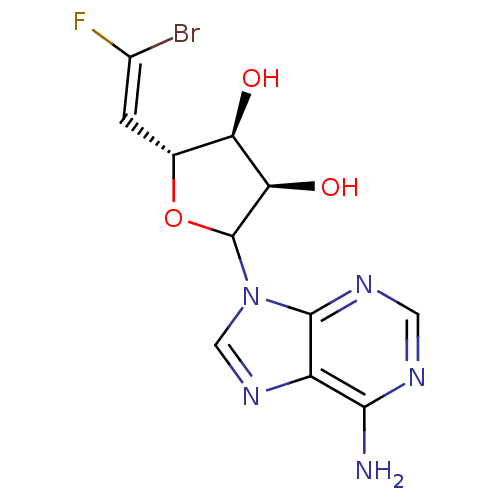
(Homo sapiens (Human)) | BDBM50369379
(CHEMBL607755)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](\C=C(\F)Br)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H11BrFN5O3/c12-5(13)1-4-7(19)8(20)11(21-4)18-3-17-6-9(14)15-2-16-10(6)18/h1-4,7-8,11,19-20H,(H2,14,15,16)/b5-1+/t4-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compound |
J Med Chem 41: 3078-83 (1998)
Article DOI: 10.1021/jm9801410
BindingDB Entry DOI: 10.7270/Q2MC90PC |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369155
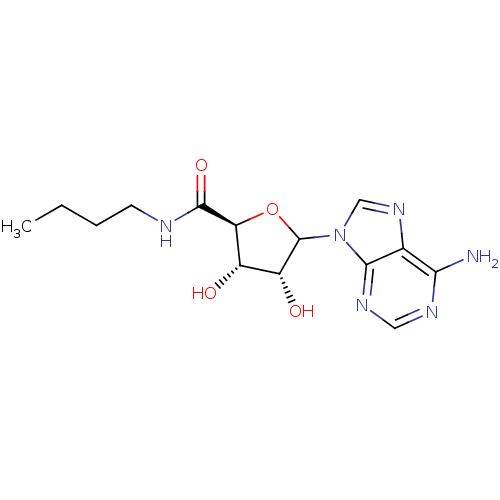
(CHEMBL609186)Show SMILES CCCCNC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H20N6O4/c1-2-3-4-16-13(23)10-8(21)9(22)14(24-10)20-6-19-7-11(15)17-5-18-12(7)20/h5-6,8-10,14,21-22H,2-4H2,1H3,(H,16,23)(H2,15,17,18)/t8-,9+,10-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM85777
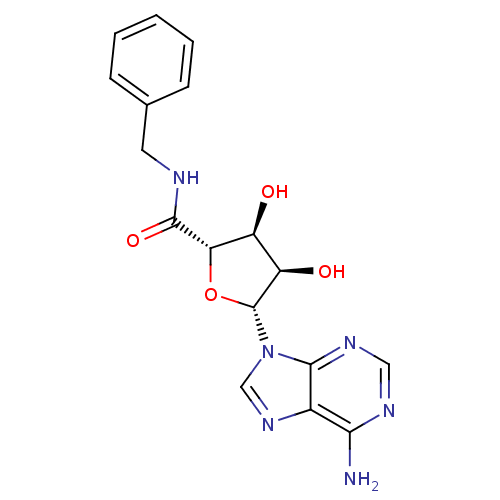
(B-NECA)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C17H18N6O4/c18-14-10-15(21-7-20-14)23(8-22-10)17-12(25)11(24)13(27-17)16(26)19-6-9-4-2-1-3-5-9/h1-5,7-8,11-13,17,24-25H,6H2,(H,19,26)(H2,18,20,21)/t11-,12+,13-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50369163
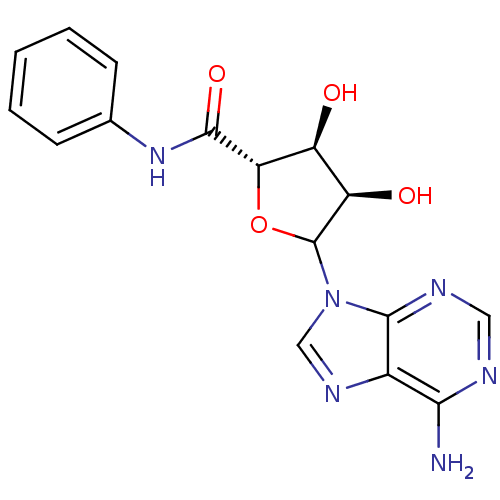
(CHEMBL610101)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H]([C@@H](O)[C@H]1O)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C16H16N6O4/c17-13-9-14(19-6-18-13)22(7-20-9)16-11(24)10(23)12(26-16)15(25)21-8-4-2-1-3-5-8/h1-7,10-12,16,23-24H,(H,21,25)(H2,17,18,19)/t10-,11+,12-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. |
J Med Chem 39: 4162-6 (1996)
Article DOI: 10.1021/jm960313y
BindingDB Entry DOI: 10.7270/Q2DN45QW |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217798
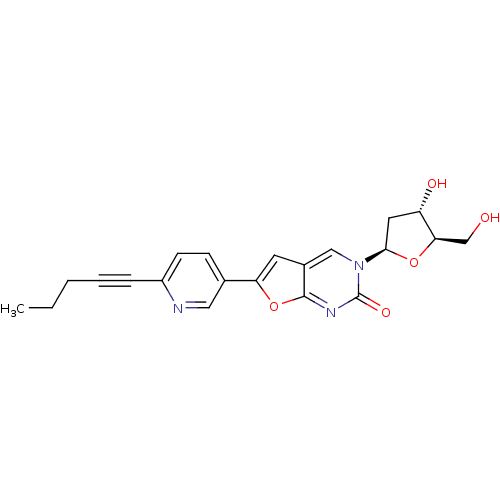
(3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-[2-(pe...)Show SMILES CCCC#Cc1ccc(cn1)-c1cc2cn([C@H]3C[C@H](O)[C@@H](CO)O3)c(=O)nc2o1 Show InChI InChI=1S/C21H21N3O5/c1-2-3-4-5-15-7-6-13(10-22-15)17-8-14-11-24(21(27)23-20(14)29-17)19-9-16(26)18(12-25)28-19/h6-8,10-11,16,18-19,25-26H,2-3,9,12H2,1H3/t16-,18+,19+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of VZV thymidine kinase |
J Med Chem 50: 3897-905 (2007)
Article DOI: 10.1021/jm070210n
BindingDB Entry DOI: 10.7270/Q2S75G19 |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217802
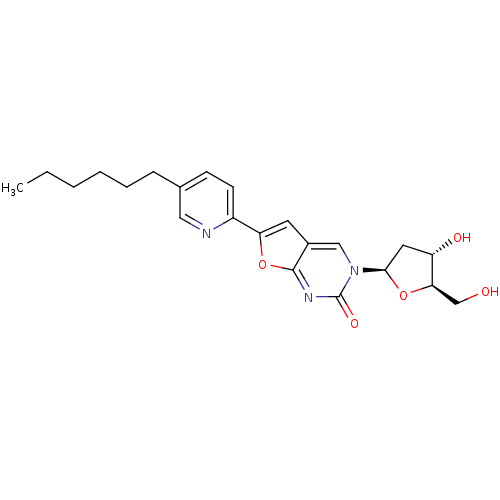
(3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(4-hex...)Show SMILES CCCCCCc1ccc(nc1)-c1cc2cn([C@H]3C[C@H](O)[C@@H](CO)O3)c(=O)nc2o1 Show InChI InChI=1S/C22H27N3O5/c1-2-3-4-5-6-14-7-8-16(23-11-14)18-9-15-12-25(22(28)24-21(15)30-18)20-10-17(27)19(13-26)29-20/h7-9,11-12,17,19-20,26-27H,2-6,10,13H2,1H3/t17-,19+,20+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of VZV thymidine kinase |
J Med Chem 50: 3897-905 (2007)
Article DOI: 10.1021/jm070210n
BindingDB Entry DOI: 10.7270/Q2S75G19 |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217799
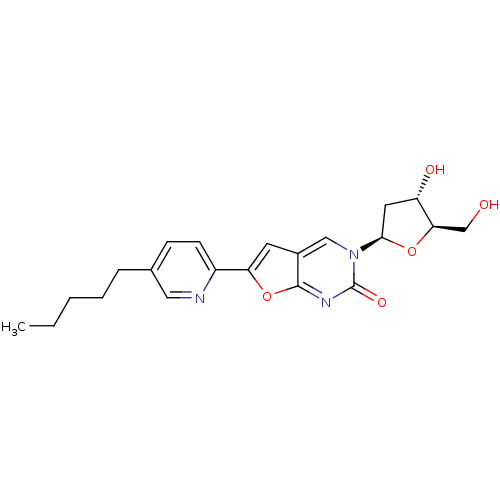
(3-(2-deoxy-D-erythro-pentofuranosyl)-6-(5-pentylpy...)Show SMILES CCCCCc1ccc(nc1)-c1cc2cn([C@H]3C[C@H](O)[C@@H](CO)O3)c(=O)nc2o1 Show InChI InChI=1S/C21H25N3O5/c1-2-3-4-5-13-6-7-15(22-10-13)17-8-14-11-24(21(27)23-20(14)29-17)19-9-16(26)18(12-25)28-19/h6-8,10-11,16,18-19,25-26H,2-5,9,12H2,1H3/t16-,18+,19+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of VZV thymidine kinase |
J Med Chem 50: 3897-905 (2007)
Article DOI: 10.1021/jm070210n
BindingDB Entry DOI: 10.7270/Q2S75G19 |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217806
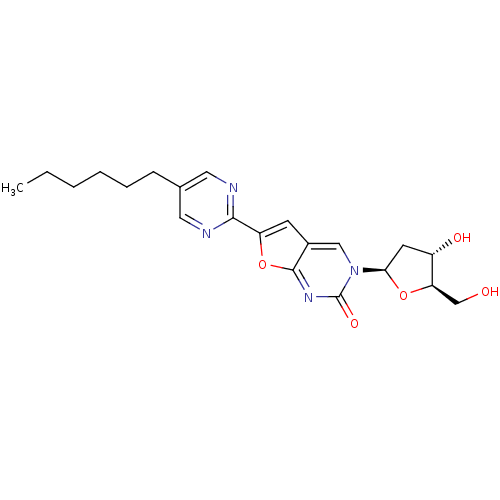
(3-(2-deoxy-D-erythro-pentofuranosyl)-6-(2-hexylpyr...)Show SMILES CCCCCCc1cnc(nc1)-c1cc2cn([C@H]3C[C@H](O)[C@@H](CO)O3)c(=O)nc2o1 Show InChI InChI=1S/C21H26N4O5/c1-2-3-4-5-6-13-9-22-19(23-10-13)16-7-14-11-25(21(28)24-20(14)30-16)18-8-15(27)17(12-26)29-18/h7,9-11,15,17-18,26-27H,2-6,8,12H2,1H3/t15-,17+,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of VZV thymidine kinase |
J Med Chem 50: 3897-905 (2007)
Article DOI: 10.1021/jm070210n
BindingDB Entry DOI: 10.7270/Q2S75G19 |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217797
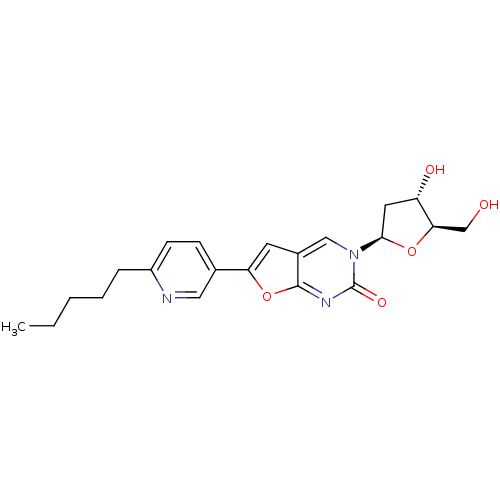
(3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(2-pen...)Show SMILES CCCCCc1ccc(cn1)-c1cc2cn([C@H]3C[C@H](O)[C@@H](CO)O3)c(=O)nc2o1 Show InChI InChI=1S/C21H25N3O5/c1-2-3-4-5-15-7-6-13(10-22-15)17-8-14-11-24(21(27)23-20(14)29-17)19-9-16(26)18(12-25)28-19/h6-8,10-11,16,18-19,25-26H,2-5,9,12H2,1H3/t16-,18+,19+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of VZV thymidine kinase |
J Med Chem 50: 3897-905 (2007)
Article DOI: 10.1021/jm070210n
BindingDB Entry DOI: 10.7270/Q2S75G19 |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217810
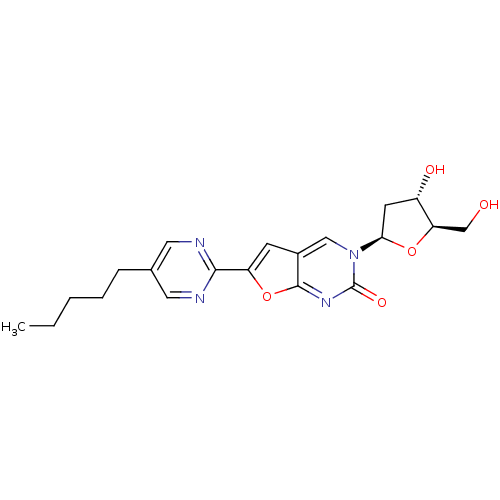
(3-(2-deoxy-beta-D-erythro-pentofuranosyl)-6-(5-pen...)Show SMILES CCCCCc1cnc(nc1)-c1cc2cn([C@H]3C[C@H](O)[C@@H](CO)O3)c(=O)nc2o1 Show InChI InChI=1S/C20H24N4O5/c1-2-3-4-5-12-8-21-18(22-9-12)15-6-13-10-24(20(27)23-19(13)29-15)17-7-14(26)16(11-25)28-17/h6,8-10,14,16-17,25-26H,2-5,7,11H2,1H3/t14-,16+,17+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of VZV thymidine kinase |
J Med Chem 50: 3897-905 (2007)
Article DOI: 10.1021/jm070210n
BindingDB Entry DOI: 10.7270/Q2S75G19 |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217804
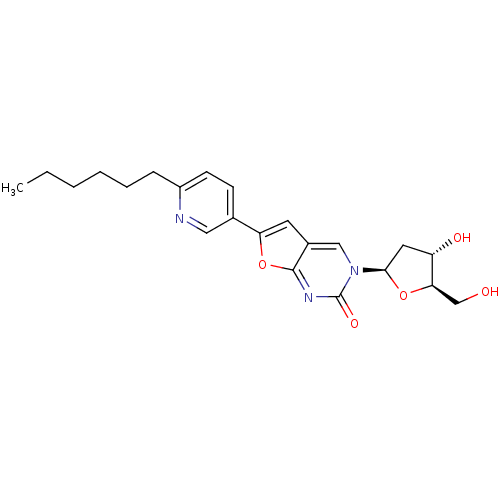
(3-(2-deoxy-D-erythro-pentofuranosyl)-6-(2-hexylpyr...)Show SMILES CCCCCCc1ccc(cn1)-c1cc2cn([C@H]3C[C@H](O)[C@@H](CO)O3)c(=O)nc2o1 Show InChI InChI=1S/C22H27N3O5/c1-2-3-4-5-6-16-8-7-14(11-23-16)18-9-15-12-25(22(28)24-21(15)30-18)20-10-17(27)19(13-26)29-20/h7-9,11-12,17,19-20,26-27H,2-6,10,13H2,1H3/t17-,19+,20+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of VZV thymidine kinase |
J Med Chem 50: 3897-905 (2007)
Article DOI: 10.1021/jm070210n
BindingDB Entry DOI: 10.7270/Q2S75G19 |
More data for this
Ligand-Target Pair | |
Thymidine kinase
(Varicella-zoster virus (strain Dumas) (HHV-3) (Hum...) | BDBM50217809
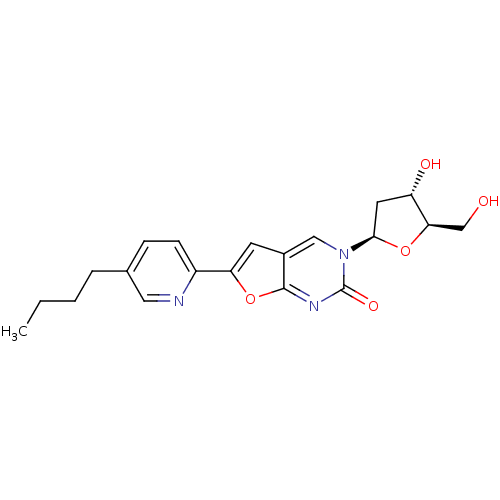
(6-(5-butylpyrid-2-yl)-3-(2-deoxy-beta-D-erythro-pe...)Show SMILES CCCCc1ccc(nc1)-c1cc2cn([C@H]3C[C@H](O)[C@@H](CO)O3)c(=O)nc2o1 Show InChI InChI=1S/C20H23N3O5/c1-2-3-4-12-5-6-14(21-9-12)16-7-13-10-23(20(26)22-19(13)28-16)18-8-15(25)17(11-24)27-18/h5-7,9-10,15,17-18,24-25H,2-4,8,11H2,1H3/t15-,17+,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University
Curated by ChEMBL
| Assay Description
Inhibition of VZV thymidine kinase |
J Med Chem 50: 3897-905 (2007)
Article DOI: 10.1021/jm070210n
BindingDB Entry DOI: 10.7270/Q2S75G19 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data